Magnetic resonance (MR) imaging has been playing an evolving role in evaluating noninflammatory small-bowel conditions, such as tumors and malabsorption syndrome. MR imaging has shown to be superior to other diagnostic methods in identifying tumors of the small bowel. MR enterography and MR enteroclysis are both valid for studying noninflammatory conditions of the small intestine, although MR enteroclysis may be considered the modality of choice because of its accuracy in the diagnosis of small-bowel neoplasms. Intraluminal and extraluminal MR findings, combined with contrast-agent enhancement and functional information, help to make an accurate diagnosis and consequently to characterize small-bowel diseases.
Key points
- •
Magnetic resonance (MR) enteroclysis provides adequate distension of the entire small bowel and can exclude small-bowel disease reliably.
- •
MR enteroclysis is an effective diagnostic technique in patients suspected of having small-bowel neoplasms.
- •
MR enteroclysis may also be used to distinguish neoplasms from inflammatory diseases.
- •
Small polypoid masses that do not cause an obstruction may be difficult to detect using MR enterography.
- •
Intraintestinal and extraintestinal features detected with MR imaging may be helpful in establishing a diagnosis of celiac disease and in clarifying the causes of nonspecific gastrointestinal symptoms in patients with previously undiagnosed celiac disease.
Introduction
Tumors of the small bowel are infrequent, accounting for about 3% to 6% of all gastrointestinal (GI) neoplasms. Diagnosis is not easy, owing to the nonspecific symptoms and because the mesenteric small bowel is traditionally the most difficult portion of the GI tract to investigate.
Most tumors are not determined by obstruction, and very often manifest with obscure GI bleeding, anemia, and abdominal pain. In the case of neuroendocrine tumors, the clinical manifestation depends on the hormones produced by the tumors.
Conventional enteroclysis and capsule endoscopy are the procedures most commonly used to visualize mucosal abnormalities, but are limited in the evaluation of mural and extramural extent of small-bowel tumors. Therefore, radiologists assume a major role in the detection of small-bowel tumors. However, inadequate radiologic studies may cause incorrect interpretation of radiologic findings, leading to crucial delay in diagnosing primary malignancies of the small intestine.
Magnetic resonance (MR) imaging has a leading role in the diagnosis not only of tumors but also of many diseases of the small intestine. The lack of ionizing radiation, the possibility of combining the morphologic information of cross-sectional imaging with functional information, the excellent soft-tissue contrast, and a relatively safe intravenous contrast agent profile make MR imaging the method of choice for the study of the small intestine. Moreover, the opportunity of studying surrounding structures (eg, for detection of any metastases in the liver) make MR imaging an excellent method not only for diagnosis but also for staging and prognosis. The intraluminal and extraluminal MR findings, combined with contrast enhancement and functional information, help to make an accurate diagnosis and, consequently, characterize small-bowel neoplasms.
Celiac disease is characterized by malabsorption of the intestine, which develops as a result of gluten and/or gluten-related protein intake. MR imaging provides morphologic information obtained noninvasively, such as fold pattern abnormalities and bowel dilatation, as well as extraintestinal findings such as mesenteric vascular congestion, lymphadenopathy, hyposplenism, and intussusception.
This article describes MR findings of primary small-bowel neoplasms and celiac disease, and discusses MR findings for the differential diagnosis.
Introduction
Tumors of the small bowel are infrequent, accounting for about 3% to 6% of all gastrointestinal (GI) neoplasms. Diagnosis is not easy, owing to the nonspecific symptoms and because the mesenteric small bowel is traditionally the most difficult portion of the GI tract to investigate.
Most tumors are not determined by obstruction, and very often manifest with obscure GI bleeding, anemia, and abdominal pain. In the case of neuroendocrine tumors, the clinical manifestation depends on the hormones produced by the tumors.
Conventional enteroclysis and capsule endoscopy are the procedures most commonly used to visualize mucosal abnormalities, but are limited in the evaluation of mural and extramural extent of small-bowel tumors. Therefore, radiologists assume a major role in the detection of small-bowel tumors. However, inadequate radiologic studies may cause incorrect interpretation of radiologic findings, leading to crucial delay in diagnosing primary malignancies of the small intestine.
