Gastric and Duodenal Emergencies
- Vincent M. Mellnick
- Christine Menias
The upper gastrointestinal (GI) tract, including the esophagus, stomach, and duodenum, is a common but potentially overlooked site of disease that may prompt presentation to the emergency department (ED), including inflammation and infection, obstruction, and perforation. Although these organs have traditionally been evaluated by fluoroscopy, which offers mucosal detail, computed tomography (CT) is now the first-line imaging modality in most EDs, warranting knowledge of the appearance of these diseases on cross-sectional imaging as well. This section will discuss inflammation, infection, obstruction, and perforation of the esophagus, stomach, and duodenum. Of note, the topics of trauma, ischemia, and hemorrhage involving these organs will be discussed in other chapters.
Normal Anatomy
The esophagus extends from the lower border of the cricoid cartilage to the stomach, passing through the diaphragmatic hiatus at T10. Spanning 25 to 30 cm in length, the esophagus deviates to the left in the neck, to the right in most of the thorax, and then back to the left as it joins the gastroesophageal junction. Normal esophageal wall thickness on CT is less than or equal to 5 mm. The esophageal wall has an adventitial layer, but no serosa, which permits esophageal diseases to more readily extend into the nearby neck, mediastinum, or upper abdomen.
Esophageal Emergencies
Esophageal Inflammation/Infection
Esophagitis
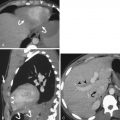
Esophagitis can arise from a number of causes, including infection, radiation, gastroesophageal reflux, and medications. Infectious esophagitis may have characteristic findings on barium fluoroscopy depending on the causative pathogen. Candida esophagitis manifests with raised mucosal plaques between which barium is trapped. Herpes esophagitis typically presents with multiple small ulcers represented by pooled barium. Larger ulcers are seen with cytomegalovirus and human immunodeficiency virus (HIV) esophagitis. Reflux esophagitis may manifest with strictures, as well as reticular-appearing mucosa distally, indicating Barrett esophagus. In contrast to the detailed mucosal evaluation afforded by barium fluoroscopy, CT findings of infectious esophagitis are nonspecific and insensitive, demonstrating a sensitivity of approximately 55% in one study. Computed tomography findings include long segment thickening of the esophageal wall (greater than 5 mm), as well as a “target” sign caused by mucosal hyperemia and submucosal edema ( Fig. 13-1 ). There is a large overlap in the CT appearance of infectious and noninfectious esophagitis, such as from reflux or radiation. Complications of esophagitis include functional obstruction, aspiration, and perforation.
Esophageal Obstruction
Foreign Bodies
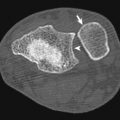
Esophageal foreign bodies are most often ingested by children and patients with cognitive defects. Commonly seen foreign bodies include impacted food boluses, fish and chicken bones, coins, and vinyl gloves. Although most esophageal foreign bodies pass spontaneously, 10% to 20% of cases require endoscopic removal and approximately 1% undergo surgery for treatment. Up to one third of affected adult patients with foreign body impaction have an underlying esophageal stricture contributing to their presentation. Diagnostic workup often starts with radiography, particularly if the ingested item is radiodense (e.g., metallic) or suspected to be lodged in the hypopharynx. Computed tomography is useful in select cases for definitive diagnosis and localization, and identifying suspected complications such as esophageal perforation ( Fig. 13-2 ).
Esophageal Strictures
Although not an emergency per se, stricture of the esophagus can cause esophageal obstruction (including impaction of food boluses as discussed earlier) and presentation to the ED. Benign strictures have smooth borders and usually involve a longer segment of the esophagus. Possible causes of benign esophageal strictures include long-standing gastroesophageal reflux, radiation, chronic medication-induced esophagitis, nasogastric intubation, epidermolysis bullosa, and eosinophilic esophagitis. Barium studies can reliably characterize benign esophageal strictures, but those with equivocal or malignant features warrant further evaluation with endoscopy. Esophageal obstruction by a malignant stricture usually manifests with short segment involvement and mucosal shouldering on fluoroscopy. Corresponding submucosal soft tissue attenuation can be seen on CT, which may also demonstrate invasion into the adjacent mediastinum and mediastinal lymphadenopathy in advanced disease.
Esophageal Perforation
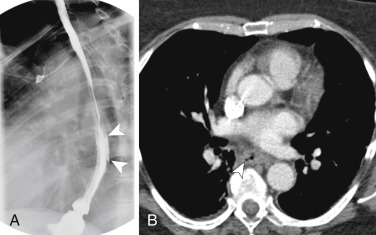
Mallory-Weiss Tears
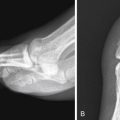
The clinical presentation and imaging findings of esophageal perforation depend on the thickness of the esophageal tear. Superficial tears, or Mallory-Weiss tears, commonly result from repeated, forceful retching and may cause “coffee-ground” emesis caused by mucosal bleeding. These tears may have no imaging findings, particularly on CT. On barium studies they may manifest as linear collections of contrast, classically in the distal esophagus, corresponding to mucosal tears identified on endoscopy ( Fig. 13-3 ).
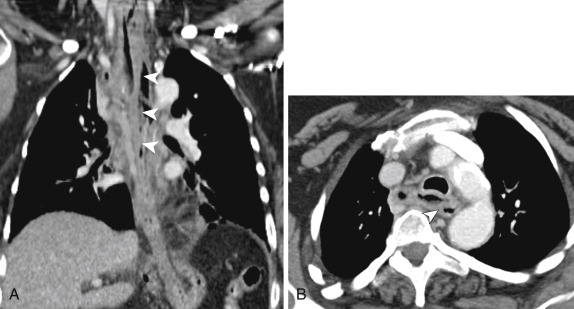
Esophageal Dissection
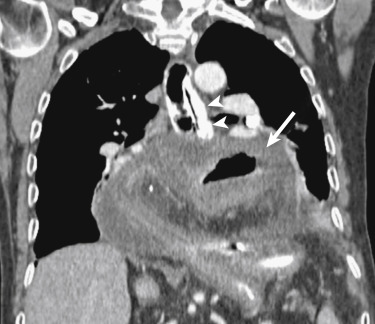
Intermediate—or intramural—perforation is a rare form of esophageal perforation also referred to as esophageal dissection . This condition can be seen after instrumentation, foreign body impaction, or forceful vomiting. Imaging findings of intramural perforation include a “double-barrel esophagus” caused by an intraluminal flap separating the true and false lumens, which may be accentuated with oral contrast, creating what has been also been termed a mucosal stripe sign . The false lumen of esophageal dissection is often posterior to the true lumen and may be better demonstrated on coronal and sagittal reformatted images. There is considerable overlap between esophageal dissection and an intramural hematoma, which may be distinguished by visualization of contrast in the false lumen with a true dissection ( Fig. 13-4 ).
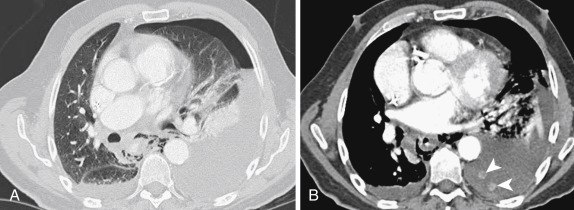
Transmural Perforation
Similar to esophageal dissection, full-thickness esophageal perforation may be iatrogenic, such as from surgery, stricture dilatation, stenting, or thermal injury. In addition, full-thickness tears may occur from vomiting (Boerhaave syndrome), caused by incomplete cricopharyngeal relaxation and increased intraluminal pressure. On imaging, full-thickness perforation may result in pneumomediastinum, pleural effusions, and leak of oral contrast into the mediastinal and/or pleural spaces ( Fig. 13-5 ). In addition to benign causes, primary or metastatic esophageal tumor may also perforate, particularly following palliative dilatation and/or stenting. Complications of esophageal perforation include mediastinitis, pneumonia (from direct spread of infection or from aspiration), and empyema/abscess formation.
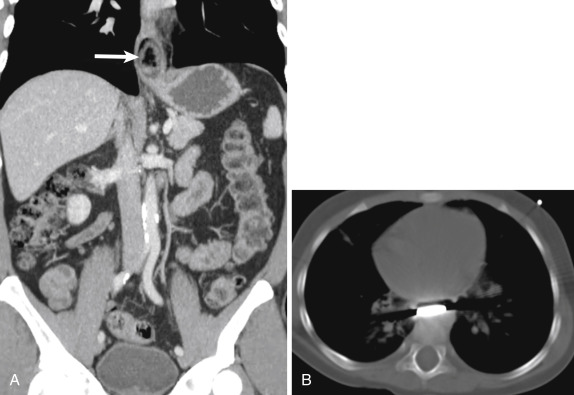
Aortoesophageal Fistulas
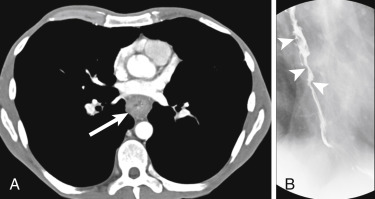
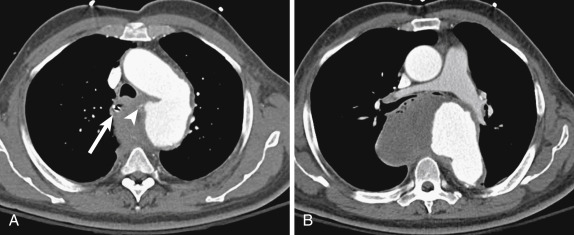
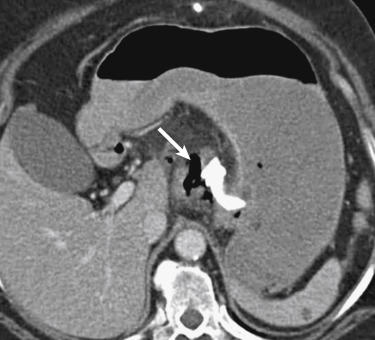
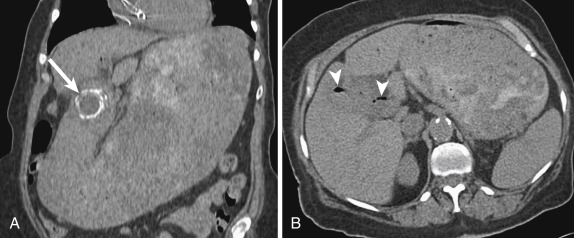
Chronically, esophageal perforation may result in fistula formation, including to the trachea, pleural space, and aorta. The majority of aortoesophageal fistulas result from aortic disease fistulizing to the esophagus, such as from rupture of a descending thoracic aortic aneurysm or an infected aortic graft. Primary esophageal disease may result in aortic fistulization as well: esophagitis, foreign body perforation, and advanced esophageal cancer have all been described as underlying causes. Clinically, patients with aortoesophageal fistulas present with the Chiari triad: midthoracic pain or dysphagia, a sentinel episode of hematemesis, and a symptom-free interval that gives way to massive upper GI bleeding. Imaging findings of aortoesophageal fistulas include a focal outpouching of the aorta toward the esophagus, adjacent esophageal wall thickening, and extraluminal gas within or abutting the aortic wall ( Fig. 13-6 ). Oral contrast or angiographic studies may also demonstrate a luminal communication.
Gastric Emergencies
Normal Anatomy
The stomach is divided into five arbitrary segments: the cardia, fundus, body, antrum, and pylorus. The lesser curvature of the stomach is at the right, posterior aspect of the organ, whereas the greater curvature of the stomach lies to the left and anteriorly. At the junction of the lesser curvature and antrum lies the incisura angularis. A line drawn from the incisura to the greater curvature of the stomach represents the transition zone from body-type to antral-type mucosa, which is a frequent site for gastric ulcers. Intraluminal rugal folds in the stomach are most prominent in the gastric fundus and body. The gastric antrum demonstrates a thicker wall on CT and can normally demonstrate mural stratification, owing to its greater muscular composition and increased peristalsis in this segment. The degree of distention within the gastric segment in question determines normal wall thickness on CT. The nondependent gastric body should be less than or equal to 5 mm in thickness when properly distended, whereas the antrum may normally measure up to 12 mm in thickness. The fundus is the most dependent portion of the stomach and is subsequently the most common site for layering intraluminal contents, notably blood products.
Gastric Inflammation and Infection
Gastritis
Gastritis has many potential underlying causes, including Helicobacter pylori infection, nonsteroidal antiinflammatory medications, and alcohol. Inflammation of the stomach is most commonly diffuse, but it can also be focal process. H. Pylori gastritis may be isolated to the antrum or greater curvature. On fluoroscopy, gastritis manifests with thickened rugal folds, erosions, and foci of ulceration, identified by persistent pooling of oral contrast. On CT, gastric inflammation appears as low-attenuation submucosal edema and mucosal hyperemia, which result in mural stratification. As previously discussed, attention to the degree of gastric distention helps discern true gastric wall thickening from artificial thickening resulting from collapsed lumen.
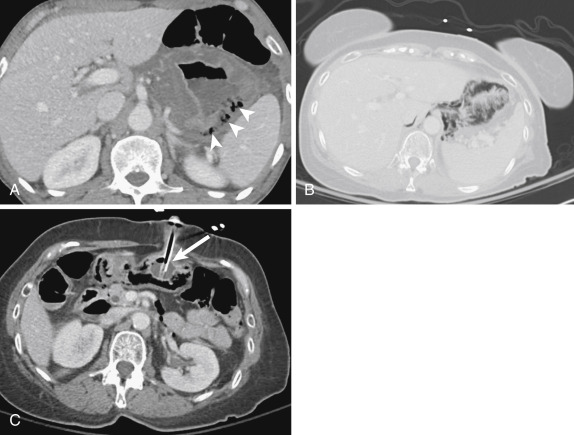
A subset of patients with gastritis can develop the emphysematous variant, an uncommon form with a high mortality rate. The underlying pathologic process in emphysematous gastritis is mucosal disruption and invasion of the gastric wall by a gas-producing organism, particularly Escherichia coli . On CT this condition presents with signs of gastritis (e.g., mural stratification), accompanied by gas bubbles in the stomach wall and potentially portal venous gas. Differentiation between emphysematous gastritis and more benign gastric emphysema may be difficult, but the latter is much more common, is typically seen in relatively asymptomatic patients, and appears with more linear intramural gas with no wall thickening to suggest gastritis ( Fig. 13-7 ).
Peptic Ulcer Disease
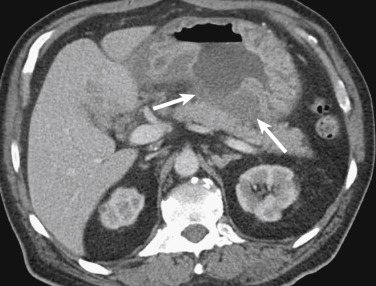
Although the incidence of peptic ulcers has decreased since the advent of H. pylori treatment and proton pump inhibitors, they remain a potential cause for presentation to the ED. Whereas superficial ulcers can be seen only with endoscopy or with barium fluoroscopy, deeply penetrating ulcers may be identified on CT as a focal mucosal outpouching, submucosal edema, perigastric fat stranding, and in the case of perforation, extraluminal gas ( Fig. 13-8 ). In patients who have undergone gastric surgery, particularly Billroth and Roux-en-Y gastric bypass procedures, marginal gastric ulcers may appear at the gastrojejunal anastomosis. These ulcers can be deeply penetrating, usually begin at the jejunal side of the anastomosis, and typically develop 2 to 4 years following surgery.