Magnetic resonance (MR) imaging has a leading role in the diagnosis not only of tumors but also of many diseases of the small intestine. The lack of ionizing radiation, the possibility of combining the morphologic information of cross-sectional imaging with functional information, the excellent soft-tissue contrast, and a relatively safe intravenous contrast agent profile make MR imaging the method of choice for the study of the small intestine. Moreover, the opportunity of studying surrounding structures (eg, for detection of any metastases in the liver) make MR imaging an excellent method not only for diagnosis but also for staging and prognosis. The intraluminal and extraluminal MR findings, combined with contrast enhancement and functional information, help to make an accurate diagnosis and, consequently, characterize small-bowel neoplasms.
Celiac disease is characterized by malabsorption of the intestine, which develops as a result of gluten and/or gluten-related protein intake. MR imaging provides morphologic information obtained noninvasively, such as fold pattern abnormalities and bowel dilatation, as well as extraintestinal findings such as mesenteric vascular congestion, lymphadenopathy, hyposplenism, and intussusception.
This article describes MR findings of primary small-bowel neoplasms and celiac disease, and discusses MR findings for the differential diagnosis.
Technical considerations
Contrast Agents
A substance to be considered a good enteral agent should guarantee uniform and homogeneous opacification, adequate distension of small-bowel lumen, high contrast between the lumen and the small bowel, low cost, and the absence of serious adverse side effects.
A several number of enteral agents have been proposed for use in MR imaging of the small bowel. All of these substances are classified according to the intensity produced: positive, negative, or biphasic contrast enteral agents.
Positive contrast enteral agents have high signal intensity on T1-weighted images; gadolinium chelates, manganese ions, ferrous ions, and foods such as blueberry juice belong to this group. Using these contrast agents it is possible to show wall thickening on T1-weighted images; their limitation is the detection of more subtle mucosal or wall hyperenhancement after the intravenous injection of gadolinium-based contrast material.
Negative contrast enteral agents have low signal intensity on T2-weighted images. These agents constitute solutions with superparamagnetic iron oxides (SPIOs), including nanoparticles of maghemite in bentonite matrix, and ultrasmall SPIOs (USPIOs). On T2-weighted images, the inflammation in the bowel wall is much more pronounced because negative contrast agents reduce the signal intensity of the bowel lumen.
However, the low signal intensity of intraluminal contrast on T2-weighted images and the associated susceptibility effects may reduce the conspicuity of the normal small-bowel wall and of low–signal-intensity lesions, such as carcinoid tumors, as well as of intraluminal abnormalities.
For suspected small-bowel neoplasms it is recommended to use biphasic agents, such as water, Volumen, sorbitol, and polyethylene glycol (PEG), which produce low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. These agents provide conspicuous distinction between the bowel wall and the lumen, both on T2-weighted images (eg, by enabling the detection of mural ulcers, which could otherwise be missed, being darkened if a negative—hypointense—contrast agent is used) and on T1-weighted postgadolinium images (eg, by increasing the conspicuity of hyperenhancing masses).
On T1-weighted images, the contrast between the bowel lumen and masses after intravenous administration of contrast material is increased. The high contrast between the lumen and the dark bowel wall on T2-weighted images improves the detection of endoluminal abnormalities and more effectively highlights transmural ulcers.
The role of gadolinium chelates for the detection of small-bowel tumors has yet to be clearly defined. The accuracy of a nonenhanced MR protocol designed for detection of small-intestinal neoplasms was recently found to be similar to that of a protocol that included contrast enhancement.
Gadolinium-enhanced fat-saturated T1-weighted pulse sequences help to characterize small-bowel tumors because of their enhancement pattern, and to stage neoplasms by evaluating the presence of liver and mesenteric metastasis. Moreover, the use of gadolinium-enhanced T1-weighted sequences allows the characterization of malignant versus benign strictures in small-bowel obstruction.
Contrast-enhanced sequences help to differentiate inflammatory from noninflammatory diseases, thereby increasing diagnostic accuracy. Dynamic contrast-enhanced MR imaging of the small bowel may be useful in evaluating and identifying bowel-wall inflammation in patients with celiac disease.
The use of gadolinium is highly discouraged in pregnant patients and in patients with renal failure, as it increases the risk of developing nephrogenic systemic fibrosis.
MR Techniques: Enterography and Enteroclysis
The first step in the evaluation of the small intestine is to achieve a good distension of the small bowels. This distension is critical because collapsed bowel loops may hide lesions or mimic disease by mistakenly suggesting that the collapsed segments are actually an abnormality-related thickened bowel wall.
At present there are 2 basic methods to obtain distension of the small bowel, namely MR enteroclysis and MR enterography; the first is obtained by infusion of the contrast material through a nasojejunal tube, the second by oral administration of contrast material.
The choice between the intubation-infusion method (enteroclysis) and the oral approach (enterography) is controversial and depends on the clinical indications, the patient population, the radiology practice, and the diagnostic algorithms of different centers.
Enteroclysis is reported to be the only method that invariably provides adequate distension of the entire small bowel, and can help exclude small-bowel disease reliably.
MR enteroclysis provides better depiction of endoluminal lesions in the small intestine than that achieved on MR enterography, especially at level of jejunal loops. All sequences should be performed in breath-holds. The patient’s position during imaging can be supine or prone. Prone positioning reduces the area to be imaged, and may help elevate small-bowel loops and separate them from the pelvis. A preprocedural fasting time of 4 hours is recommended ; fasting reduces the amount of food residue and debris in the intestinal lumen, which may be mistaken for mass lesions or polyps. Most centers perform MR enterography with a torso coil at 1.5 T to enhance access and reproducibility of image quality. The pulse sequences used for both MR enteroclysis and MR enterography are essentially the same, the only difference being that breath-hold 2-dimensional T2-weighted fast spin-echo images are acquired continuously during the infusion of intraluminal contrast agent for MR enteroclysis, but only once for MR enterography.
Antiperistaltic agents are used to eliminate peristalsis and reduce motion artifact. Achieving reduced peristalsis is of considerable importance for the T1-weighted 3-dimensional sequences performed after the administration of intravenous contrast material, and may help to limit intraluminal flow artifacts on images obtained with the half-Fourier acquisition.
The T2-weighted sequence based on the half-Fourier reconstruction technique, termed either half-Fourier RARE or single-shot fast spin-echo, allows each image to be obtained in less than 1 second, thereby overcoming or limiting artifacts arising from small-bowel peristalsis. The coronal and axial balanced gradient-echo MR images that are obtained yield contrast that is intermediate relative to contrast on T1-weighted and T2-weighted images.
Balanced gradient-echo sequences (fast imaging employing steady-state acquisition [FIESTA] and true fast imaging with steady-state precession [TrueFISP]) are particularly effective as a means of obtaining information about mural and extraintestinal abnormalities.
Coronal gradient-echo fat-saturated T1-weighted sequences are performed after gadolinium-based contrast material is administered (0.2 mmol per kilogram of body weight at a rate of 2 mL/s). The entire protocol for MR enterography takes 20 to 25 minutes, whereas 30 to 35 minutes are required for the MR enteroclysis protocol (excluding the time required for the fluoroscopically guided nasojejunal intubation), owing to the intraluminal administration of contrast medium through a nasojejunal catheter.
MR enteroclysis is a validated technique for the detection of small-bowel tumors and low-grade obstruction of small bowel, whereas MR enterography has not yet demonstrated its potential for these indications. MR enteroclysis provides optimal small-bowel distension and allows more accurate detection of strictures. Moreover, small polypoid masses that do not cause an obstruction may be difficult to detect using oral contrast material for distension.
Image interpretation
The transit of the PEG solution through the small bowel is considered normal when unimpeded flow of intraluminal solution from the duodenojejunal junction to the ascending colon is observed, with no evidence of transit delay or stenosis during MR fluoroscopy.
Bowel-wall thickness of more than 3 mm must be considered abnormal. MR imaging is able to assess the morphology of the lesion and the endoluminal, mural, and extramural abnormalities; these findings are relevant for the differential diagnosis of small-bowel neoplasms.
Small-bowel tumors are commonly mildly hypointense or isointense to the intestinal wall on precontrast T1-weighted sequences, show a variable grade of enhancement on postgadolinium images, and are better depicted with the use of fat-suppression techniques, which increase the conspicuity between the enhancing mass and the surrounding mesenteric fat.
The high signal intensity of the intraluminal fluid and mesenteric fat on TrueFISP and half-Fourier acquisition single-shot turbo spin-echo (HASTE) images allows the depiction of tumors exhibiting intermediate signal intensity. High contrast between the tumors and surrounding bright fat enables MR imaging to accurately demonstrate the local extension of the lesions.
Benign tumors
Adenoma
Adenomas are the most common benign and asymptomatic tumors of the small intestine, which occur frequently in the duodenum, in particular near the ampulla of Vater. Adenomas may have malignant potential, and the presence of numerous adenomas in the duodenum should lead one to consider familial adenomatous polyposis.