Crohn Disease
Gastroduodenal Crohn disease (CD) is rare, causing clinical symptoms in 0.5% to 4% of all patients with CD. Most of these patients have simultaneous involvement of the distal small bowel or the colon. The most common manifestation of gastroduodenal CD is contiguous involvement of the antrum, pylorus, and proximal duodenum, resulting in a nondistensible distal stomach and a characteristic “ram’s horn” appearance. Other imaging findings include mucosal “cobblestoning,” thickening folds, pseudopolyps, ulcers (including postbulbar ulcers in the duodenum), and strictures. Like CD elsewhere in the GI tract, gastroduodenal involvement may be complicated acutely by obstruction, perforation, abscess, and fistula formation.
Gastric Obstruction
Gastric Volvulus
An uncommon cause of gastric obstruction, gastric volvulus presents clinically with Borchardt triad: sudden epigastric pain, intractable retching, and inability to pass a nasogastric tube. The two subsets of gastric volvulus are organoaxial and mesenteroaxial, although many are seen as a combination of these. Organoaxial gastric volvulus is the most common type and results from rotation about the long axis (line connecting the pylorus to the cardia) of the stomach with the antrum anterosuperiorly and the fundus posteroinferiorly ( Fig. 13-9 ). This type of gastric volvulus is more commonly associated with diaphragmatic defects and vascular compromise. Mesenteroaxial gastric volvulus is less common and results from rotation of the stomach about its short axis, resulting in the antrum being positioned above the gastroesophageal junction ( Fig. 13-10 ). These are more commonly chronic and less likely to be associated with diaphragmatic hernias.
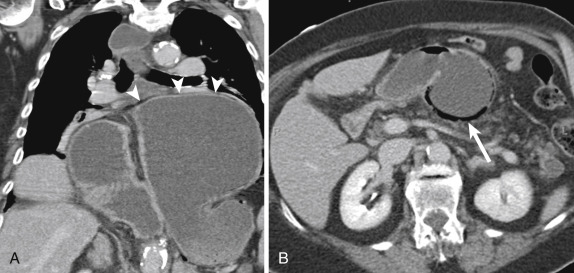
On imaging, gastric volvulus presents with a distended stomach, nonpassage of oral contrast, and an abnormal lie to the stomach. Gastric volvulus requires at least 180 degrees of rotation and gastric outlet obstruction. Asymptomatic patients with large hiatal hernias may present with an abnormal lie, that is, organoaxial positioning of the stomach without volvulus. These patients are predisposed to both gastric volvulus and hiatal hernia incarceration, particularly when the fundus “redescends” into the abdomen, obstructing the mouth of the hernia sac.
Peptic Ulcer Disease
Similar to peptic ulcer disease in general, the incidence of gastric outlet obstruction due to peptic ulcer disease is decreasing. Acutely, obstruction from peptic ulcer disease occurs because of mucosal ulceration and submucosal edema. More commonly, in the chronically affected patient, fibrosis may also cause luminal narrowing and obstruction. On imaging this appears as narrowing of the affected segment with associated soft tissue thickening, most often the pylorus. However, because of its overlap with malignant causes of gastric outlet obstruction, exclusion of an underlying mucosal lesion is often warranted.
Malignancy
Primary tumors in or abutting the stomach may be associated with gastric outlet obstruction. This is most commonly seen with gastric adenocarcinoma but can be seen with pancreatic and biliary tumors, primary duodenal tumors, and rarely lymphoma. In the case of malignant duodenal obstruction, many such cases are generally classified as causing “gastric outlet obstruction,” leading to ambiguity as to the precise level of obstruction. After gastric adenocarcinoma the second most common tumor to obstruct the stomach and/or duodenum is pancreatic head adenocarcinoma, which can cause gastric outlet obstruction in 15% to 25% of cases. Imaging clues to a malignant cause for gastric or duodenal obstruction include mucosal shouldering on barium studies, soft tissue thickening at the transition point of the obstruction on CT, and evidence of metastatic disease, such as enlarged lymph nodes, liver lesions, or omental deposits.
Bezoars
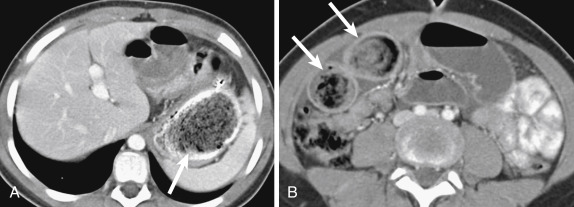
Ingested foreign bodies may be a cause of gastric or small bowel obstruction and often fall into two major categories: trichobezoars and phytobezoars. Trichobezoars are composed of hair and are most common in women and psychiatric patients, often those with long hair. When the hair extends from the stomach into the small and/or large bowel this has been termed Rapunzel syndrome ( Fig. 13-11 ). Phytobezoars are composed of fruit or vegetable matter, commonly oranges and persimmons. Patients with decreased gastric motility, such as those who have undergone gastric surgery, are predisposed to formation of phytobezoars, which may also pass into the small bowel and cause obstruction more distally as well. Patients who have undergone organ transplantation also have an increased risk for developing bezoars, which is hypothesized to be secondary to decreased gastric motility, either due to vagus nerve injury or a side effect of cyclosporine.
Gastric Banding
The increasing rate of obesity in the United States has resulted in a rising number of bariatric surgery techniques, one of which is gastric banding. The gastric banding device is composed of the band itself, an access port, and connecting tube. The band is secured around the cranial portion of the stomach, approximately 2 cm from the gastroesophageal junction, forming a small pouch and limiting food intake. The port is usually placed outside the peritoneal cavity, either within the rectus abdominis muscles sheath or under the external thoracic fascia. If the band slips distally, it may surround a larger portion of the stomach and lead to gastric outlet obstruction. Normal gastric band orientation is assessed with the phi angle, which is increased with a slipped band. Measured in the anteroposterior dimension, the phi angle (angle between the spinal column and gastric band) has a normal range of 4 to 58 degrees.
Gastric Perforation
Peptic Ulcer Disease
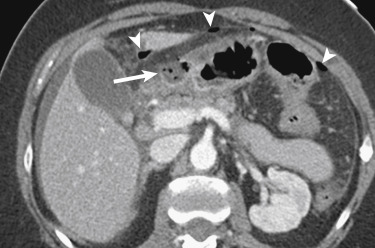
Perforation complicates peptic ulcer disease in approximately 2% to 10% of affected patients. The imaging findings of perforation can vary depending upon the location of the perforated ulcer. Ulcers on the anterior wall and greater curvature perforate freely into the peritoneal space, whereas more posterior ulcers may have a relatively contained perforation into the lesser sac. Perforation of peptic ulcers within the duodenal bulb and stomach remains one of the most common causes of GI perforation, warranting close examination of the stomach and proximal duodenum for ulcers when free intraperitoneal gas is identified on CT ( Fig. 13-12 ).
Malignancy
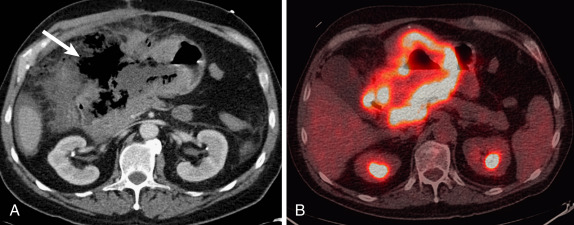
Gastric adenocarcinoma is complicated by perforation in 0.4% to 6.0% of cases and is more common in patients older than 65 years of age. Although typically found in locally advanced gastric cancer, a focal ulcerated malignancy may perforate if the ulcer crater is deeply penetrating. Perforation of GI lymphoma is most commonly seen in the small bowel, but gastric lymphoma may also perforate; perforation is seen in a higher percentage of T-cell lymphoma involving the GI tract than with B-cell lymphoma ( Fig. 13-13 ). Clues to an underlying malignancy as a cause of gastric perforation include focal gastric wall thickening with soft tissue attenuation in the submucosa, as well as evidence of tumor outside of the stomach such as lymphadenopathy or metastases.
Gastric Banding
Perforation related to gastric banding can be seen acutely in 0.1% to 0.8% of patients and may have a varied clinical presentation. Computed tomography may demonstrate free or loculated extraluminal gas or abscess formation. Chronic perforation of the stomach complicates 1% to 3% of patients with gastric bands and results from erosion of the band into the stomach lumen. On fluoroscopy, demonstration of contrast outlining the band can confirm erosion, which may also manifest on CT with gas surrounding the band. In addition, CT may show inflammation or fluid tracking along the connector tubing and/or port; when seen, this should prompt evaluation of the band as the site of erosion or infection ( Fig. 13-14 ).
Duodenal Emergencies
Normal Anatomy
The first segment of the small intestine, the duodenum is typically 25 to 38 cm in length and extends from the gastric pylorus to the duodenojejunal flexure (ligament of Treitz). The duodenum consists of four segments: the intraperitoneal bulb, followed by the retroperitoneal descending, transverse, and ascending portions. The second (descending) portion of the duodenum contains the ampulla of Vater and abuts the pancreas, forming the pancreaticoduodenal groove.
Duodenal Inflammation and Infection
Peptic Ulcer Disease
Duodenal peptic ulcers are more common than gastric ulcers, typically solitary, and located in the duodenal bulb in 5% to 11% of patients. In contrast to peptic ulcers in the stomach, duodenal ulcers have very low malignant potential and typically occur because of increased peptic acid secretion, including in the setting of chronic H. pylori infection. However, similar to their gastric counterparts, duodenal peptic ulcers show persistent pooling of oral contrast on fluoroscopy and on CT show a focal outpouching with mucosal hyperemia, submucosal edema, and adjacent fat stranding.
Zollinger-Ellison Syndrome
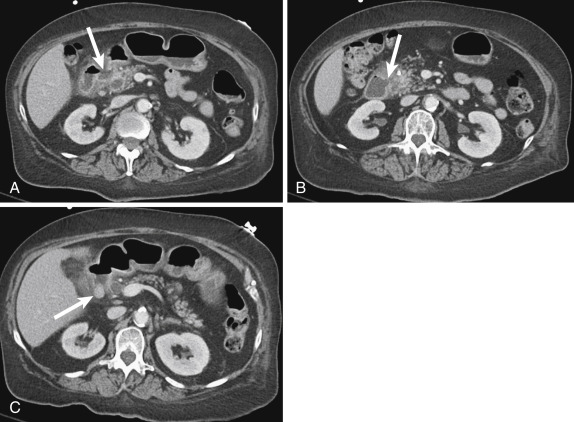
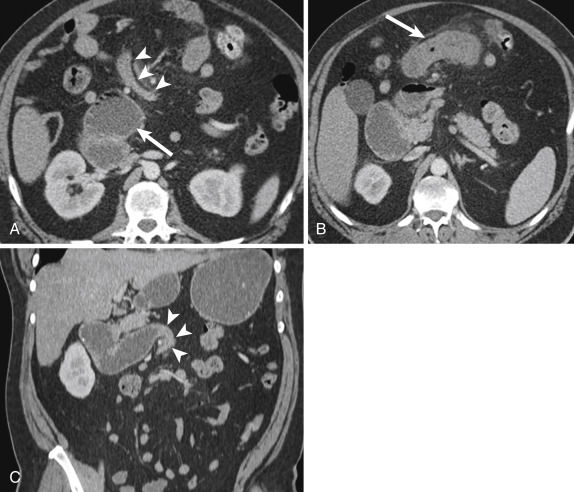
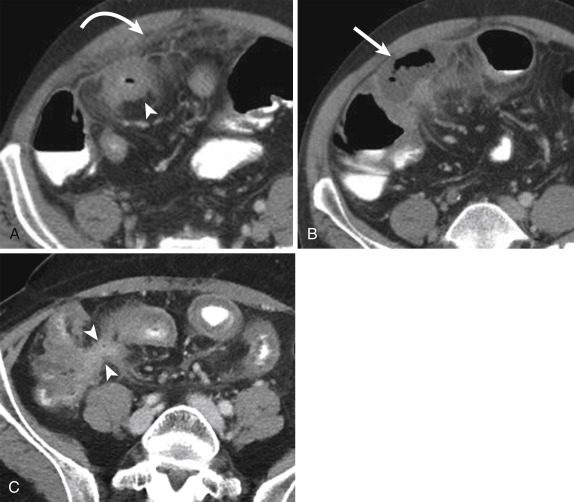
A subset of patients with duodenal ulcers will have Zollinger-Ellison syndrome, typically caused by a gastrin-secreting tumor in the gastrinoma triangle, an anatomic space defined by the junctions of the cystic and common bile ducts, the neck and body of the pancreas, and the second and third portions of the duodenum. The increased serum gastrin levels stimulate peptic acid secretion and lead to duodenal mucosal ulceration. Computed tomography findings of Zollinger-Ellison syndrome include reflux esophagitis, gastritis, and multiple duodenal ulcers, including distal to the duodenal bulb ( Fig. 13-15 ). This conglomerate of findings should prompt a search for an adjacent hyperenhancing nodule representing a gastrinoma, although these may be difficult to identify on routine portal venous phase CT and may require multiphase phase CT, endoscopic ultrasonography (EUS), or octreotide scan to aid in detection.
Diverticulitis
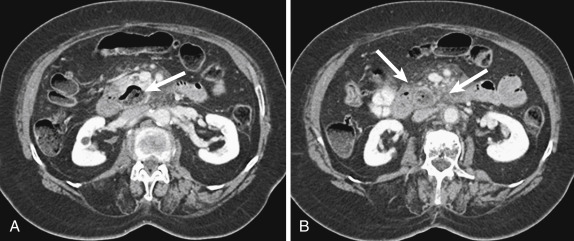
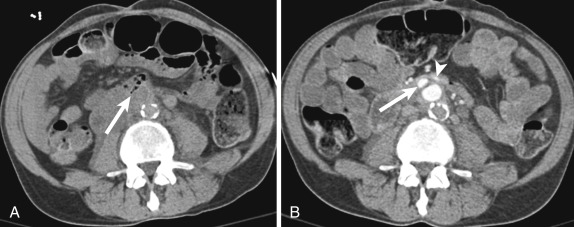
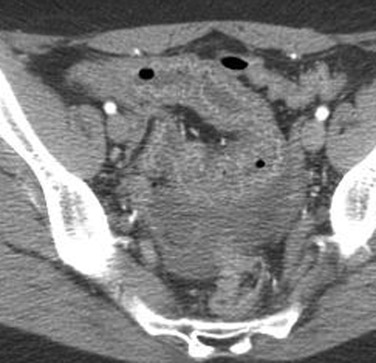
Duodenal diverticula are very common, occurring in approximately 23% of patients, most common in the second portion and typically arising medially. Although a relative minority of duodenal diverticula cause symptoms, up to 1% of patients will have a complication from a duodenal diverticulum that requires treatment, including diverticulitis, perforation, and obstruction in the case of intraluminal diverticula. Computed tomography findings of duodenal diverticulitis resemble those seen in inflamed diverticula elsewhere in the GI tract: a focal, round outpouching from the duodenal lumen with wall thickening and adjacent fat stranding and fluid ( Fig. 13-16 ). Treatment of duodenal diverticulitis may be operative or nonoperative (i.e., antibiotics), depending on the clinical condition and stability of the patient.
Groove Pancreatitis
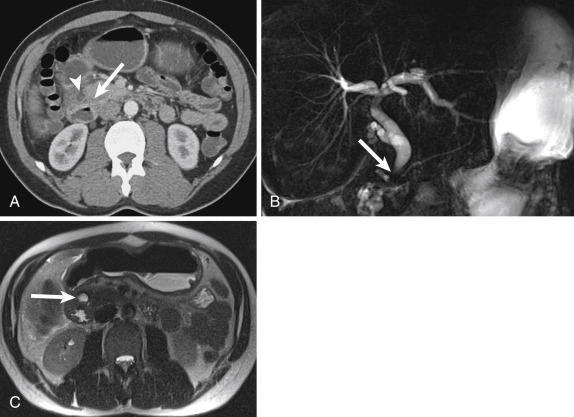
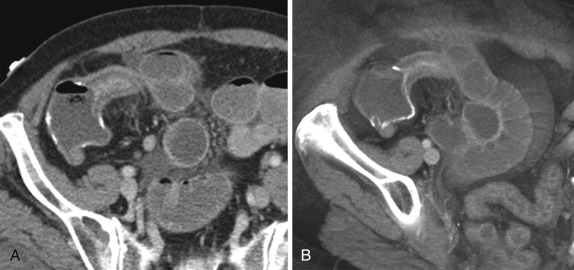
A rare form of chronic pancreatitis, groove pancreatitis occurs because of inflammation in the pancreaticoduodenal groove, including the pancreatic head and pancreatic rests in the duodenal wall. Clinically patients may present with normal serum amylase and lipase levels. The focal inflammation in the duodenal wall may result in benign-appearing common bile duct strictures and duodenal obstruction. The imaging appearance of groove pancreatitis may overlap with pancreatic adenocarcinoma considerably, but groove pancreatitis classically manifests with low-attenuation cystic areas (“cystic degeneration”) in the descending duodenal wall and soft tissue in the pancreaticoduodenal groove resulting from fibrosis. This disease can be particularly well characterized on magnetic resonance cholangiopancreatography (MRCP), which can demonstrate both the well-marginated T2-hyperintense cystic areas and biliary strictures without underlying mass lesion. These findings, along with a lack of vascular invasion, help differentiate between groove pancreatitis and adenocarcinoma of the pancreaticoduodenal groove ( Fig. 13-17 ). Associated fibrosis, which can result from chronic inflammation, may demonstrate delayed contrast enhancement on CT and magnetic resonance imaging (MRI).
Duodenal Obstruction
Malrotation and Volvulus
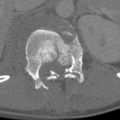
Although malrotation is rare in adulthood—80% of patients will present in the first month of life and 90% within the first year—some cases remain quiescent for years and present later in life. Affected patients may present clinically with symptoms of proximal bowel obstruction, namely, severe abdominal pain and bilious vomiting. On imaging studies the findings of malrotation are the same as those seen in childhood: a duodenum that does not cross the spine, reversal of the relationship between superior mesenteric artery (SMA) and vein, colon situated on the left side of the abdomen, and small bowel on the right. When complicated by volvulus, CT shows a distended stomach and duodenum and an abrupt transition to decompressed bowel with a focal twist causing a “whirlpool” sign ( Fig. 13-18 ). There may also be occlusion of mesenteric vessels with associated bowel ischemia, indicated by bowel wall thickening, hypoenhancement, and/or pneumatosis with adjacent mesenteric edema and fluid.
Superior Mesenteric Artery Syndrome
Duodenal obstruction may result from compression of the transverse portion as it crosses between the aorta and SMA. Patients predisposed to this condition include those with prior surgery causing loss or distortion of normal retroperitoneal fat, rapid weight loss, or a lean body habitus. Superior mesenteric artery syndrome can be diagnosed with an abnormally acute aortomesenteric angle (normal range is 28 to 65 degrees) or a decreased aortomesenteric distance (normally 10 to 34 mm) resulting in extrinsic compression of the duodenum. Treatment can either be conservative, including nasogastric decompression and nutritional supplementation, or surgical such as duodenojejunostomy.
Gallstones
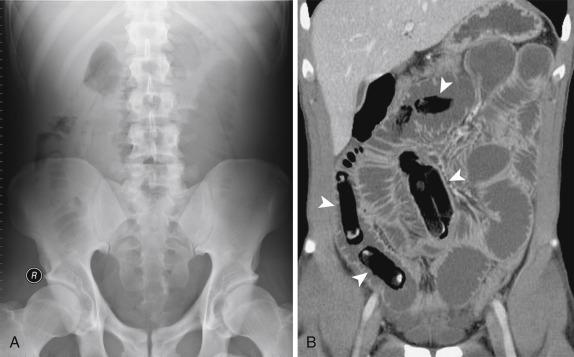
Gastric outlet obstruction due to gallstones, termed Bouveret syndrome , is a rare subset of gallstone ileus that presents classically with a triad of pneumobilia, an ectopic gallstone, and bowel obstruction ( Fig. 13-19 ). Although the majority, approximately 85% of patients with cholecystenteric fistulas, have stones pass spontaneously, 15% of these patients will have a resulting bowel obstruction, most commonly in the terminal ileum and least commonly in the stomach and duodenum. Bouveret syndrome has a high mortality rate and is typically treated surgically.
Malignancy See Gastric Obstruction, Malignancy
Duodenal Perforation
Peptic ulcer disease, trauma, and iatrogenic causes lead the list of potential causes of duodenal perforation. The imaging appearance depends on the segment of duodenum affected: bulb perforations present with free intraperitoneal gas and/or fluid, whereas the remaining segments perforate into the retroperitoneum. As such, one important space to examine for extraluminal fluid and gas is the right anterior pararenal space, which immediately abuts the descending and proximal transverse duodenum. Although in some cases a focal wall discontinuity or extraluminal gas may be found, many times a duodenal perforation may simply present with an intramural hematoma and/or adjacent fluid. In such cases of equivocal findings for duodenal perforation, oral contrast, either for fluoroscopy or repeat CT, may be a useful adjunct because a delay in diagnosis may portend a poor outcome.
Aortoduodenal Fistulas
Chronic perforation of the duodenum, similar to that of the esophagus, may result in fistulization to nearby structures, including small and large bowel, bile ducts, kidneys and ureters, inferior vena cava (IVC), and aorta. These fistulas can result from primary duodenal perforation, such as from malignancy, ulcer disease, or trauma, or from an extrinsic source, as is often the case with aortoduodenal fistulas. The duodenum is the most common site for aortoenteric fistulas, owing to the proximity of these two structures in the retroperitoneum. This condition most frequently occurs in the setting of an abdominal aortic aneurysm (AAA), either arising as a primary event or a complication of surgical repair. Uncommonly, fistulas between the aorta and duodenum may result from vasculitis or radiation. Clinically patients may present with abdominal pain, GI bleeding, and a pulsatile mass. Similar to those with aortoesophageal fistulas, affected patients may have a “herald bleed” preceding life-threatening voluminous hemorrhage. Although diagnostic imaging options for aortoenteric fistulas include barium studies, aortography, and tagged red blood cell (RBC) scintigraphy, these options have limitations compared to CT. Computed tomography findings of aortoduodenal fistulas include focal tethering of the duodenum to the aortic wall with associated duodenal wall thickening, ectopic perigraft gas and fluid remote from the perioperative period, pseudoaneurysm formation, and possibly active contrast extravasation into the bowel lumen ( Fig. 13-20 ).
Small Bowel Obstruction and Inflammation
- Daniel Souza
- Aaron Sodickson
Small bowel obstruction (SBO) and inflammation are common conditions, often presenting with nonspecific signs and symptoms, similar to those seen in other acute abdominal disorders. Because an accurate clinical diagnosis remains challenging, imaging plays a central role in providing comprehensive information about bowel anatomy, the presence (and site) of the abnormality, distribution, severity, associated complications, and probable cause. The goal of this section is to provide an overview of the normal bowel anatomy, discuss the diagnostic approach, and describe common examples of SBO and inflammation. Acute disorders of vascular origin, such as acute hemorrhage, ischemia/infarction, and vasculitis are discussed in the nontraumatic vascular emergencies section.
Anatomy of the Small Bowel and Mesentery
The small bowel has always been a challenging organ for clinical and radiologic evaluation. It is a long and tortuous tube that extends for approximately 3 to 5 m from the pylorus to the ileocecal valve. The duodenum is the shortest segment, with retroperitoneal location and lacking a mesentery. It is a C-shaped structure that extends to the ligament of Treitz. The jejunum and ileum are mobile and intraperitoneal organs, attached to the posterior abdominal wall by the mesentery, which contains fat and provides the vasculature and lymphatics to the small bowel. The ileum has an abundance of lymphoid follicles, which are nearly absent in the jejunum. The layers of the visceral peritoneum encase the small bowel and associated mesentery. The visceral and parietal peritoneum enclose the large potential space referred to as the peritoneal cavity . The SMA supplies the jejunum and ileum. The venous return parallels the arterial supply, with drainage occurring to the superior mesenteric vein and then to the portal vein.
Normal Imaging Appearance of the Small Bowel and Mesentery
The small bowel has a central location in the abdomen. There is no exact point of transition between the jejunum and ileum, but differences in their usual location, caliber, fold pattern, and degree of vascularity allow distinction between the two. The small bowel extends in a roughly clockwise direction from the right upper quadrant to the left upper and lower quadrants, tapering progressively from proximal to distal. The jejunum is located more proximal (and superior), with larger caliber, thicker folds, and greater vascularity than the jejunum. When the proximal small bowel is distended by pathologic processes, the mucosal folds (or valvulae conniventes) become visible. There is an inverse relationship between the normal bowel wall thickness and the degree of bowel distention. However, a bowel wall thickness greater than 5 mm should always be considered pathologic. In rare cases of midgut malrotation the third part of the duodenum is not visualized across the midline from the right to the left, there is reversal of the normal positions of the SMA and superior mesenteric vein, and the small bowel is completely right sided with a left-sided colon.
The vascular supply of the small and large bowel is supplied by the celiac trunk, which provides the blood supply from the distal esophagus to the descending duodenum; the SMA, which supplies the distal duodenum, jejunum, ileum, and the large bowel to the splenic flexure; and the inferior mesenteric artery (IMA), which supplies the more distal colon. There are important collateral pathways providing connection between these arteries, including between the celiac trunk and the SMA via the gastroduodenal artery and between the SMA and IMA via the marginal artery of Drummond and the arcade of Riolan.
Imaging Modalities for Small Bowel
Plain Radiography
Despite overall low diagnostic accuracy and specificity, the kidney, ureter, and bladder (KUB) radiographic examination is still sometimes used as an initial imaging examination in patients with abdominal symptoms. It is widely available, inexpensive, and reasonably sensitive for the diagnosis of high-grade bowel obstruction and radiopaque foreign bodies. Typical radiographic views include supine and upright views of the abdomen and an upright chest radiograph. The supine abdominal view demonstrates the bowel pattern to better extent, whereas air-fluid levels within the bowel are evident on the upright view. The upright view of the chest is best for the detection of free air under the diaphragm (pneumoperitoneum), which is an ominous sign of bowel perforation.
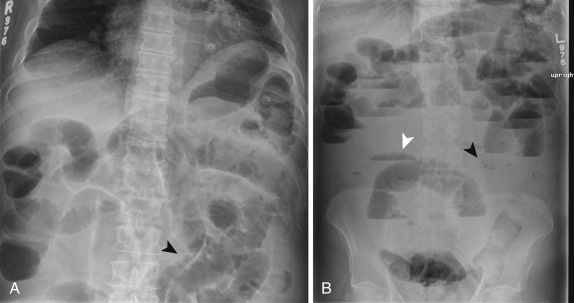
Although a fair amount of gas is normally seen within the stomach and colon, the small bowel should contain little gas. When bowel motility is affected by mechanical obstruction or nonobstructive adynamic ileus, gas accumulates within the small bowel. Small bowel obstruction is suspected when multiple gas- or fluid-filled loops of dilated small bowel are present. The presence of an unusual quantity of colonic gas in this setting usually indicates nonobstructive ileus, partial SBO, or early complete SBO. Common predisposing factors for ileus include sepsis, electrolyte disturbances, GI infection, and recent surgery. Differentiation between partial and early SBO can be challenging in the absence of clinical information. Although subjective, features favoring high-grade obstruction include (1) the presence of multiple air-fluid levels, particularly when discrepant levels are seen within the same loop, (2) dilated loops averaging more than 2.5 cm in diameter and/or exceeding 50% of the caliber of the largest visible colonic loop, and (3) a large number of distended loops of bowel. Although patients with SBO can present with normal KUB findings, a confident diagnosis can be made in 50% to 60% of cases when dilated gas or fluid-filled loops of small bowel are present in the setting of a gasless colon ( Fig. 13-21 ).
Ultrasonography
The potential advantages of ultrasonography (US) include widespread availability, fast, dynamic real-time acquisition, low cost, and lack of ionizing radiation. Although US is often used in the diagnosis of intussusception in children, its role in the assessment of the small bowel in the adult population is very limited, particularly in the United States. Disadvantages include operator variability and incomplete evaluation of the small bowel due to intervening gas and obese habitus. However, it may be indicated in selected cases, such as in the follow-up of patients with inflammatory bowel disease (IBD) or critically ill patients.
Barium Contrast Studies
Although small bowel follow-through studies can provide spatial resolution in the evaluation of the mucosa that is superior to that of cross-sectional studies, and these studies have been performed in the past to triage patients with suspected SBO into surgical versus nonsurgical management, the widespread use of abdominal CT has largely replaced this practice. Rapid transit of contrast material within normal-caliber small bowel loops excludes SBO. However, in cases of SBO, the duration of the small bowel follow-through examination is lengthy, and retained fluid dilutes the barium, often limiting assessment of the point of obstruction, with delayed films needed if one wishes to identify the transition point or determine whether contrast passes distally.
Computed Tomography and Computed Tomography Enterography
The role of CT in the diagnosis of SBO cannot be overemphasized. It is fast and widely available, and it allows for the concurrent assessment of the mesentery, mesenteric vessels, and peritoneal cavity. In the setting of SBO, it can be performed without oral contrast administration, because the retained intraluminal fluid serves as a natural negative contrast agent (and delays transit of any ingested contrast). In the assessment of small bowel disease, CT enterography, with negative or neutral oral contrast material, can be used to achieve bowel distention and to improve evaluation of bowel wall morphologic characteristics, thickness, and enhancement. Multiplanar reformations (MPRs) and postprocessing techniques aid in a confident diagnosis and can be helpful to depicting findings. Coronal reformatted images are most intuitive because they are analogous to a frontal radiograph and the course of the bowel loops can be most readily followed in this plane. Maximum intensity projection images and other postprocessing techniques, such as volume-rendering techniques, can improve depiction of the vasculature and bowel anatomy and improve the communication of findings ( Fig. 13-22 ).
Magnetic Resonance Imaging
Magnetic resonance imaging provides accurate anatomic and functional information about the small bowel without the use of ionizing radiation. A potential advantage over CT that has been proposed is the characterization of malignant versus benign strictures. In addition, because MR enterography can be used to monitor small-bowel filling in real time without radiation exposure, it is an alternative to CT enterography as a means of evaluating low-grade obstruction. It is an exceptional technique to evaluate patients with IBD because these patients are often young and require serial examinations due to acute exacerbations. Magnetic resonance imaging is more controversial in the setting of suspected SBO but may have a role in children and pregnant patients.
Small Bowel Obstruction
Small bowel obstruction is common, accounting for 20% of surgical admissions and 4% of ED visits for abdominal pain. Although the diagnosis is often suspected based on clinical history, CT has become the primary modality in evaluation and management of patients with suspected bowel obstruction. Conservative management is preferred when possible, and surgery is performed much more selectively than in the past. As a result the radiologist plays a central role in answering specific questions to diagnose SBO and to guide conservative versus surgical management. These include (1) confirming (or excluding) SBO and elucidating alternative diagnoses in the absence of SBO; (2) assessing complexity/severity of the obstruction (simple versus closed-loop, complete versus partial, low-grade versus high-grade), and identifying the presence of complications (strangulation, perforation); (3) determining the presence and location of the transition point; and, whenever possible, (4) establishing the underlying cause ( Box 13-1 ).
Extrinsic Lesions
Adhesions
Hernias (external, internal)
Tumors (serosal disease)
Hematoma
Endometriosis
Intrinsic Lesions
Inflammatory diseases (Crohn, tuberculosis)
Neoplastic diseases (adenocarcinoma, gastrointestinal stromal tumor, lymphoma, metastatic disease)
Vascular lesions (radiation, ischemia)
Hematoma (anticoagulants, blood dyscrasia, trauma)
Intussusception
Intraluminal
Gallstones
Bezoars
Foreign bodies
Confirming the Presence and Severity of Obstruction
Typical findings of SBO include continuous loops of dilated (>2.5 cm) proximal small bowel, associated with normal caliber or collapsed bowel distal to a transition point. When oral contrast medium is used, the absence of contrast in distal loops of bowel is indicative of complete obstruction provided that enough time has been allowed for delayed passage. In selected cases, delayed scans can be performed to confirm complete obstruction, although this is rarely performed because the patient’s clinical progression is the primary determinant of management approach in the absence of clear signs of high-grade or closed-loop obstruction.
Multiple conditions can result in small bowel dilatation (or abdominal distention) and be confused with SBO, particularly before clinical and radiologic information are integrated. Adynamic ileus is associated with bowel distention that may include the colon, in addition to the small bowel and stomach. Shock bowel can present with dilated loops of small bowel, but history, involvement of the colon, and other imaging findings such as a flattened IVC, and hyperenhancement of the adrenal glands, should help differentiate it from SBO. Bowel ischemia can likewise cause significant distention of atonic small bowel loops and simulate the appearance of obstruction, but ancillary findings of bowel thickening, segmental bowel perfusion abnormalities, and evidence of arterial atherosclerotic disease help suggest an ischemic cause for the dilatation.
Differentiation between partial low-grade or high-grade obstruction is subjective. High-grade obstruction should be suggested when there is a substantive caliber difference between proximal and distal loops of bowel (and absent passage of contrast material through the transition point if delayed imaging is performed). The diagnosis of partial low-grade obstruction is more challenging and considered a relative “blind spot” for CT because the degree of caliber change is generally less striking.
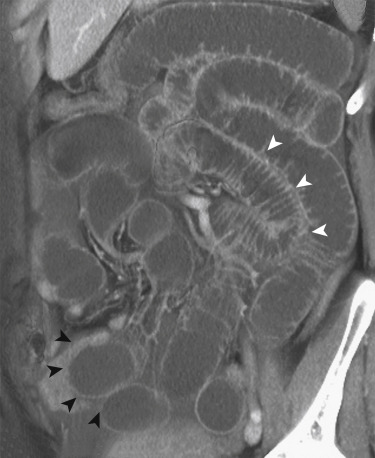
The use of oral contrast for the diagnosis of SBO is controversial. Potential advantages include the indirect assessment of bowel transit time, which may aid in the diagnosis of partial low-grade obstruction, and the progressive dilution of contrast as the contrast column approaches the transition point, which may help to determine its location ( Fig. 13-23 ). Delayed images can provide confirmation of a complete obstruction in the appropriate setting. However, oral contrast delays the diagnosis in the emergency setting and is generally not tolerated by obstructed patients presenting with nausea and vomiting. In addition, the fluid within the distended loops of bowel acts like a natural neutral oral contrast, as long as nasogastric tube decompression is not performed before imaging ( Fig. 13-24 ).
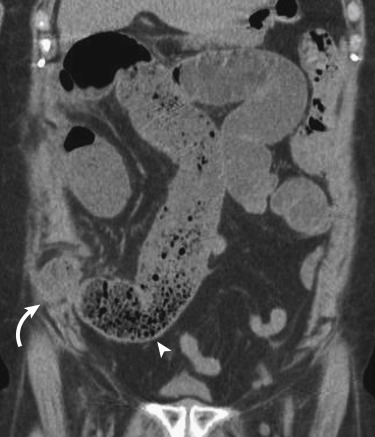
Another helpful imaging feature in SBO is the “small bowel feces sign,” or the presence of mottled, particulate matter and gas within the lumen that simulates the appearance of feces. It is often associated with high-grade obstruction but has been described in nonobstructed patients. When present, it is generally located immediately proximal to the transition point and is thus extremely helpful to locating the site of obstruction, which is the next step in the evaluation of SBO ( Fig. 13-25 ).
Determining the Location and Cause
A transition zone or point is the region at which the bowel caliber changes and greatly increases confidence that a true mechanical obstruction is present by identifying the site of the obstruction. In the absence of a focal transition point, dilated loops are nonspecific and may be due to other conditions such as IBD, scleroderma, or ischemia. It is often easiest to identify a transition point by careful inspection of the course of the bowel loops, usually by an antegrade approach starting in the distended proximal loops. Distally located SBO can, however, be most readily evaluated by a retrograde approach starting at the decompressed terminal ileum. Careful and systematic travel through the bowel loops in multiple planes is the key to success. When the transition point is identified, a search for intraluminal, intrinsic, or extrinsic causes of obstruction should be performed. Intraluminal causes include foreign bodies, gallstones, and bezoar. Intrinsic bowel lesions typically manifest as mural or focal thickening. Extrinsic causes of obstruction, such as hernias and CD, are associated with other extraintestinal findings. It should be noted that adhesions, the most common cause of SBO in the United States, are typically not visible on CT and can be suggested in regions of an abrupt transition point when no clear cause is seen.
Determining the Presence of Closed-Loop Obstruction and Complications
In simple obstruction the bowel is occluded at one or more points along its course. The diagnosis of closed-loop obstruction is crucial because it carries a higher risk for strangulation and bowel infarction. Most often, an adhesion or hernia will cause partial or complete occlusion of a segment of bowel loop at two adjacent points. Computed tomography findings depend on the length, degree of distention, and orientation of the closed loop but include a characteristic fixed radial distribution of several dilated, usually fluid-filled bowel loops with stretched and prominent mesenteric vessels converging toward a point of torsion. The configuration is usually U shaped or C shaped. A narrow pedicle can be formed leading to torsion of the loops and producing a small bowel volvulus. A “beak” sign is seen at the site of the torsion as a fusiform tapering. Occasionally a “whirl” sign of the mesenteric vessels can be seen, reflecting the rotation of the bowel loops around the fixed point of obstruction. Strangulation is defined as closed-loop obstruction associated with intestinal ischemia, and its occurrence depends on the time and degree of rotation of the incarcerated loops. The findings that indicate strangulation include bowel wall thickening and hyperattenuation, a halo or target sign, mesenteric fat stranding and/or fluid, pneumatosis intestinalis, and mesenteric or portal venous gas, but these findings are not entirely specific. More specific findings include absent, asymmetric, or delayed bowel wall enhancement, with or without focal mesenteric fluid or hemorrhage ( Fig. 13-26 ).
Causes of Small Bowel Obstruction
Adhesions
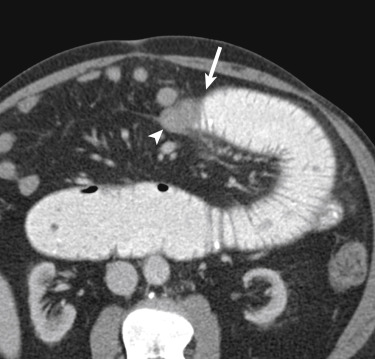
Adhesions are the most common cause of SBO in the United States, ranging from 50% to 80% of cases. The diagnosis should not be excluded even in the absence of prior surgery because adhesions can occur as a result of prior peritonitis. Adhesions are typically not seen, and the diagnosis is one of exclusion. Computed tomography findings include an abrupt change in the caliber of the bowel, with focal kinking and tethering, in the absence of an underlying mass lesion, and significant inflammation or bowel wall thickening at the transition point ( Fig. 13-27 ).
Hernias
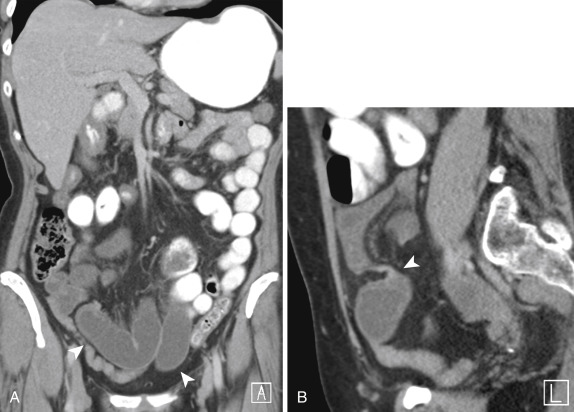
Hernias are the most common cause of SBO in developing countries. An external hernia occurs as a result of a defect in the abdominal wall at sites of congenital weakness or previous surgery. Although the diagnosis can be obvious at clinical examination, it may be challenging in very obese patients ( Fig. 13-28 ).
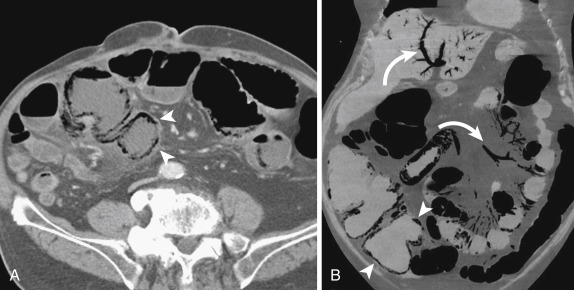
The less common internal hernia occurs when there is protrusion of the viscera through a defect in the peritoneum or mesentery into another compartment within the abdominal cavity. Closed-loop obstruction can result ( Fig. 13-29 ). Early diagnosis in this scenario becomes critical because these patients will often not benefit from conservative management because the involved segment will not be decompressed with nasogastric drainage, and bowel ischemia and necrosis can rapidly develop ( Fig. 13-30 ).

Endometriosis
Endometriosis is common and underdiagnosed. It is estimated to affect approximately 5% of women of reproductive age. Endometrial implants may manifest as contiguous or penetrating soft tissue nodules along the antimesenteric border of the bowel wall.
Malignancy
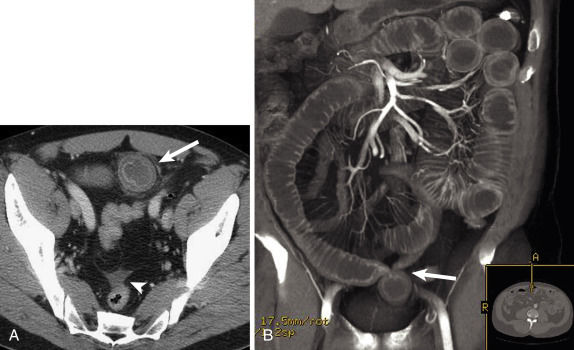
Metastatic involvement is far more common than primary disease. It can result in SBO by different mechanisms (intrinsic and extrinsic). It can be secondary to intraperitoneal seeding, hematogenous spread, or direct extension from an adjacent visceral malignancy. Although SBO can result from intrinsic obstruction from submucosal implants, such as from malignant melanoma and breast cancer, it most often occurs in the setting of peritoneal carcinomatosis, when extensive serosal disease will determine extrinsic compression and/or circumferential involvement at the transition point ( Fig. 13-31 ).
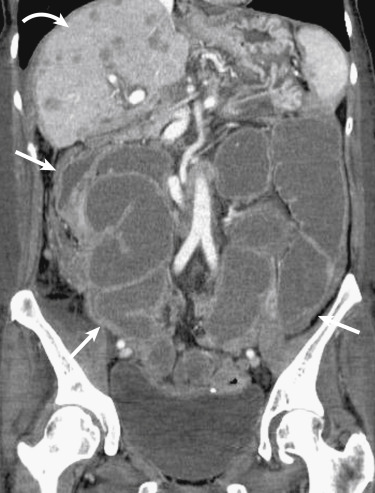
Primary small bowel neoplasms are rare. Advanced cases of adenocarcinoma can present with SBO, with pronounced asymmetric and irregular mural thickening noted at the transition point ( Fig. 13-32 ). Conversely, primary lymphoma of the small bowel, even when extensive or annular, is a soft tumor and does not generally cause obstruction unless it is associated with adhesions or posttreatment change ( Fig. 13-33 ). Small bowel carcinoid is a relatively rare cause of SBO because the primary lesion is often occult, and it usually will manifest as spiculated mesenteric masses with calcification resulting from a desmoplastic reaction ( Fig. 13-34 ).
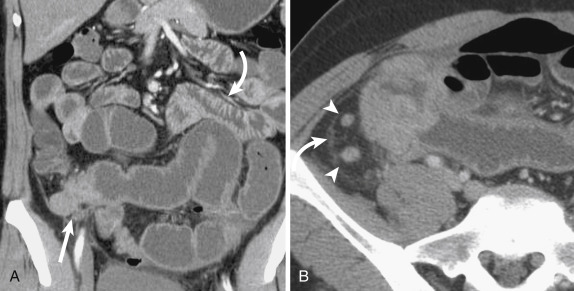
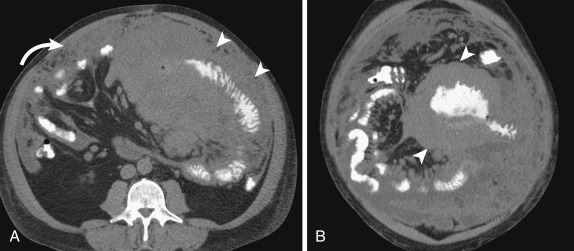
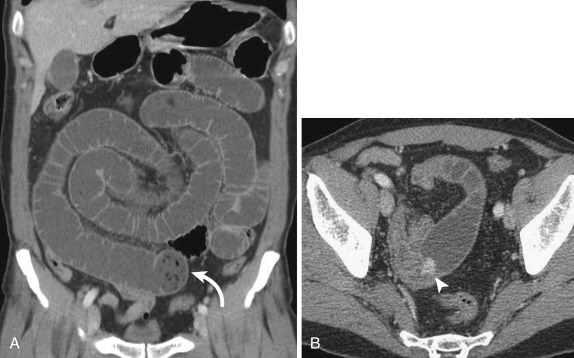
Intussusception
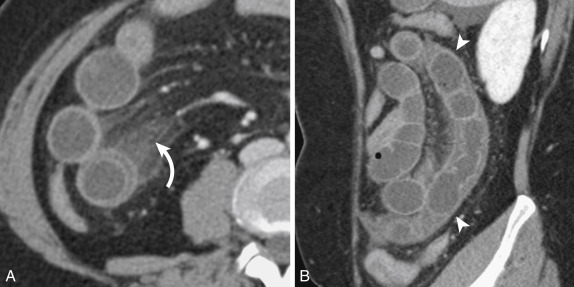
Intussusception is a benign and common condition in children younger than 3 years old, but a rare cause of bowel obstruction in adults. When found in older children and adults, it should trigger a search for a lead point, such as an underlying neoplasm, inciting adhesion or foreign body. Computed tomography findings include the pathognomonic bowel-within-bowel configuration, with or without mesenteric fat and mesenteric vessels. The finding of a leading mass should be interpreted with caution because the intussusception itself can present as a pseudotumor ( Fig. 13-35 ).
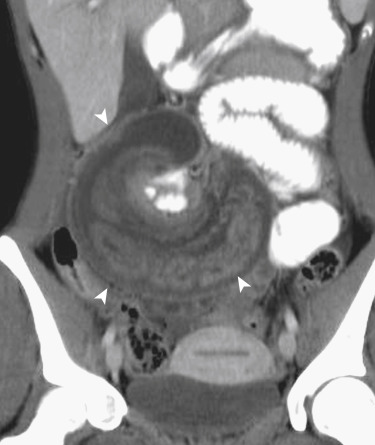
Functional disturbances that affect bowel motility, such as scleroderma, celiac disease, and cystic fibrosis, can cause transient intussusception. Clinical history can be helpful in these patients, and careful evaluation of the intussusception will confirm the absence of a neoplastic lead point. When multiphasic CT is performed, resolution of the intussusception can be often observed, confirming the transient nature of this phenomenon ( Fig. 13-36 ).
Inflammation and Fibrosis
Acute and chronic inflammation, such as CD and radiation enteritis, can lead to fibrosis, strictures, and, in more severe cases, obstruction. Small bowel obstruction can occur during the acute phase of CD when the intense acute transmural inflammation causes narrowing of the bowel lumen, during the chronic phase due to fibrotic stenosis, postoperative adhesions, incisional hernias, and exacerbation of the inflammatory condition (acute flare). Small bowel obstruction occurs in the late phase of radiation enteritis, most often in the distal small bowel as a result of adhesive and fibrotic changes that develop several months after therapy. Radiation serositis can lead to narrowing of the lumen and abnormal peristalsis. Computed tomography findings include adhesions, narrowing of the lumen secondary to mural thickening, mesenteric retraction, and abnormal bowel enhancement within the radiation field.
Hematoma and Vascular Causes
Hematoma can mimic neoplasm and result in significant luminal narrowing. It is usually associated with anticoagulant therapy, iatrogenic intervention, or trauma. In the setting of severe stenosis or occlusion of the mesenteric arterial or venous supply, bowel ischemia can lead to significant bowel wall thickening, resulting in SBO. Although relatively benign, in advanced cases, pneumatosis and portomesenteric venous gas can signal the presence of infarction.
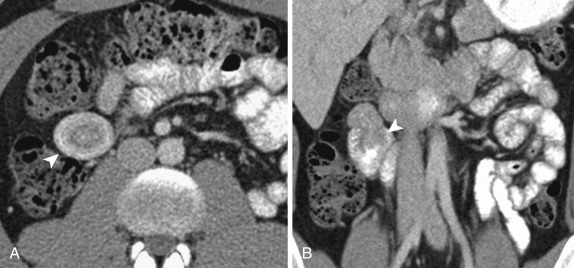
Gallstone Ileus
Gallstone ileus is a rare complication of chronic cholecystitis. It is the result of migration of a gallstone into the small bowel with subsequent impaction, most often at the ileocecal valve. The imaging features of the “Rigler triad” include SBO, an ectopic gallstone, and pneumobilia because a cholecystenteric fistula is needed for the migration of a large obstructing gallstone to occur ( Fig. 13-37 ). In the event of proximal obstruction, at the level of the stomach or duodenum, gastric outlet obstruction may result (Bouveret syndrome).
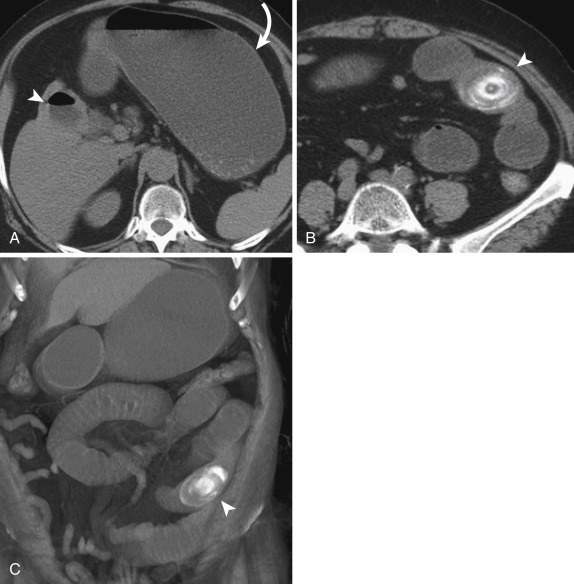
Bezoar
Bezoar was considered a rare cause of SBO, but the number of reported cases is increasing in recent years as a result of gastric outlet surgery, because it prevents adequate digestion of vegetable fibers, which may become impacted. Computed tomography findings include an elongated and intraluminal mass demonstrating a mottled appearance at the transition point ( Fig. 13-38 ).
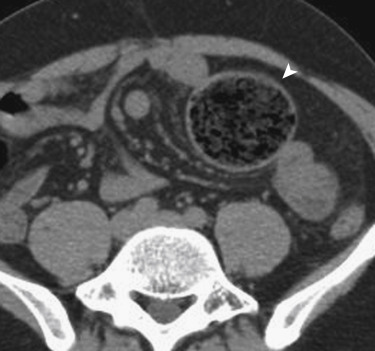
Foreign Bodies
A wide range of foreign bodies can cause SBO with variable imaging appearances. Clinical history is very important in helping to establish preoperative diagnosis ( Fig. 13-39 ). In children and, emotionally disturbed, or mentally disabled patients, foreign bodies should be suspected.
Iatrogenic SBO can occur as a result of endoscopic capsule retention because they have been used more often in the evaluation of the small bowel, particularly in patients with CD. Diagnosis is made by identifying an ovoid metallic object impacted at the transition point.
Small Bowel Inflammation
Infectious Enteritis
Infectious enterocolitis can cause mild symptoms resembling common viral gastroenteritis, but it can present with clinical findings that are indistinguishable from appendicitis and other surgical conditions. Although imaging findings are often nonspecific, including mural thickening, mesenteric fat stranding, and moderate mesenteric lymphadenopathy, the clinical history and distribution can be helpful in narrowing the differential diagnosis. Giardia lamblia is a protozoan known to cause severe diarrhea and malabsorption. The organism tends to inhabit the duodenum and jejunum. Imaging findings consist of mural thickening involving the proximal small bowel, increased luminal secretions, and disordered motility.
Immunocompromised patients can be affected with systemic infection with Mycobacterium avium-intracellulare . It affects multiple organs and has been called pseudo-Whipple disease because of clinical, histologic, and radiologic similarities. Computed tomography findings include thickened small bowel more pronounced in the jejunum, hepatomegaly, and splenomegaly. Focal, low-density lesions can be present in the spleen. Cytomegalovirus can be reactivated in the setting of immunosuppression and can result in hemorrhagic enteritis. Definitive diagnosis may require biopsy with immunohistochemical stains. Cryptosporidium is a GI parasite that results in watery diarrhea in patients with acquired immunodeficiency syndrome (AIDS). Clinical history is important in establishing the diagnosis. Computed tomography findings demonstrate wall thickening more often involving the proximal small bowel and stomach, similar to that of giardiasis. Other common pathogens that often affect the small bowel, typically the distal ileum and cecum, include Yersinia , Campylobacter , and Salmonella species ( Fig. 13-40 ).
Crohn Disease
Inflammatory bowel disease and infectious enteritis can have similar imaging findings, with distinction made based on the clinical history, as well as the distribution of findings within the bowel. Crohn disease is part of the IBD spectrum, characterized by chronic, relapsing inflammation of unknown cause. Despite abundant research in this field, it remains a diagnostic and therapeutic challenge. Crohn disease can affect any part of the GI tract but predominantly affects the small bowel (up to 80% of cases) and right colon. As opposed to ulcerative colitis (UC), rectal involvement is very rare in CD.
Crohn disease is characterized by the presence of granulomatous inflammation at histologic evaluation, with chronic erosions, ulceration, and transmural bowel inflammation, which predisposes to complications such as fistulas and abscess formation. The presence of “skip lesions” is one of the hallmarks of the disease. Crohn disease has a tendency toward remission and relapse, and acute and chronic changes often coexist within the same affected bowel segment. Fatty infiltration of the bowel wall may occur in chronic IBD and was thought to be pathognomonic of this disease. However, it has been demonstrated in healthy individuals, particularly in association with obesity.
Endoscopy and classic barium studies are critical in the initial diagnosis and staging of IBD in general because they are superior to cross-sectional imaging in the evaluation of the mucosa, particularly in early-stage disease. However, CT and MRI can depict several abnormalities that are associated with acute bowel inflammation, such as mural thickening and stratification, thickened folds and/or reduction or distortion of folds due to ulceration and cobblestoning. Additional findings include pseudosacculations or pseudodiverticulum formation, which occur as a consequence of relative sparing of the antimesenteric border within an affected segment. The major advantage over endoscopy and classic barium studies is the assessment of extraenteric findings, which are relatively common in CD. In addition, cross-sectional imaging is reserved for the follow-up evaluation of established disease, assessment of treatment response, diagnosis of relapse, detection of complications, and assessment before surgical intervention. In the presence of abscesses, percutaneous drainage under CT guidance is favored over surgery.
Computed tomography findings of active inflammation in CD include significant mural enhancement and stratification due to submucosal edema (target or “double-halo” appearance), adjacent mesenteric fat stranding, and engorged vasa recta (“comb” sign). The presence of fibrofatty proliferation along the mesenteric border of the affected bowel is considered pathognomonic of this disease. Extraintestinal manifestations include mesenteric inflammatory changes and the presence of phlegmon, abscess, or lymphadenopathy. As described earlier, deformity of bowel loops, such as pseudodiverticulum formation, is caused by asymmetric involvement by longitudinal ulcers and scars, both of which are well demonstrated on both axial and coronal images. Lymphadenopathy is not uncommon, and although controversial, it has been suggested that lymphadenopathy can allow differentiation between active inflammatory and chronic (fibrostenosing) disease. Nonetheless, lymph nodes larger than 10 mm in short axis should prompt evaluation for superimposed lymphoma or carcinoma ( Figs. 13-41 and 13-42 ).
Magnetic resonance enterography is an attractive modality for the assessment of CD because it can offer dynamic evaluation of bowel motility, superior soft tissue contrast, and excellent depiction of fluid and edema, without associated ionizing radiation. Magnetic resonance enterography can be particularly useful in young patients that require serial examinations. Protocols are variable but generally include multiphasic coronal fluid-sensitive sequences. Optimal bowel distention is achieved by use of enteric contrast material, but the type of contrast material and method of administration are controversial. Most patients require a delay of at least 40 to 60 minutes from contrast material ingestion to imaging.
Magnetic resonance enterography can play an important role in the follow-up of patients with established IBD, or it can be used to exclude IBD in a young patient who presents with symptoms suggesting the disease. Although early changes, such as aphtoid ulceration, are beyond the resolution of MR, it can be useful in the evaluation of fixed stenoses and segmental dilatation and to detect adhesions. Because of high contrast, CT can better assess extent of fistulas in penetrating disease, mesenteric vascularity, and lymphadenopathy. Fistulas, sinus formation, and abscesses are features of penetrating disease. They are visible as wall-enhancing structures. Adhesions can be differentiated from fistulas because they are fibrotic and tend to be thinner and enhance later. Over time, chronic inflammation within the bowel wall leads to stricture formation, and bowel obstruction may develop. These findings are important to identify because they are not responsive to medical therapy and typically require surgical resection if associated with symptomatic bowel obstruction. On dynamic images, fibrotic strictures appear as aperistaltic bowel segments that often demonstrate fixed mural thickening and luminal narrowing. T2 hyperintensity is typically absent in these fibrotic regions, unless there is associated mural inflammation and edema ( Fig. 13-43 ).
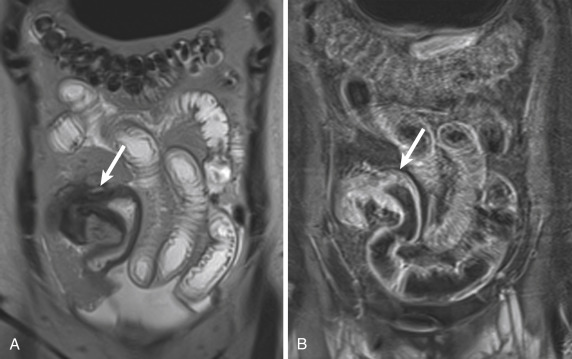
Radiation Enteritis
Radiation-induced enteropathy has distinct acute and chronic manifestations. Acute radiation enteritis occurs within the first few days or weeks after exposure. The most characteristic findings include mucosal hyperenhancement, submucosal edema, and bowel wall thickening. Chronic radiation enteritis is a transmural process that develops months to years after exposure in up to 16% of patients after radiation treatment to the pelvis. It is characterized by stricture formation and obstruction. Typical morphologic changes include diffuse bowel wall thickening, strictures and fistula formation, tethering, and impaired peristalsis.
Angioedema of the Bowel
Angioedema is not a true inflammatory disease, but it can mimic inflammation because it presents with bowel wall thickening. It is characterized by episodes of increased capillary permeability, with extravasation of intravascular fluid and subsequent edema of the skin, mucosa of the upper airways, or GI tract. Gastrointestinal involvement sometimes mimics an acute abdomen or rarely can cause life-threatening hypovolemic shock. Characteristic imaging features include reversible bowel and mesenteric edema, characterized by multiple dilated small-bowel loops with regular thickened mucosal folds, a stacked-coin appearance with bowel wall thickening, and thumbprinting. The small and large intestine may be involved, including jejunum, ileum, duodenum, and colon, in descending frequency of involvement. The findings are usually transient and segmental, returning to normal after an acute attack. A high degree of clinical suspicion is needed, particularly in patients using angiotensin-converting enzyme inhibitors and a variety of other medications, in the setting of typical imaging findings. A definite diagnosis can be confirmed by elevated serum levels of C4 and C1 esterase inhibitor and C1 esterase inhibitor functional activity complement levels ( Fig. 13-44 ).
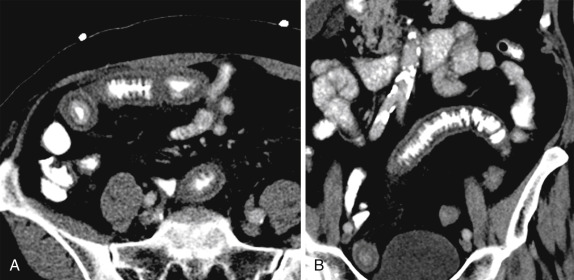
Celiac Disease
Celiac disease in now recognized as a common but largely underdiagnosed disease, with typical diagnostic delay of more than 10 years from the onset of symptoms. It is a chronic autoimmune disorder induced in genetically susceptible individuals after ingestion of gluten proteins. The small bowel mucosa is primarily affected, resulting in progressive villus inflammation and destruction, with resulting induction of crypt hyperplasia. It may be asymptomatic or characterized by diverse symptoms of malabsorption with varying severity. Characteristic findings include duodenitis with dilution, slow transit, and flocculation of oral contrast, small bowel dilation, transient small bowel intussusception, villous atrophy and reversal of the fold pattern, with jejunization of the ileum, as reflected by a decrease in normal jejunal folds in contrast to the increasing fold pattern in the ileum.
Whipple Disease
Whipple disease is a rare multisystemic bacterial infection caused by the Whipple bacillus (Tropheryma whipplei) , involving the small bowel (particularly the jejunum), lymph nodes, joints, and central nervous system. Barium studies can demonstrate to better extent the thickened, irregular folds and tiny nodules in the jejunum and, to a lesser degree, the ileum, due to accumulation of the Whipple bacilli and periodic acid-Schiff–positive macrophages in the submucosa and lamina propria. Characteristic imaging findings include mesenteric and retroperitoneal lymphadenopathy and fat-attenuation lymph nodes measuring between 10 and 20 HU. Whipple disease is an infectious condition, and these patients often demonstrate significant response to treatment with antibiotics.
Small Bowel Diverticulitis
Small bowel diverticulosis is far less common than colonic diverticulosis and characterized by the presence of multiple saclike mucosal herniations, or pseudodiverticula, through weak points in the intestinal wall. Duodenal diverticula are relatively common and have been found in up to 5% of the population undergoing barium studies. Jejunoileal diverticula are less common but usually multiple and localized to the proximal jejunum. They are frequently associated with disorders of intestinal motility such as scleroderma and visceral neuropathies. The major clinical manifestation of jejunoileal diverticula is malabsorption. Complications are more common in jejunoileal diverticula than duodenal diverticula and, similar to their colonic counterpart, include bleeding, intestinal obstruction, and diverticulitis.
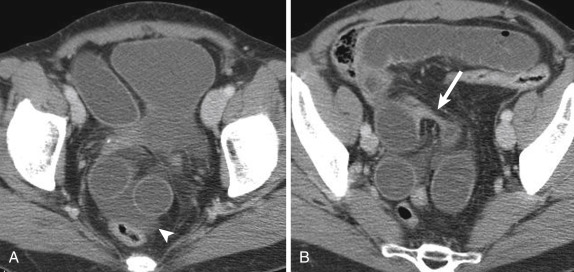
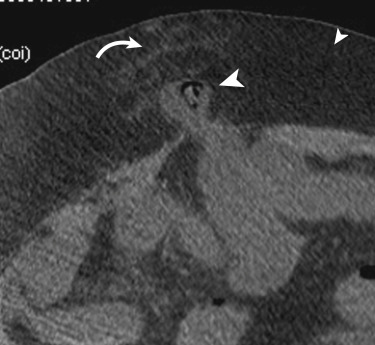
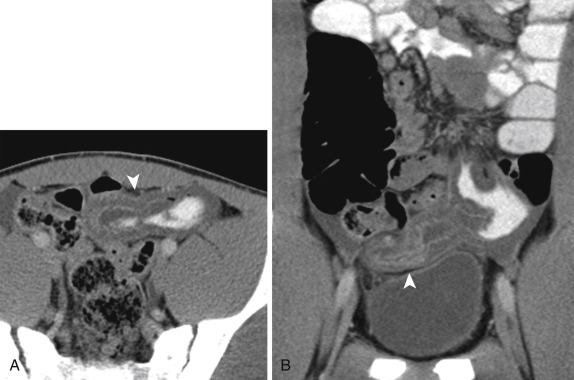
Meckel diverticulum is the most common congenital anomaly of the GI tract, occurring in 2% of the population. It is a true diverticulum that contains all layers of the intestinal wall and has its own blood supply. It results from incomplete absorption of the omphalomesenteric duct and is frequently associated with heterotopic gastric or pancreatic mucosa in up to 50% of cases, with gastric heterotopia being most common. It is typically located in the distal ileum approximately 2 feet from the ileocecal valve. Although usually asymptomatic, approximately 2% of patients can present with complications, more common in children than adults. Inflammation (Meckel diverticulitis) is one of the most common complications, after hemorrhage and SBO. Computed tomography diagnosis relies on the identification of a blind-ending, tubular, round, or oval structure in the right lower quadrant or periumbilical region, with surrounding inflammation. The diverticulum can be associated with mural thickening and hyperenhancement, with focal calcifications at the base (enteroliths), and adjacent mesenteric fat stranding and fluid collections. In some instances the diverticulum will extend toward the umbilicus. The identification of a normal appendix can be very helpful because patients will often present with right lower quadrant pain. A Meckel scan can confirm the presence of gastric mucosa within the diverticulum in equivocal cases ( Fig. 13-45 ).
Other Inflammatory Conditions of the Small Bowel
There are numerous additional inflammatory conditions of the small bowel that will present with nonspecific bowel wall thickening, including eosinophilic gastroenteritis, amyloidosis, Behçet syndrome, and primary lymphangiectasia. Differentiation will depend on the clinical history, distribution pattern, and often biopsy.
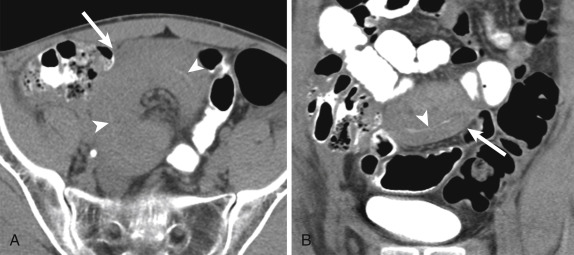
Small Bowel Hemorrhage
Small bowel hemorrhage can be the result of ischemia, trauma, vasculitis, coagulopathies, and anticoagulation therapy. Bleeding usually occurs within the submucosal layer. It can cause bowel wall thickness of 1 cm or more, but it will often extend for less than 15 cm in length. The CT appearance of regular fold thickening, also known as the picket fence appearance on fluoroscopic studies, can be diffuse or segmental, depending on the underlying cause. It can present on CT as an intramural mass that narrows the lumen. If the hemorrhage is acute, the hematoma may have high density. Mesenteric fluid or hemoperitoneum is often associated with bowel wall hemorrhage or injury ( Fig. 13-46 ).
Colorectal Emergencies
- Jennifer W. Uyeda
- Jorge A. Soto
- Stephan W. Anderson
- Jorge A. Soto
Rapid and accurate imaging and diagnosis is essential in the appropriate management of patients who present in the emergency setting with acute nontraumatic GI conditions. Appropriate diagnosis and subsequent treatment rely on accurate imaging diagnosis and radiologists’ familiarity with the vast spectrum of GI diseases. This section will review the pathophysiology and pertinent imaging features of nontraumatic emergencies that affect the colon and the appendix.
Acute Inflammation of the Colon and Appendix
Acute Appendicitis
Acute appendicitis is the most common acute abdominal emergency requiring surgery and typically presents as periumbilical pain that migrates to the right lower quadrant with other characteristic clinical signs and symptoms, including nausea, anorexia, and fever, although the clinical presentation is highly variable. Initially the appendiceal lumen becomes obstructed by fecal material (appendicolith or fecalith), undigested material such as vegetables or seeds, hypertrophied Peyer patches, or neoplasm, and progressive accumulation of secretions leads to intraluminal distention and increased intramural pressure. The initial periumbilical abdominal pain represents referred pain from visceral innervation due to appendiceal luminal distention, and localized right lower quadrant pain is secondary to inflammation of the parietal peritoneum. Ongoing luminal distention and bacterial overgrowth result in lymphatic and venous obstruction leading to mucosal ischemia.
Plain abdominal radiographs have limited utility in the diagnosis of acute appendicitis. However, appendicoliths may be seen on abdominal radiographs and, in the appropriate clinical setting, are suggestive of acute appendicitis. In addition, free intra-abdominal air and bowel obstruction may readily be excluded on plain abdominal radiographs. In certain patients, such as young women or generally thin patients, US of the right lower quadrant may be the first-line imaging modality. Acute appendicitis on US is seen as a noncompressible, thick-walled, dilated, blind-ending tubular structure in the right lower quadrant measuring 7 mm or more in diameter with graded compression, with or without a visible appendicolith. The utility of US for the diagnosis of acute appendicitis is highly operator dependent, and this modality is limited in obese patients and in the presence of gas-filled bowel. Typically, in cases of ongoing clinical suspicion, negative or inconclusive US examination results warrant a subsequent CT examination. In the majority of patients, CECT represents the first-line imaging modality of choice and is the most accurate imaging study with well-demonstrated sensitivity, specificity, and diagnostic accuracy exceeding 95% for the diagnosis of acute appendicitis. On CT, acute appendicitis is seen as a distended, circumferentially thickened, fluid-filled structure with mucosal hyperenhancement and surrounding fat stranding consistent with periappendiceal inflammation ( Fig. 13-47 ). Although a strict size limit for normal appendices, similar to ultrasonographic imaging, is not applicable to CT given the lack of compression, acutely inflamed appendices are typically dilated and approach or exceed 1 cm in diameter. Furthermore, CECT is the imaging modality of choice to detect complications of acute appendicitis such as appendiceal perforation and periappendiceal abscess formation. On unenhanced CT examinations, a thickened appendix with surrounding inflammatory changes, with or without a calcified appendicolith (see Fig. 13-47 ), is diagnostic of acute appendicitis. In cases in which positive oral contrast agents are administered, opacification of the appendiceal lumen with oral contrast effectively excludes a diagnosis of appendicitis. However, the converse does not hold true, and a lack of appendiceal opacification with oral contrast material does not definitively indicate a diagnosis of acute appendicitis because this is often the case with normal appendices.
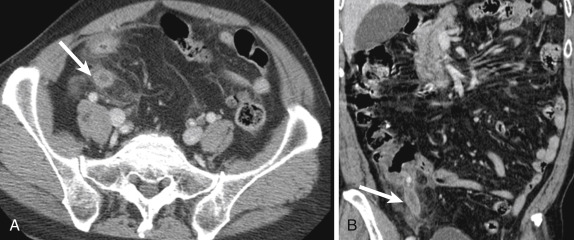
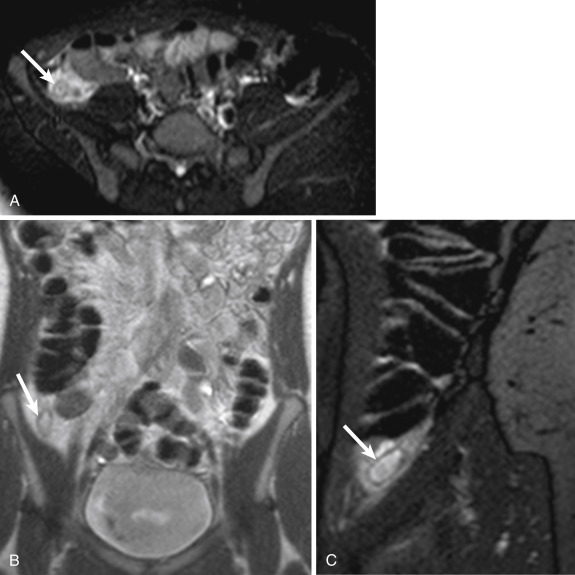
In cases of suspected acute appendicitis in pregnant women, an abdominal US is often the initial imaging study obtained to evaluate for possible appendicitis. However, because the sensitivity of this modality for the detection of acute appendicitis is limited, especially in pregnant women, a noncontrast MRI of the abdomen is often employed after an inconclusive US. With a reported sensitivity and specificity comparable to that of CT, with the benefits of a lack of ionizing radiation exposure to the fetus, MRI has gained wide acceptance for this application. With the use of MRI, the need for CT in cases of suspected acute appendicitis may be markedly decreased or eliminated altogether. Typically MRI examinations in cases of suspected appendicitis include multiplanar T1- and T2-weighted sequences. In pregnancy, intravenous gadolinium should not be administered for this application and the utility of oral contrast is a subject of ongoing investigation. Appendicitis on MRI is readily depicted on T2-weighted sequences acquired with and without fat suppression. The inflamed and edematous appendix is dilated and fluid filled and exhibits surrounding periappendiceal edema and fat inflammation ( Fig. 13-48 ).
Appendectomy is the treatment of choice in simple acute appendicitis, and laparoscopic appendectomy is increasingly common. Antibiotic treatment for small abscesses is feasible, whereas image-guided percutaneous drainage may be performed for larger abscesses.
Acute Colonic Diverticulitis
Acute diverticulitis results from the obstruction and subsequent perforation of a colonic diverticulum. Colonic diverticula are small, focal outpouchings that occur at weak areas of the bowel wall, typically between the mesenteric and antimesenteric teniae where the vasa recta penetrate the circular muscle layer of the bowel wall. Diverticular disease is the most common pathologic process that involves the colon, with the sigmoid colon most commonly affected in up to 90% of the population. Initially the neck of a colonic diverticulum becomes obstructed by stool, undigested food particles such as seeds, or inflammation, eventually resulting in microscopic or macroscopic perforation and inflammation, contamination, and infection of the pericolic fat. Patients with acute diverticulitis typically present with left lower quadrant pain and fever. Less common signs and symptoms include nausea, vomiting, constipation, and urinary symptoms. Importantly, the location of the abdominal pain varies depending on the segment of colon that is affected.
On imaging, acute diverticulitis may yield abnormalities on abdominal radiographs, including pneumoperitoneum, bowel obstruction, or the presence of a focal region of increased soft tissue density related to the presence of an abscess. Although rarely employed currently, barium enema with water-soluble contrast may demonstrate extravasation of contrast material, as well as the presence of an intramural sinus tract or a fistula. Contrast-enhanced CT of the abdomen and pelvis is currently the study of choice in patients with suspected acute diverticulitis, which manifests with pericolic inflammation, engorgement of the adjacent mesenteric vasculature, and focal colonic wall thickening with or without abscess formation ( Fig. 13-49 ). In many cases the offending diverticulum may be directly visualized on CT, further increasing reader confidence in the diagnosis of acute diverticulitis.
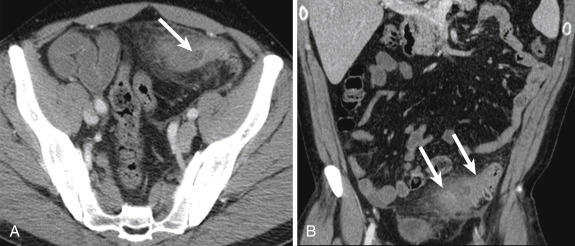
Complications, including diverticular perforation with frank abscess formation, fistulas, (e.g., vesicocolonic or colocolonic among others), pyelophlebitis, or liver abscesses, may also be readily visualized with CT. A pericolic abscess appears as a hypoattenuating, pericolic collection with peripheral enhancement, often containing air or an air-fluid level. Acute diverticulitis represents the most common cause of vesicocolonic fistulas, which often occur along the left posterolateral aspect of the bladder in cases of sigmoid diverticulitis and may be suspected based on the presence of intravesicular air and focal bladder wall thickening adjacent to an inflamed diverticulum.
Treatment options in cases of acute diverticulitis range from conservative management with antibiotics and bowel rest to emergent surgery in cases of complications. Computed tomography-guided percutaneous abscess drainage is a viable treatment option in patients with abscesses related to diverticulitis in which CT readily depicts the anatomy and may be used to guide catheter placement.
An important differential diagnostic consideration in patients with focal colonic wall thickening includes colon carcinoma, which may have similar imaging findings to that of acute diverticulitis. Marked wall thickening out of proportion to the degree of pericolic inflammation, obliteration of the expected mural enhancement pattern of the colon, the presence of mesenteric lymphadenopathy, and acute bowel obstruction are imaging findings that are suggestive of colonic carcinoma. In contradistinction, the presence of fluid within the root of the sigmoid mesentery, visualization of an offending diverticulum, preservation of the expected mural enhancement pattern, and a relatively long segment of circumferential colonic wall thickening favor the diagnosis of acute diverticulitis.
Infectious Colitis
Infectious colitis refers to colonic inflammation caused by a variety of bacterial, viral, fungal, or protozoan infections. Presenting symptoms typically include acute onset of crampy abdominal pain and tenderness and watery or bloody diarrhea. Additional signs and symptoms include fever, rash, nausea, vomiting, headache, malaise, and weight loss. Patients with infectious colitis may be found to have electrolyte imbalances and leukocytosis.
Bacterial causes of infectious colitis include Salmonella , Shigella , Campylobacter , Yersinia , Staphylococcus , E. coli (O157:H7), Staphylococcus , and Clostridium difficile . Viral organisms known to cause colitis include herpes, cytomegalovirus, Norwalk virus, and Rotavirus , and associated fungal organisms include Histoplasma and Mucor . Finally, parasitic infections known to cause colitis include Entamoeba histolytica , Schistosoma , Strongyloides , Trichuris , and Anisakis , all of which are more commonly seen in underdeveloped countries. Some of the more commonly encountered causes of infectious colitis include Campylobacter jejuni , Yersinia enterocolitica , Salmonella typhi , and Clostridium difficile . C. jejuni is one of the most common causes of infectious diarrhea in the United States and is the leading cause of infectious colitis worldwide. Y. enterocolitica typically involves the ileocecal valve and usually affects infants, young children, and young adults. S. typhi causes enterocolitis, usually involving the cecum and ascending colon, and is the cause of typhoid fever. Salmonella may penetrate the mucosa and submucosa of bowel, thereby resulting in the release of endotoxins into the bloodstream leading to severe septicemia. Complications of Salmonella infection include bowel perforation, toxic megacolon, gangrenous cholecystitis, and massive lower GI bleeding. C. difficile overgrowth is normally suppressed by colonic bacteria; however, antibiotic use suppresses normal bowel flora, enabling the overgrowth of C. difficile . Therefore C. difficile colitis often occurs 4 to 9 days after the initiation of antibiotics.
The clinical diagnosis of infectious colitis is based on visualizing the organisms in stool cultures, blood cultures, and/or serologic studies. Proctoscopy or flexible sigmoidoscopy may demonstrate inflamed mucosa, and endoscopic biopsies may be used to establish the diagnosis. Imaging is reserved for cases in which blood and stool cultures are negative and in cases of failed colonoscopies. Imaging may also used in cases of infectious colitis to assess for complications such as colonic strictures, bowel gangrene, and perforation.
Although CT has largely replaced barium enema, imaging findings that have been well described on barium studies include a narrowed bowel lumen and focal or diffuse bowel wall thickening. Thumbprinting, nodules, inflammatory polyps, and ulcers are additional findings that may be identified in cases of infectious colitis on barium enema. On CT, abnormally increased mucosal and serosal enhancement of affected colonic segments, bowel wall thickening, and ascites are suggestive of infectious colitis ( Fig. 13-50 ). In addition, depending on the underlying cause, air-fluid levels, pericolic inflammation, and mesenteric lymphadenopathy may also be visualized on CT in cases of infectious colitis.
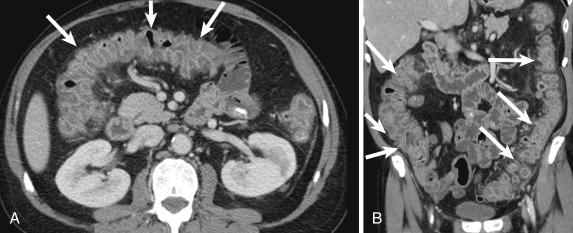
The treatment for infectious colitis varies depending on the organism; however, most cases are self-limiting. Bacterial-induced colitis, which typically persists for 1 to 2 weeks and up to 1 month, may require antibiotic treatment. Viral causes are self-limited; however, fungal and parasitic organisms are typically treated with antifungal and antihelminthic drugs, respectively.
Typhlitis (Neutropenic Colitis)
Typhlitis, also known as neutropenic colitis , is seen in profoundly neutropenic and severely immunocompromised patients. Patients with typhlitis typically present with abdominal pain, fever, nausea, and diarrhea. Patients susceptible to typhlitis most commonly are receiving chemotherapy for acute leukemia; however, typhlitis may occur in the setting of AIDS, aplastic anemia, bone transplantations, and multiple myeloma. Bacteria, viruses, and fungi may penetrate the damaged colonic mucosa and proliferate in the setting of a compromised immune system and neutropenia, leading to subsequent colonic edema and inflammation. In the absence of appropriate treatment, there may be progressive colonic inflammation with transmural necrosis, perforation, and even death. Although the pathophysiologic process is not fully understood, typhlitis is likely secondary to a combination of ischemia, infection, and mucosal hemorrhage.
On radiography, ileocecal dilatation can be seen with thumbprinting secondary to edema. Computed tomography is the imaging study of choice. Findings in typhlitis include circumferential mural thickening, predominantly of the cecum and ascending colon. Hypoattenuation secondary to submucosal edema, pericolic inflammatory stranding, and ascites are also frequently identified on CT. Pneumatosis intestinalis may be present in severe cases, presumably related to disruption of mucosal integrity. Computed tomography may be employed to confirm a suspected diagnosis of typhlitis, to monitor disease progression based on the mural thickness, and to detect complications such as pneumoperitoneum in cases of silent perforation or necrosis. Direct visualization with endoscopy or the use of barium enema is typically contraindicated in cases of suspected typhlitis given the high risk for bowel perforation. Complications related to typhlitis include the formation of abscesses or fluid collections, bowel perforation, bowel necrosis, and sepsis.
The treatment of patients with typhlitis typically includes high doses of antibiotics and intravenous fluids to prevent transmural necrosis and perforation. Surgical resection may be indicated when complications such as transmural necrosis, intramural necrosis, abscess, uncontrolled sepsis, and/or GI hemorrhage are present.
Toxic Megacolon
Toxic megacolon represents acute transmural fulminant toxic colitis resulting in colonic dilatation greater than 6 cm in a patient with clinical signs of toxicity. Patients with toxic megacolon are typically quite ill with abdominal pain and tenderness, fever, leukocytosis, dehydration, altered mental status, and tachycardia. The inflammation involves the mucosa, but unlike uncomplicated colitis, in cases of toxic megacolon the inflammation extends through the submucosa and serosa of the bowel wall. The nitrous oxide created from the inflammation is believed to inhibit smooth muscle tone, resulting in bowel distention. Toxic megacolon may be a complication of infectious colitis such as C. difficile colitis, IBD, ischemic colitis, radiation colitis, and colonic volvulus. Medications such as anticholinergics, chemotherapy, and opiates have been associated with the development of toxic megacolon, and various procedures, including colonoscopies and barium enemas, have been implicated as causes. Overall the most common causes of toxic megacolon are C. difficile and UC.
On plain abdominal radiographs, marked focal or diffuse colonic dilatation, commonly of the transverse colon, greater than 6 cm (mean dilatation is 8 to 9 cm) in a patient exhibiting signs of sepsis is diagnostic of toxic megacolon. Although colonic dilatation is nonspecific, additional imaging findings that may be identified in patients with toxic megacolon include thickened bowel wall and markedly edematous haustra. Pseudopolyps, which are nodular masses of inflamed mucosa, may be seen projecting into the lumen of affected colon. On CT a distended colon with a markedly thickened and nodular bowel wall and submucosal edema may be seen ( Fig. 13-51 ). Furthermore, the complications of toxic megacolon, including abscess formation, pneumoperitoneum, and pneumatosis intestinalis, may be readily identified on CT. In addition, imaging findings of septic thrombophlebitis may be visualized on CT, including thrombus in mesenteric and portal veins.
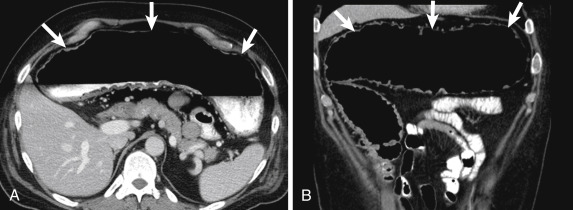
Treatment in patients with toxic megacolon may include operative intervention with colectomy and treatment of associated complications. Ultimately the prognosis varies and is highly dependent on the presence of complications, including frank bowel perforation and abscess formation.
Epiploic Appendagitis
Epiploic appendagitis represents acute inflammation or infarction of an epiploic appendage. Located on the antimesenteric side of the colon, the epiploic or omental appendages are small, lobulated masses containing adipose tissue and blood vessels, arising from the serosal surface of the colon. The epiploic appendages may become inflamed or torsed, resulting in infarction. Other reported causes of epiploic appendagitis include incarcerated hernia or bowel obstruction. Patients with epiploic appendagitis typically present with an acute onset of abdominal pain with localized tenderness over the portion of the affected colon with the left lower quadrant being the most commonly affected site. Marked abdominal tenderness to palpation with significant rebound tenderness is elicited on physical examination. Both a normal white blood cell count and body temperature are commonly found, and constitutional symptoms such as nausea, vomiting, and anorexia are uncommon.
Computed tomography is the imaging modality of choice to diagnose acute epiploic appendagitis, which is visualized as an ovoid, fat-attenuating pericolic mass situated along the antimesenteric border of the colon, ranging in size from 1 to 4 cm ( Fig. 13-52 ). In addition, surrounding inflammatory changes with a thin hyperattenuating rim due to thickening of the visceral peritoneum may be identified. Finally, a central “dot” of increased attenuation within the inflamed appendage may be identified and represents an engorged or thrombosed central vein. On US a targeted evaluation of the area of pain may reveal a noncompressible, hyperechoic mass adherent to the colon without central vascularity. Finally, MRI and T1- and T2-weighted images demonstrate a small pericolic mass that exhibits fat signal. Similar to CT, a hypointense central dot with a thin, surrounding rim of edema and inflammation, as well as peripheral enhancement, may be appreciated on MRI.
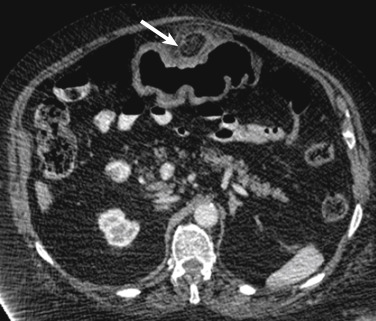
Acute epiploic appendagitis is usually self-limited with resolution of symptoms in 1 week, and conservative management with analgesics is the treatment of choice. Although rare, complications occasionally occur and include the formation of adhesions, bowel obstruction, peritonitis, and intraperitoneal abscesses.
Acute Presentations of Inflammatory Bowel Disease
Crohn Disease
Crohn disease is one of the major forms of IBD and represents a chronic and recurrent granulomatous inflammatory disease. Acute presentations and exacerbations of this disease are common source of symptoms seen in ED patients. Crohn disease is more common in white and Jewish populations and in northern Europe and North America and typically occurs in the second and third decades of life, affecting both sexes equally. The cause is unknown, but recent advances in knowledge revolve around multiple factors, including genetics with a familial predisposition, intestinal microbial flora and infection, nutritional factors, and immunologic factors.
The most common presenting symptoms in patients with CD include chronic diarrhea and abdominal pain. Weight loss, malabsorption, and perianal fistulas and fissures are also frequently observed. Extraintestinal manifestations are common, particularly when the colon is involved, and they include abnormalities of the skin, joints, eyes, kidneys, and liver and biliary tree. Crohn disease, unlike UC, is patchy and segmental with skip lesions and transmural involvement. Any part of the GI tract may be involved, from the mouth to the anus, and the disease process is commonly discontinuous.
Colonoscopy with the retrieval of multiple biopsy specimens is the first-line study for diagnosing this disease. Aphthous ulcers with a target appearance, deep fissuring ulcers, and lymphoid hyperplasia are characteristic findings on colonoscopy. The role of CT and MRI in CD has expanded with recent advances in technology allowing for rapid acquisition of high-resolution images of the bowel. Computed tomography and MRI may identify disease, localize and characterize the severity and extent of disease, indicate the presence of acute complications, assess the severity of inflammation, and monitor disease progression. In the acute stage, bowel wall thickening greater than 3 mm is the most consistent cross-sectional imaging finding ( Fig. 13-53 ). Mural stratification with a target appearance consisting of intense enhancement of the mucosa, hypoattenuation of the submucosa, and an outer enhancing muscularis propria may be identified (see Fig. 13-53 ). Mucosal hyperenhancement reflects inflammation, and the degree of enhancement correlates with the degree of inflammation. Mesenteric fibrofatty proliferation, comb sign (engorgement of the vasa recta), and mesenteric lymphadenopathy are additional findings (see Fig. 13-53 ). Complications of CD include abscesses, fistula formation, anal fissures, and colon cancer. Many of these complications are first diagnosed in the ED setting.
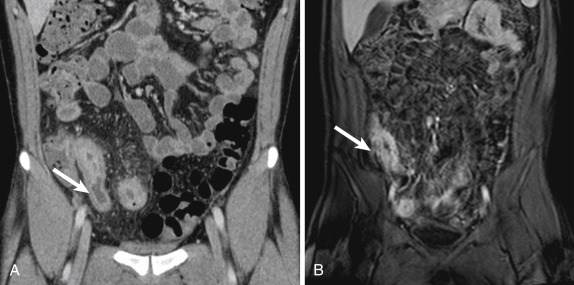
Ulcerative Colitis
Ulcerative colitis, another form of IBD, represents a chronic, idiopathic, diffuse inflammatory process in the colon. Ulcerative colitis typically affects patients between 15 and 25 years of age with women slightly more frequently affected than men. Similar to CD, UC is more common in white and Jewish populations and in northern Europe and North America. Although the cause is not entirely clear, genetic susceptibility, host immunity, and environmental factors have been implicated. Patients with UC usually present with bloody diarrhea, passage of mucus, abdominal pain, tenesmus, and urgency of defecation. Ulcerative colitis is also associated with extraintestinal manifestations, similar to those seen in CD. Not uncommonly, UC is first diagnosed when patients present with these symptoms to the ED. Acute exacerbations also require emergent medical attention.
At presentation a majority of patients (>95%) are found to have rectosigmoid involvement, which not uncommonly progresses to pancolitis and rarely affects the small bowel. In UC the inflammation is limited to the mucosa and typically ends at the muscularis mucosae, which is in contradistinction to the transmural involvement in CD. Continuous and symmetrical involvement is the hallmark of UC with distinct transitions between diseased and unaffected segments of colon, which again is in contrast to CD, in which the entire digestive tract may be affected in a discontinuous manner with transmural involvement. In early disease, mucosal edema and hyperemia are encountered, and with disease progression the mucosa develops punctate ulcers that enlarge and may extend into the lamina propria. The epithelium regenerates between acute inflammatory attacks, resulting in the formation of pseudopolyps, usually seen in long-standing disease. In chronic UC the colon becomes foreshortened, and featureless, with the loss of haustral folds, and exhibits luminal narrowing. In 15% to 20% of patients with UC a fulminant form of the disease may develop that is characterized by extensive inflammation with severe symptoms and colonic dilatation.
On abdominal radiography there is bowel wall thickening and colonic distention. In cases of fulminant UC severe colonic dilatation or toxic megacolon may be seen. As in all cases of IBD, CT (and increasingly MRI) is employed for determining disease activity. On CT a target or double-halo appearance due to mural stratification is commonly identified. However, this imaging finding is nonspecific and may be seen in patients with CD, pseudomembranous colitis, ischemic and radiation enterocolitis, infectious colitis, and bowel edema. With disease progression the bowel wall thickens and becomes featureless due to the loss of haustral folds.
Acute complications of UC that are particularly relevant in the ED setting include toxic megacolon, perforation with peritonitis and abscess formation, and venous thrombosis. Urgent surgery may be necessary when these complications develop. More chronic complications include colorectal cancer and stricture formation.
Perirectal Fistula and Abscess
Perirectal abscess and fistulization are inflammatory conditions of the rectum and anus that can cause severe perirectal pain and sinus discharge, often sending patients to seek emergency medical attention. A fistula results from an abscess derived from an infection originating in the anal canal glands at the dentate line. Sepsis from a perirectal abscess usually resolves after antibiotic treatment and surgical drainage; however, in approximately 25% of patients the abscess cavity does not resolve completely, and the infection decompresses to the skin. There is often a skin opening with erythema and focal granulation tissue with purulent or serosanguineous discharge. An inflammatory tract develops between a primary internal opening in the anal crypt at the dentate line and extends to a secondary external opening in the perianal skin. The tract decompresses in the anatomic plane of least resistance, the most common being the plane located between the internal and external sphincters, extending into the ischiorectal fascia and to the perineal skin. The fistulous tract may also extend circumferentially in the ischiorectal fossa with the tract passing into the contralateral fossa through the posterior rectum, forming a horseshoe fistula. Perirectal fistulas are classified into four types based on the anatomic relation of the tract to the sphincter muscles. However, a precise definition of the specific type of fistula requires a dedicated MRI examination with high-resolution imaging and is seldom necessary in the emergency setting.
Computed tomography is the imaging study typically requested in the ED. Computed tomography should be performed with intravenous contrast. The use of rectal contrast may be necessary for proper detection of perianal fistulas. Abscesses are identified as loculated low-attenuation fluid collections, possibly containing foci of air or an air-fluid level, with peripheral enhancement. Magnetic resonance imaging is the imaging modality of choice to depict the anatomy of the anal sphincter and perianal structures. However, as mentioned previously, emergent MRI is usually not required for this indication.
Acute Colonic Obstruction
Large bowel obstructions are less common than SBOs and account for approximately 20% of all obstructions. Acute colonic obstructions are emergencies requiring early detection to prevent complications such as perforation or ischemia. The most common cause of acute colonic obstruction is malignancy, usually occurring in the sigmoid colon ( Fig. 13-54 ). Other causes of large bowel obstruction include acute sigmoid diverticulitis and colonic volvulus. Abdominal pain, failure to pass stool or gas, and abdominal distention are common presenting symptoms of acute colonic obstruction. Differentiating between mechanical obstruction and Ogilvie syndrome, also known as colonic pseudo-obstruction , has therapeutic implications and should be accomplished with barium enema. A false diagnosis of colonic obstruction, particularly in patients with obstructive symptoms, may lead to inappropriate surgical exploration.
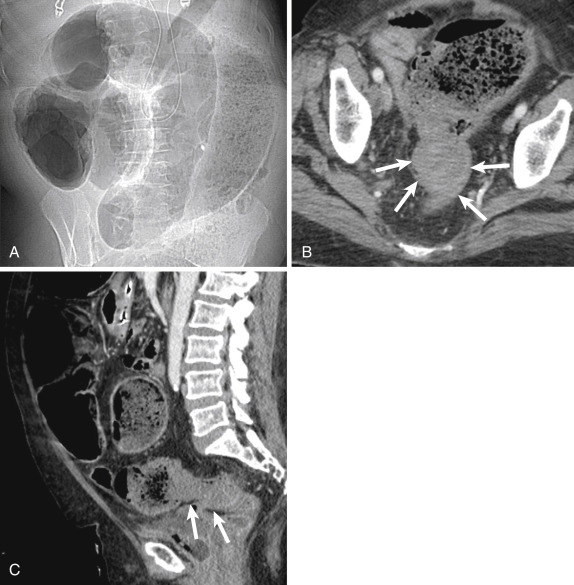
Imaging, particularly CT, is essential in assessing crucial information that has therapeutic implications. Computed tomography can help determine whether small or large bowel is affected, assess the location and severity of obstruction, and identify the cause and potential complications. On plain radiography, dilated colon (dilation being a caliber greater than 6 cm and 9 cm in the cecum) is seen. The presence of free air is indicative of bowel perforation. On CT the cause can be identified, and imaging appearances vary depending on whether colonic malignancy, acute diverticulitis, volvulus, or other pathologic process is present. Large bowel obstruction usually warrants surgical exploration, particularly when complications such as perforation or ischemia are present.
Sigmoid Volvulus
Sigmoid volvulus represents torsion or twisting of the sigmoid colon around the mesenteric axis. Patients typically present with acute or insidious onset of abdominal pain, nausea, abdominal distention, and obstipation. Vomiting usually occurs in late stages of disease when abdominal distention is severe. The presence of tachycardia, rebound tenderness, and severe abdominal pain are predictors of poor outcome. Patients with sigmoid volvulus are at increased risk for developing bowel ischemia by two mechanisms: arterial occlusion from mesenteric arterial torsion and mural ischemia due to increased wall tension of distended bowel. Colonic volvulus is associated with a high morbidity, particularly in cases of late presentation.
Sigmoid volvulus accounts for up to 75% of cases of volvulus of the bowel but accounts for less than 10% of all cases of intestinal obstruction in the United States. Predisposing factors for sigmoid volvulus include chronic constipation, obstipation from medications, high-fiber diet resulting in bulky stool causing elongation of bowel, and prior abdominal surgeries. Comorbidities include psychiatric conditions, advanced age, and institutionalization in medical facilities.
Imaging is particularly useful in diagnosing colonic volvulus. Abdominal radiographs are diagnostic in a majority of cases when classic findings of a distended, inverted U-shaped loop of sigmoid colon is seen, along with an absence of haustral markings ( Fig. 13-55 ). The “coffee bean” sign refers to the inverted U shape of the sigmoid colon with a dense white line formed by the apposed colonic walls, which is directed from the pelvis to the right upper quadrant. A single contrast enema typically reveals a smoothly tapered narrowing of the rectosigmoid junction at the distal aspect of the volvulus, resulting in a “bird beak” appearance. On CT a whirl sign may be visualized in which twisting of the sigmoid colon (see Fig. 13-55 ) and the mesentery is present and “beaking” due to tapered narrowing of the afferent and efferent bowel loops may be seen. Computed tomography may readily identify complications of sigmoid volvulus, including bowel ischemia or perforation.
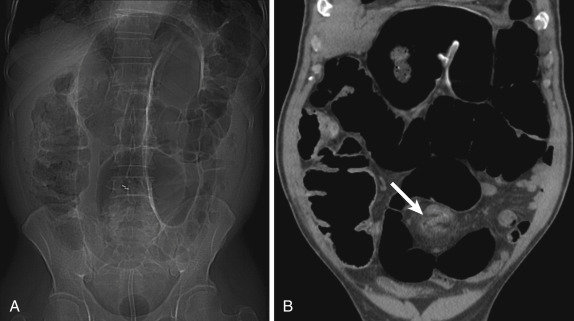
Sigmoid volvulus may be treated nonoperatively with proctoscopic/colonoscopic decompression with a high success rate. In some cases nonoperative treatment of sigmoid volvulus may be followed with an elective sigmoidectomy. In cases of sigmoid volvulus with complications such as bowel ischemia or perforation, emergent surgery is often performed.
Cecal Volvulus
Cecal volvulus refers to torsion or twisting of the cecum around a fixed and twisted mesenteric axis, resulting in massively dilated bowel with the tip pointing to the left upper quadrant. Patients present with abdominal pain of acute or insidious onset, nausea, and vomiting. Like sigmoid volvulus, patients are at increased risk for developing bowel ischemia, and cecal volvulus is associated with a high morbidity, especially in cases of late presentation.
As opposed to sigmoid volvulus, in which the cause is usually acquired, the most common predisposing factor to cecal volvulus is an abnormal embryologic connection of the right colon to retroperitoneum resulting in increased mobility of the cecum. Other causes of cecal volvulus include cecal bascule with anterior folding of the cecum, postpartum ligamentous laxity, colonic distention, and chronic constipation. Cecal bascule refers to abnormal positioning of the cecum in the midabdomen secondary to loose mesenteric attachment, which results in folding of the cecum.
On abdominal radiographs, cecal volvulus has a characteristic appearance with a dilated loop of inverted U-shaped bowel eccentrically located in the left upper quadrant that is directed from the pelvis to the left upper quadrant. The diagnosis of cecal volvulus may be confirmed on contrast enema or CT. On contrast enema examination a beaklike tapering of the cecum is seen at the level of the volvulus, and contrast usually does not pass into the proximal colon or small bowel. On CT the cecum is abnormally positioned in the mid and left abdomen ( Fig. 13-56 ). Progressive narrowing of the afferent and efferent limbs of colon is seen, leading to a whirl sign, which represents a tight twisting of the mesentery and “beaking” due to tapered narrowing of the afferent and efferent bowel loops. Computed tomography may readily identify complications, including bowel ischemia or perforation. Colonoscopy can be performed to reduce the volvulus, but surgical intervention, including cecopexy or resection, is indicated in complicated cases.
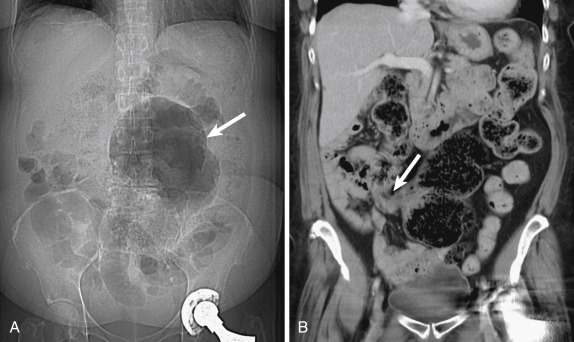
Intussusception
Colonic intussusception refers to invagination or telescoping of a proximal loop of bowel (intussusceptum) into the lumen of an adjacent, distal segment of bowel (intussuscipiens). Intussusception may be intermittent, particularly in the absence of a lead point. Patients typically present with abdominal pain, nausea, and vomiting, and in patients with an underlying malignancy, weight loss, palpable abdominal mass, melena, and constipation may be seen.
The cause and pathophysiologic process of intussusception are not well understood, particularly intussusception without a lead point. Dysrhythmic bowel contractions have been implicated. Intussusceptions may be caused by lesions within the intestinal wall, or, alternatively, intraluminal lesions may act as lead points. Large bowel intussusception is commonly associated with lead points. Benign lesions such as lipomas, adenomatous polyps, colitis, epiploic appendagitis, and postoperative adhesions, as well as malignant lesions such as metastatic disease, primary malignancy, and lymphoma, may act as lead masses causing intussusception.
Four major forms of intussusception have been described, including enteroenteric, ileocolic, ileocecal, and colocolic. The majority of cases involve the small bowel. Ileocolic or ileocecal intussusception is often associated with small bowel metastatic disease; the most common primary malignancies include melanoma, breast, and lung. The most common benign causes of colocolic intussusception are lipomas, followed by adenomatous polyps, whereas colonic adenocarcinoma is the most common malignant cause.
Contrast-enhanced CT is the imaging modality of choice to evaluate the bowel in cases of suspected intussusception. The appearance of the bowel on CT varies widely depending on various factors such as the presence and configuration of the lead mass, presence of bowel dilatation and degree of obstruction, degree of bowel wall edema, and amount of invaginated mesenteric fat and blood vessels. Intussusceptions on CT typically demonstrate a targetlike appearance, with or without the presence of mesenteric fat or vessels, with the outer layer representing the intussuscipiens and the inner layer representing the intussusceptum ( Fig. 13-57 ). Typically the cross-sectional diameter of an intussusception with a lead mass is greater than that of normal bowel. In addition, signs of obstruction may also be seen, including proximal bowel dilatation with distally collapsed loops. A relatively short length of intussusception and the absence of proximal obstruction, typically in the small bowel, support the diagnosis of a transient, nonobstructive intussusception.
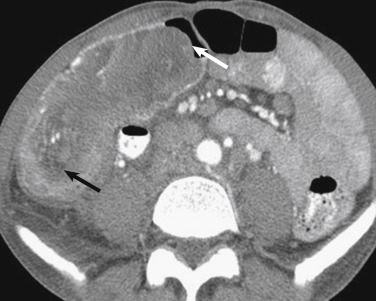
Treatment for transient, nonobstructive intussusception is conservative, whereas surgical reduction or resection is the usual treatment for obstructive intussusception and for malignant lead masses.
Ogilvie Syndrome
Ogilvie syndrome, also known as colonic pseudo-obstruction , is characterized by colonic dilatation with obstructive symptoms in the absence of a true mechanical obstruction. Patients present with obstructive symptoms of abdominal pain and distention, nausea, vomiting, and failure to pass stool. Although no underlying mechanical obstruction exists, Ogilvie syndrome is a significant cause of morbidity and death with possible progression to bowel ischemia and perforation. Although the pathogenesis of colonic pseudo-obstruction is not fully understood, abnormal parasympathetic autonomic regulation of the colon and intramural ganglion damage are among proposed mechanisms. A false diagnosis of colonic obstruction, particularly in patients with obstructive symptoms, may lead to inappropriate surgical exploration. The term Ogilvie syndrome has been applied to both the acute and chronic forms of colonic pseudo-obstruction, though some authors believe this term applies to the acute form, which represents a reversible condition, associated with major surgery or severe medical illness. The chronic form of pseudo-obstruction is characterized by repeated episodes of bowel dilatation and obstructive symptoms.
On imaging, marked dilatation of the colon is seen on abdominal radiography with the cecum and ascending and transverse colons more severely affected. In patients with Ogilvie syndrome, CT readily demonstrates marked colonic distention with a long segment of relative transition to more collapsed bowel occurring in the absence of an obstructing lesion. This relative transition often occurs at the splenic flexure where the parasympathetic bowel innervation changes from the vagal nerve to the sacral nerve, lending support to the hypothesis that transient impairment of the sacral plexus leads to functional obstruction of the proximal colon. Computed tomography also is useful for accurately depicting the degree of colonic distention that may warrant surgical decompression. In addition, complications of pseudo-obstruction, including bowel ischemia and perforation, may be assessed with CT. The treatment of Ogilvie syndrome is based on the clinical and imaging assessment and includes supportive treatment, endoscopic decompression, and surgical decompression. The presence of bowel ischemia and perforation typically warrant surgical intervention.
Acute Colonic Ischemia and Pneumatosis
Ischemic Colitis
Ischemic colitis is the most common vascular disorder of the GI tract and is caused by compromise of the mesenteric vascular supply. Ischemic colitis commonly occurs in the so-called watershed areas supplied by the SMA and IMA at the splenic flexure and decreased perfusion between the IMA and hypogastric artery at the rectosigmoid junction. The rectum is typically spared secondary to extensive collateral blood supply. In older adults, left-sided ischemic colitis is more typical secondary to hypoperfusion, whereas right-sided ischemic colitis due to hemorrhagic shock is more common in younger patients. Presenting signs and symptoms in patients with acute ischemic colitis include the acute onset of mild to severe crampy abdominal pain, nausea, and vomiting; bloody diarrhea and rectal bleeding may also occur several hours after the onset of abdominal pain. Predisposing factors to ischemic colitis include, among others, hypotension from hemorrhagic or septic shock, cardiogenic shock, arrhythmias, congestive heart failure, vasculitis, mechanical bowel obstruction, and certain medications.
The degree of bowel wall involvement ranges from isolated mucosal to transmural pathologic process depending on the severity and duration of ischemia. Because the mucosa receives a majority of the vascular supply to the colonic wall, it is most susceptible to ischemia. The sequelae of acute ischemic colitis include reversible ischemic colitis, chronic ulcerative ischemic colitis, ischemic colonic stricture, or colonic necrosis with perforation and sepsis.
Abdominal radiographs demonstrate thumbprinting in up to 75% of patients with ischemic colitis. This sign reportedly appears within 24 hours of the onset of ischemia. Thumbprinting manifests as smooth, round, polypoid, and scalloped filling defects projecting into the colonic lumen, which correspond to thickened mucosal folds related to submucosal edema or hemorrhage. Additional radiographic findings that may be appreciated in patients with acute ischemic colitis include the presence of a nonspecific ileus, loss of haustral folds, pneumatosis intestinalis, and luminal narrowing. Contrast-enhanced CT has become the imaging modality of choice in cases of suspected ischemic colitis with imaging features of circumferential bowel wall thickening and pericolic inflammation ( Fig. 13-58 ). A target sign resulting from hyperenhancement of the mucosa and serosa secondary to hyperemia and relative submucosal hypoattenuation related to edema may be identified. Following reperfusion, hypoattenuation of the bowel wall due to edema or hyperattenuation of the bowel wall due to hemorrhage may be seen. In cases of continued occlusion of the colonic vasculature without reperfusion, the bowel wall may remain hypodense with nonenhancement of the bowel wall after intravenous contrast administration. In cases of suspected ischemic colitis the mesenteric vessels should be closely scrutinized for obstructing arterial or venous thrombi. In the setting of ischemic colitis, pneumatosis intestinalis or the presence of portomesenteric venous air is highly suggestive of frank bowel wall necrosis.