Adenomas have different types of growth, and may appear as polypoid pedunculated mass on a stalk, a sessile mass (broad-based and without a stalk), or a mural nodule within the mucosa. Adenomatous small-bowel polyps appear as intraluminal homogeneous enhancing masses on gadolinium-enhanced fat-suppressed images, confined within the boundaries of intestinal lumen.
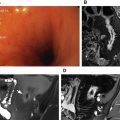
On HASTE and TrueFISP sequences, polyps appear as rounded low–signal-intensity intraluminal masses ( Fig. 1 ). MR fluoroscopy sequences show an intraluminal filling with no evidence of prestenotic dilatation.
Polyposis Syndromes
Peutz-Jeghers syndrome is an autosomal dominant disease characterized by multiple hamartomatous polyps involving the small bowel, colon, and stomach, and mucocutaneous pigmentation involving the mouth, fingers, and toes.
Polyps have malignant potential and usually are asymptomatic, sometimes being determined by obstruction or bleeding. Another common complication of Peutz-Jeghers syndrome is intussusception by a Peutz-Jeghers polyp. Although this concerns intussusceptions with a lead point, some of these resolve spontaneously.
Because of the possible malignant evolution of polyps, surveillance of patients with Peutz-Jeghers syndrome has always been considered a major problem. International guidelines recommend biennial examination of the small bowel. Conventional enteroclysis is no longer used because of the risk of cumulative radiation exposure associated with these tests.
Capsule endoscopy is probably the best method to visualize the mucosal abnormalities, and is usually the patient’s preference.
MR enterography offers a promising alternative to capsule endoscopy for surveillance of patients with large polyps, given that it is more accurate in the estimation of location and size of the intraluminal abnormalities.
No significant difference between MR enterography and wireless capsule endoscopy were found when used to detect large (>15 mm) clinically important polyps in patients with inherited polyposis syndromes, although MR imaging allowed more accurate localization of the polyps. MR enterography may thus be used for routine surveillance in patients with large polyps alone, because small polyps are not clinically relevant.
In adults with Peutz-Jeghers syndrome, MR enterography may be less prone than capsule endoscopy to miss large polyps and may also provide more reliable size assessments of these polyps. In addition, MR enterography offers the potential for detecting extraluminal cancers in this patient group.
MR enterography performed by combining prone and supine position was accurate in the detection of Peutz-Jeghers syndrome polyps, with 93% concordance with enteroscopy for larger and more risky polyps.
FISP and gadolinium-enhanced fat-suppressed volumetric interpolated breath-hold examination (VIBE) are the most useful MR imaging sequences for detecting small-bowel polyps. Polyps appear as hypointense filling defects on FISP images, and typically show marked enhancement similar to that of the bowel-wall mucosa after the intravenous administration of a gadolinium chelate.
Lipoma
Lipomas are mature adipose tissue proliferations that arise in the submucosa of the bowel wall and frequently are seen in the distal small bowel. Lipomas are asymptomatic and are found incidentally, but when large they may produce ulceration and present iron-deficiency anemia or positive fecal occult blood testing. These lesions usually grow intraluminally but can occasionally extend outward in the mesenteric surface, and the ileum is the most frequent location.
Lipomas are high in signal intensity on T1-weighted images and have signal intensity comparable with that of intra-abdominal fat on T2-weighted images. On T1-weighted and T2-weighted fat-suppressed images these lesions show a loss of signal intensity, without enhancement on postgadolinium images.
Hemangioma
Intestinal hemangiomas are congenital submucosal tumors, mostly located in the jejunum; they may consist of either capillaries or cavernous vessels, and most commonly manifest with acute or chronic bleeding of the GI tract. Hemangiomas can be sessile or pedunculated.
Hemangiomas may appear as multiple nodules that show low signal intensity on T1-weighted images and marked hypersignal on T2-weighted images; central nodular enhancement is seen within the tumor in the arterial phase, with centrifugal enhancement on delayed phase. It may be difficult to differentiate hemangiomas from other vascular tumors or malformations based on imaging criteria alone.
Leiomyoma
Leiomyomas are mesenchymal tumors that do not express the c-KIT protein; they manifest themselves with nonspecific symptoms and bleeding. Leiomyomas are oval or round masses with a maximal diameter of 1 to 10 cm; they have intense uniform enhancement greater than that of adjacent bowel on postgadolinium images, reflecting similar imaging findings ( Fig. 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree