Carotid Duplex Ultrasound
Indications for carotid duplex US:
(1) Screening for suspected extracranial carotid disease
(a) high-grade flow-limiting stenosis
(b) low-grade stenosis with hemorrhage
(2) Nonhemispheric neurologic symptomatology
(3) History of transient ischemic attack / stroke
(4) Asymptomatic carotid bruit
(5) Retinal cholesterol embolus
(6) Preoperative evaluation before major cardiovascular surgery
(7) Intraoperative monitoring of vascular patency during endarterectomy
(8) Sequential evaluation after endarterectomy
(9) Monitoring of known plaque during medical treatment
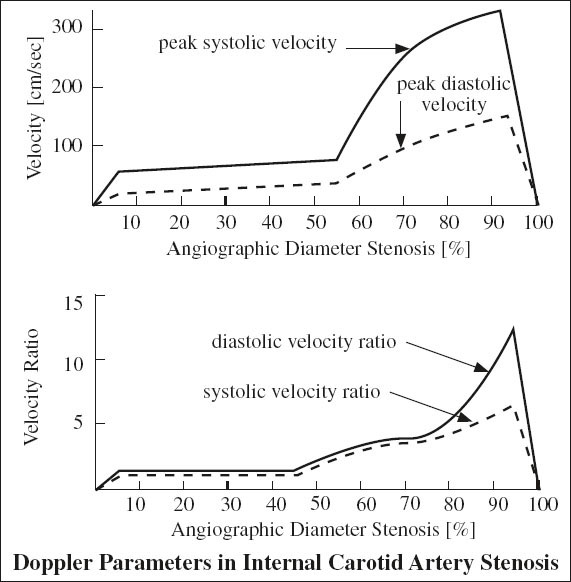
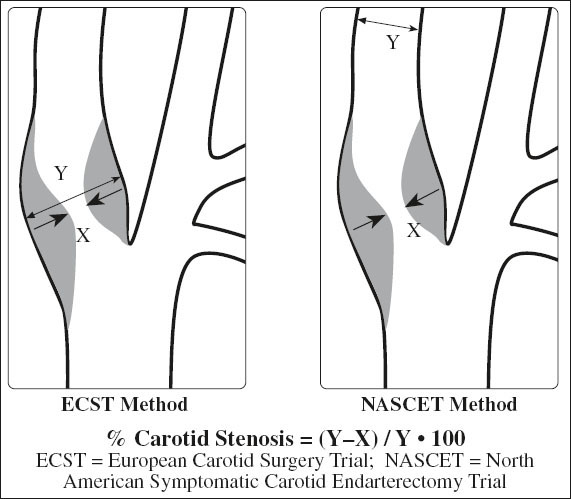
Grading of Internal Carotid Stenosis
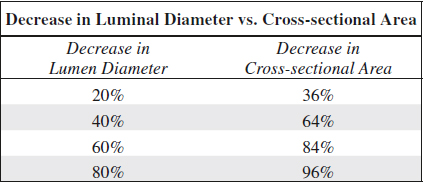
= severity of stenosis is primarily graded as a ratio of lumen diameter narrowing NOT reduction in cross sectional area
Limitations of assessment by US:
1. Calcification > 1 cm in length obscures vessel lumen
◊ A jet associated with a stenosis of > 70% usually travels at least 1 cm downstream allowing conclusion about degree of obscured stenosis!
2. Contralateral high-grade stenosis
= ipsilateral ICA functions as collateral → increased blood flow velocities
◊ Use velocity ratios to compensate for this effect!
3. Tortuosity of artery
4. Increased depth of artery
5. “High” bifurcation → possibly obscured by mandible
Accuracy of duplex scans (compared to arteriography) for ICA lesions:
91–94% sensitivity, 85–99% specificity, 90–95% accuracy for > 50% ICA diameter stenosis
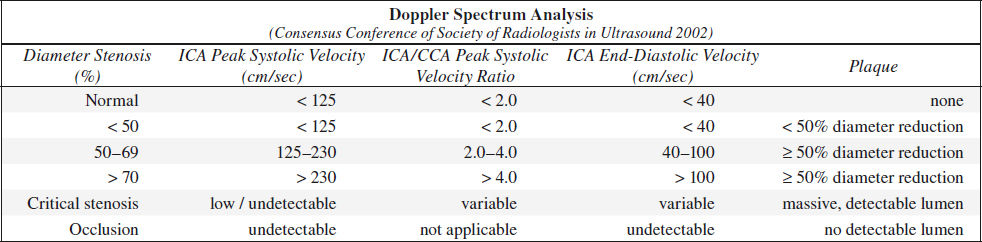
A. NORMAL (0% diameter reduction)
√ no evidence of plaque
√ peak systolic velocity (PSV) < 125 cm/sec
√ no spectral broadening (= clear window under systole)
B. MINIMAL DISEASE (0–15% diameter reduction)
√ minimal amount of plaque
√ PSV < 125 cm/sec
√ minimal spectral broadening in deceleration phase of systole
C. MODERATE DISEASE (16–49% diameter reduction)
√ moderate amount of plaque
√ peak systole < 125 cm/sec
√ end-diastolic velocity (EDV) < 40 cm/sec
√ poststenotic spectral broadening throughout systole
D. SEVERE DISEASE = hemodynamically SIGNIFICANT LESION
(a) 50–69% stenosis
√ PSV 125–230 cm/sec
√ EDV 40–100 cm/sec
(b) > 70% stenosis (→ benefit of endarterectomy documented in NASCET study)
√ peak systole > 230 cm/sec
√ end diastole > 100 cm/sec
√ peak systolic velocity ratio of ICA÷CCA > 4.0
E. CRITICAL STENOSIS (> 95% diameter reduction)
√ PSV + EDV return to normal range / flow undetectable
√ “string” sign on color Doppler with slow-flow sensitivity setting
F. OCCLUSION
√ NO signal in ICA on longitudinal / transverse images (color sensitivity + velocity scale must be set low enough to clearly discern flow signals within internal jugular vein)
√ absence of diastolic flow / diastolic flow reversal in CCA (= high impedance flow)
√ increased diastolic flow in ECA (if ECA assumes the role of primary supplier of blood to brain)
√ increase in peak systolic velocities in contralateral ICA ← collateral flow
Common Carotid Waveform Analysis
A. DISTAL OBSTRUCTION
√ high-pulsatility waveform
◊ Pulsatility changes occur only with > 80% stenosis
√ reduced amplitude
B. PROXIMAL OBSTRUCTION
√ low-amplitude damped waveform
Hemodynamic Variations of Carotid Stenosis
A. MORPHOLOGY OF STENOSIS
1. Degree of stenosis: velocities increase up to a luminal diameter of 1.0–1.5 mm
2. Length of stenosis: peak velocities decrease with length of stenosis
√ use the same angle + beam steering direction when following a patient for disease progression
B. PHYSIOLOGIC VARIABILITY
◊ A range of velocities may be encountered with a given degree of stenosis!
◊ ICA÷CCA ratio obviates effects of physiologic variability!
◊ Compare left with right waveforms to avoid errors!
◊ Measure volume flow (more sensitive because of contralateral compensatory flow increase)
Cause:
1. Cardiac output
2. Pulse rate
3. Flow velocity: ↑ with obstruction in collateral vessels, ↓ with proximal obstruction in same vessel
4. Normal helical nature of blood flow with many different velocity vectors + nonaxial blood flow NOT detectable by color Duplex imaging
5. Peripheral resistance
6. Arterial compliance
7. Hypertension
8. Blood viscosity
Carotid Plaque
1. Asymptomatic patients with diffuse atherosclerotic disease:
Prevalence of > 50% stenosis: 18–20%
2. Symptomatic patients with stroke, TIA, amaurosis fugax ← emboli from atheromas at carotid bifurcation
Prevalence of > 50% stenosis: 14%
Formation Theory of Carotid Plaque
1. Stagnant eddy that rotates at outer vessel margin (away from and opposite to flow divider = area of flow separation + low shear stress) → net influx of fluid into subendothelial tissue with progressive deposition of lipids + smooth muscle cell proliferation
2. Increased likelihood of intraplaque hemorrhage ← vascularization of plaque with fragile vessels derived from vasa vasorum / from lumen) + fissuring at a critical size
◊ As the degree of stenosis increases, it is more likely that plaques become denser + more heterogeneous demonstrating an irregular luminal surface!
Density of Carotid Plaque
1. Hypoechoic = low-echogenicity plaque
= fibrofatty plaque / hemorrhage
√ echogenicity less than sternocleidomastoid muscle
√ flow void / flow disturbance on color Duplex
2. Isoechoic plaque
= smooth muscle cell proliferation / laminar thrombus
√ echogenicity equal to sternocleidomastoid muscle + lower than adventitia
3. Hyperechoic = moderately echogenic (fibrous) plaque
√ echogenicity higher than sternocleidomastoid muscle + similar to adventitia
4. Calcification = strongly echogenic plaque
√ acoustic shadow impairs visualization of intima
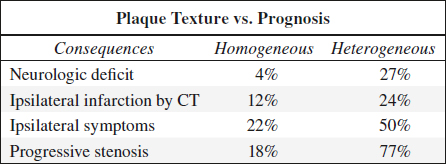
Texture of Carotid Plaque
1. Homogeneous (stable) plaque
Histo: deposition of fatty streaks + fibrous tissue; rarely shows intraplaque hemorrhage / ulcerations
√ homogeneous uniform echo pattern with smooth surface (acoustic impedance similar to blood)
2. Heterogeneous (unstable) plaque
= mixture of high, medium, and low-level echoes with smooth / irregular surface; may fissure / tear resulting in intraplaque hemorrhage / ulceration + thrombus formation → embolus / increasing stenosis
B-mode ultrasound has 90–94% sensitivity, 75–88% specificity, 90% accuracy for intraplaque hemorrhage!
MRI depicts intraplaque hemorrhage (methemoglobin) on T1 fat-suppressed SPGR / FLASH sequence
Histo: lipid-laden macrophages, monocytes, leukocytes, necrotic debris, cholesterol crystals, calcifications
√ anechoic areas within plaque (= hemorrhage / lipid deposition / focal plaque degeneration)
√ heterogeneous complex echo pattern
Surface Characteristics of Carotid Plaque
◊ US unreliable due to poor visualization of intima
| 4 Categories: | › | smooth |
| › | mildly irregular | |
| › | markedly irregular | |
| › | ulcerated |
1. Intimal thickening
Histo: fatty streaks
√ wavy / irregular line paralleling vessel wall extending > 1 mm into vessel lumen
2. Ulcerated plaque
Accuracy: 60% sensitive, 60–70% specific
◊ The presence of intraplaque hemorrhage is much more common than normally appreciated (neither arteriography nor US has proved reliable)!
√ isolated crater of > 2 mm within surface of plaque demonstrated on transverse + longitudinal images
√ reversed flow vortices extending into plaque crater demonstrated by color Doppler
√ proximal + distal undercutting of plaque
√ anechoic area within plaque extending to surface
Errors In Duplex Ultrasound
1. Error in proper localization of stenosis (6%)
Cause: ECA stenosis placed into ICA / carotid bifurcation or vice versa
2. Mistaking patent ECA branches for carotid bifurcation (4%)
Cause: complete occlusion of ICA not recognized
√ disparity in position of bifurcation between left and right
√ no difference in waveform pulsatility
√ high-resistance waveform in CCA
3. Interpreter error in estimating severity of stenosis (2.5%):
» usually overestimation, rarely underestimation
√ absence of one / more components for diagnosis which are
(a) significant elevation of peak velocity
(b) poststenotic turbulence
(c) extension of high velocity into diastole
4. Superimposition of ECA + ICA (2%)
Cause: strict coronal orientation of ECA + ICA
√ superimposition can be avoided by rotation of patient’s head to opposite side
5. Severe stenosis mistaken for occlusion
√ minimal flow not detectable
√ angiogram necessary with delayed images
6. Weak signals misinterpreted as occlusion
7. Normal / weak signals in severe stenosis
Cause: severe stenosis causes a decrease in blood flow and peak velocity → return to normal velocity levels
√ high resistivity in CCA
8. Point of maximum frequency shift not identified
Cause: extremely small lumen / short segment of stenosis
√ unexplained (poststenotic) coarse turbulence
√ collateral flow in ipsilateral ECA
√ abnormal CCA resistivity
9. Stenosis obscured by plaque / strong Doppler shift in overlying vessel
10. Inaccessible stenosis
√ abnormal CCA resistivity
√ abnormal oculoplethysmography
11. Unreliable velocity measurements
(a) higher velocities: HTN, severe bradycardia, obstructive contralateral carotid disease, anemia, hyperthyroidism
(b) lower velocities: arrhythmia, aortic valvular lesion, CHF, severe cardiomyopathy, proximal obstructive carotid lesion (“tandem lesion”), > 95% ICA stenosis
(c) aliasing = high velocities are displayed in reversed direction below zero baseline due to Doppler frequency exceeding half the pulse repetition frequency
Remedy: shift zero baseline; increase pulse repetition frequency; increase Doppler angle; decrease transducer frequency; use continuous-wave Doppler probe
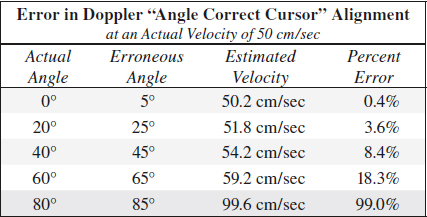
(d) misalignment of Doppler “Angle Correct” cursor
= cursor not aligned with blood stream line introducing a wrong Doppler angle
◊ Doppler angle larger than actual angle means velocity measurement are higher than actual velocity!
CHOANAL ATRESIA
◊ Most common cause of neonatal nasal obstruction!
Frequency: 1÷5,000 to 1÷8,000 neonates; M < F
Etiology: failure of perforation of buccopharyngeal + oronasal membrane, which normally perforates by 7th week EGA
Associated anomalies (in 50–75%):
acrophalyngosyndactyly, amniotic band syndrome, malrotation of bowel, Crouzon syndrome, fetal alcohol syndrome, DiGeorge syndrome, Treacher-Collins syndrome, chromosome 18 / 12 anomalies, polydactyly, coloboma, facial cleft, CHD, tracheoesophageal fistula, craniosynostosis
Location: bilateral÷unilateral atresia = 3÷2 to 2÷1
• respiratory distress in bilateral choanal atresia (relieved by crying in neonates who are obligatory nasal breathers during first 2–6 months)
• nasal stuffiness, rhinorrhea, infection in unilateral choanal atresia
Types:
A. OSSEOUS / BONY SEPTATION (85–90%)
Cause: incomplete canalization of choanae
√ fusion of hard palate + vomer + ventral clivus with nasopharyngeal atresia
B. MEMBRANOUS SEPTATION (10–15%)
Cause: incomplete resorption of epithelial plugs
C. OSSEOMEMBRANOUS
CT (preceded by vigorous suctioning + administration of topical decongestant):
√ narrowing of choanal orifice to a width of < 3.4–3.7 mm (in children < 2 years of age)
√ inward bowing of posterior maxilla
√ deviation / bowing of nasal septum
√ fusion / thickening of vomer
√ bone / soft-tissue septum extending across posterior choanae
Dx: nasal catheter cannot be advanced to beyond 32 mm
Cx: bilateral choanal atresia is LIFE-THREATENING
Rx: endoscopic perforation, choanal reconstruction
CHOLESTEATOMA
= KERATOMA = EPIDERMOID
= epithelium-lined sac characterized by accumulation of desquamated keratin epithelium → bone destruction by pressure + increase in osteoclastic activity
Location: middle ear cavity, other pneumatized portions of temporal bone
Histo: (a) acellular keratin debris = content of sac
(b) matrix = biologically active sac lining consisting of
› inner layer of keratinizing squamous epithelium
› outer layer of subepithelial connective tissue (= perimatrix) producing proteolytic enzymes → bone resorption
√ NO enhancement
• pearly white lesion diagnosed otoscopically in 95%
Cx: (1) Infection → malodorous discharge
(2) Erosion of ossicular chain, scutum, mastoid bone, Körner septum
DDx of soft-tissue attenuation:
(1) Fluid attenuation of chronic otitis media
(2) Inflammation / infection ( NO restricted diffusion)
Primary Cholesteatoma (2%)
= CONGENITAL CHOLESTEATOMA = EPIDERMOID CYST
= derived from aberrant embryonic ectodermal rests in temporal bone (commonly petrous apex) / epidural space / meninges
• conductive hearing loss (hypoacusis) in child with NO history of otorrhea, membrane perforation, otologic procedure
• cholesteatoma seen through intact tympanic membrane (usually difficult due to stenosis of EAC)
Associated with: dysplasia / atresia / stenosis of EAC /middle ear cavity
Location:
(a) epitympanum (commonly in anterior superior quadrant of middle ear cavity just above opening of eustachian tube)
(b) petrous pyramid: internal auditory canal first involved
(c) meninges: scooped out appearance of petrous ridge
(d) cerebellopontine angle: erosion of porus, shortening of posterior canal wall
(e) jugular fossa: erosion of posteroinferior aspect of petrous pyramid
Cx: facial palsy, mastoiditis, intracranial disease
Secondary Cholesteatoma (98%)
= INFLAMMATORY CHOLESTEATOMA = ACQUIRED EPIDERMOID
Pathophysiology:
(a) invagination theory = eustachian tube dysfunction → vacuum phenomenon in middle ear cavity → formation of posterosuperior retraction pocket in pars flaccida → repeated episodes of ear inflammation (= chronic otitis media)
(b) epithelial invasion theory = marginal perforation of eardrum → ingrowth of keratinizing stratified squamous epithelium
Location: middle ear cavity
Age: usually > 40 years
• chronic foul-smelling otorrhea
• facial paralysis ← compression of nerve VII at geniculate ganglion
• conductive hearing loss ← compromise of CN VIII in internal auditory canal / involvement of cochlea or labyrinth
• severe vertigo (labyrinthine fistula)
Type:
1. Pars flaccida cholesteatoma (80%)
= PRIMARY ACQUIRED CHOLESTEATOMA
= Attic cholesteatoma
Site: centered in Prussak space (pars flaccida invaginates toward Prussak space)
√ rounded expansile lobulated lesion
√ increasing width of attic
√ initially destruction of lateral wall of attic, particularly erosion of drum spur (scutum)
√ medial displacement + erosion of auditory ossicles
√ extension superiorly into attic + mastoid air cells through aditus ad antrum
√ destruction of Körner septum
2. Pars tensa cholesteatoma (20%)
= SECONDARY ACQUIRED CHOLESTEATOMA
Site: centered in sinus tympani / facial recess
√ lateral displacement of auditory ossicles
√ erosion of ossicular chain: first affecting long process of incus
CT:
√ nondependent homogeneous mass
√ perforation of tympanic membrane posterosuperiorly (= pars flaccida)
√ poorly pneumatized mastoid (frequent association)
√ erosion of tegmen tympani (with more extensive cholesteatoma) → extradural mass ± formation of meningoencephalocele
√ destruction of labyrinthine capsule (less common) involving the lateral semicircular canal first → labyrinthine / perilymphatic fistula
√ erosion of bony facial canal in its tympanic / mastoid pars
MR (adjunct to CT ONLY if doubt in diagnosis):
√ iso- / hypointense relative to cortex on T1WI
CAVE: occasional T1-hyperintensity similar to “white” epidermoid cyst ← high protein content
√ mildly hyperintense lesion on T2WI + FLAIR
√ restricted diffusion (highly hyperintense) on DWI ← high keratin content
FN: mural / evacuated cholesteatoma, lesion < 2–3 mm
FP: residual hemorrhage after recent surgery; silastic sheet; bone pâté; cerumen in EAC; cholesterol granuloma; artifact ← dental brace; middle ear / mastoid abscess
√ relatively hypointense ADC map (DDx: cholesterol granuloma very hyperintense)
√ NO delayed enhancement 45–60 min after Gd-DTPA (DDx: cholesterol granuloma enhances ← inflammatory / granulation / scar tissue)
| Cx: | (1) Intratemporal: ossicular destruction; facial nerve paralysis (1%); labyrinthine fistula; automastoidectomy; complete hearing loss |
(2) Intracranial: meningitis; cerebritis; subdural / epidural empyema; temporal lobe abscess; sigmoid sinus thrombosis; CSF rhinorrhea | |
| Rx: | canal-wall-down / canal-wall-up tympanoplasty with recurrence rates of 7% and 20%, respectively |
| DDx: | chronic otitis media; granulation tissue (= cholesterol granuloma); brain herniation through tegmen defect; neoplasm (rhabdomyosarcoma, squamous cell carcinoma) |
Cholesteatoma of External Auditory Canal
Incidence: 0.1–0.5%
Cause: spontaneous (most), prior trauma, surgery, radiation
Path: local invasion of EAC by squamous epithelial lining into underlying bone → periostitis, canal wall erosion
Age: older age group
• chronic dull pain, otorrhea
CT:
√ focal soft-tissue within EAC (typically inferior wall)
√ canal wall erosion into underlying bone
DDx: carcinoma, otitis externa
CHOLESTEROL GRANULOMA
= CHOLESTEROL CYST
= acquired benign chronic inflammatory mass in petrous bone
◊ Most common lesion arising in petrous apex!
Pathophysiology:
mucosal edema → exposure of bone marrow → hemorrhage + obstruction → breakdown of erythrocytes + tissue → release of cholesterol → foreign body giant cell reaction (= chronic granuloma) → cyst formation + bone expansion
Histo: cholesterol crystals surrounded by foreign-body giant cells; embedded in fibrous connective tissue with varying proportions of hemosiderin-laden macrophages, chronic inflammatory cells and blood vessels; brownish cyst fluid contains cholesterol crystals + blood (= “chocolate cyst” = “blue-domed cyst”)
Location: in pneumatized petrous apex; (occasionally in)mastoid segment, middle ear, orbitofrontal region
• blue (vascular) tympanic membrane without pulsatile tinnitus
• hemotympanum; conductive hearing loss, headache
• long-standing history of otitis media
√ bone gaps ← bone remodeling
√ ossicles remain intact
√ NO enhancement
CT:
√ expansile homogeneous middle ear lesion with smooth well-defined / imperceptible bone margins
√ nonenhancing mass
MR:
√ hyperintense signal on T1WI ← methemoglobin
√ heterogeneously hyperintense signal on T2WI ← cholesterol crystals + methemoglobin from repeated hemorrhage
√ remains hyperintense with fat-suppression
√ ± hypointense rim on T2WI ← hemosiderin deposition / preserved rim of bone
| DDx: | (1) Asymmetric petrous apex pneumatization (hyperintense fatty marrow on T1WI, hypointense with fat suppression) |
(2) Petrous apex effusion (no expansion / destruction of petrous air cells) | |
(3) Cholesteatoma (isointense to brain on T1WI) | |
| Rx: | conservative Rx for asymptomatic lesion; surgical drainage / cyst resection for large symptomatic lesion |
◊ Loss of T1-hyperintensity after successful drainage! |
CHRONIC SIALADENITIS
• often painful intermittent swelling of gland
√ normal-sized / small hypoechoic heterogeneous texture
√ multiple small round / oval hypoechoic areas
Chronic Sclerosing Sialadenitis
= KÜTTNER TUMOR = IgG4-RELATED SIALADENITIS
[Hermann Küttner (1870–1932), German surgeon in Breslau]
= non-neoplastic uni / bilateral hard swelling of submandibular gland considered part of IgG4-related disease
Histo: markedly fibrous sclerotic lesion containing IgG4-positive plasma cells
√ diffuse / focal swelling of both submandibular glands
US:
√ multiple small hypoechoic foci scattered on a heterogeneous background of salivary tissue
CT:
√ homogeneous attenuation
MR:
√ low to intermediate SI on T2WI
√ intermediate SI on T1WI + homogeneous enhancement
DDx: lymphoma, acute Sjögren syndrome
Granulomatous Sialadenitis
Organism: TB, actinomycosis
√ single / multiple hypoechoic areas in enlarged / normal-sized gland of diffusely low echogenicity
√ ± increased blood flow
COGAN SYNDROME
= AUTOIMMUNE INTERSTITIAL KERATITIS
= rare autoimmune multisystem disease characterized by nonsyphilitic interstitial keratitis + audiovestibular dysfunction + vascular inflammation
Age: young white adult
• eye redness. photophobia, eye pain (from interstitial keratitis)
• audiovestibular manifestations (similar to Ménière syndrome)
• nerve deafness
@ Cardiovascular (10–15%%)
Histo: prominent lymphocytic infiltration of aortic wall, destruction of media elastica, fibrosis, aneurysm formation, neovascularization
√ aortitis → aortic insufficiency
√ necrotizing vasculitis → coronary / iliac / renal artery stenosis
@ CNS (29%)
• psychosis, headache, convulsion, stroke, coma
√ ischemic change or infarction, meningoencephalitis, cerebral venous sinus thrombosis, cranial neuropathy
MR:
√ membranous labyrinthine obliteration / narrowing
√ enhancement of membranous labyrinth
CONGENITAL ATRESIA OF EAC
= failure of EAC to fully develop
Prevalence: ~ 1÷10,000
Often associated with: abnormalities of pinna
Classification: osseous / membranous / mixed
Location: unilateral > bilateral
Imaging assessment:
(1) Size + pneumatization of middle ear
(2) Abnormalities of ossicles
(3) Location of mastoid segment of facial nerve (often displaced anteriorly)
CROUP
= ACUTE LARYNGOTRACHEOBRONCHITIS = ACUTE VIRAL SPASMODIC LARYNGITIS
= lower respiratory tract infection
◊ Most common cause of airway obstruction in young children!
Organism: parainfluenza, respiratory syncytial virus
Season: fall and winter months
Age: > 6 months of age; peak incidence 2–3 years
• prodromal symptoms of viral infection
• “brassy / barking” cough → worse at night and while crying
• inspiratory difficulty with stridor, fever, hoarse cry
√ thickening of vocal cords
√ distension of cervical trachea on expiration
AP radiograph (most helpful image):
√ “steeple” / subglottic “inverted V” sign = symmetrical funnel-shaped narrowing 1–1.5 cm below lower margins of pyriform sinuses (= loss of lateral convexities / normal “shouldering” of air column)
Cause: subglottic mucosal edema affecting most severely the conus elasticus + external restriction by cricoid
√ accentuated on expiration
√ paradoxical inspiratory collapse → less pronounced during expiration
LAT radiograph:
√ NORMAL epiglottis + aryepiglottic folds (→ NO epiglottitis)
√ subglottic narrowing + increased density of subglottic region
√ narrow + indistinct subglottic trachea
√ inspiratory ballooning of hypopharynx (= nonspecific sign of any acute upper airway obstruction)
DDx: normal at end of inspiratory phase in a crying child
With clinically suspected croup imaging is performed to determine if another cause of inspiratory stridor is present.
Viral croup is unlikely to occur in children > 3 years of age. With radiographic findings of croup outside this age range, consider membranous croup and foreign-body aspiration.
Prognosis: usually self-limiting
Rx: supportive
DERMOID / EPIDERMOID OF HEAD AND NECK
= benign congenital or acquired (eg, posttraumatic) inclusions of dermal elements at site of embryonic 1st + 2nd branchial arches
◊ Dermoids of the head and neck comprise 7% of all dermoids
Definition:
Teratoma = neoplasm whose tissue is foreign to the part of the body from which the tumor arises
Teratoid cyst
Origin: ectoderm + mesoderm + endoderm
Histo: inclusion cyst lined by squamous / respiratory epithelium containing derivatives of ectoderm + endoderm + mesoderm (skin appendages, nervous / GI / respiratory tissue)
Dermoid cyst
Origin: ONLY ectoderm = epidermis + dermal substructure of subcutaneous tissue + skin appendages (= annexa consisting of hair follicles, sebaceous glands, sweat glands)
Histo: inclusion cyst lined by a thick keratinizing squamous epithelium containing a variable number of skin appendages ± dystrophic calcifications; lumen filled with keratin + sebaceous material + hair (occasionally)
Path: cheesy tumor, fat content often collects in globules
√ greater signal heterogeneity ← combination of solid and cystic elements
√ intralesional fat = DISTINGUISHING feature:
√ “sack-of-marbles appearance” = globules of coalesced fat (nearly PATHOGNOMONIC)
Epidermoid cyst
Origin: ONLY epithelial elements WITHOUT skin appendages
Histo: inclusion cyst lined by thin simple squamous epithelium ± calcifications (rarely); lumen filled with debris of keratin and some cholesterol
Path: “pearly tumor” = shiny smooth waxy character
√ usually lacks observable solid components
√ ± CHARACTERISTIC restriction at DWI (= high diffusion + moderately low apparent diffusion coefficient)
Mean age: 30 years (range, 5–50 years); M÷F = 3÷1
Location:
(1) Periorbital (50%): lateral eyebrow (most common)
(2) Oral cavity (25%): floor of mouth (12%), root of tongue
(3) Nasal cavity (13%)
(4) Submental: neck
√ well-circumscribed lesions of high T2 signal
√ NO / only rim enhancement
Nasal Dermoid
= dermal inclusion in cranium through patent suture
Associated with: craniofacial malformation (in 41%)
Location: anywhere between glabella (= depressed space between eyebrows above nose) and columella (= fleshy external part of nasal septum); lower third of bridge of nose (most commonly)
• nasal pit (skin dimple) / fistula / fluctuant swelling
• hair protruding from cyst / sinus tract (< 50%)
√ bone defect in cranium (= dermal sinus)
MR:
√ variable nonspecific appearance of sinus ± cyst
Cx: infection with abscess in nasal septum
DDx: normal fat in crista galli / nasal septum
Cervical Dermoid / Epidermoid Cyst
Age: 2nd–3rd decades; M = F
• slowly growing soft mobile mass in the suprahyoid midline (no movement with tongue protrusion!)
Site: (a) sublingual space (superior to mylohyoid muscle) = intraoral surgical approach (more frequent)
Type: frequently epidermoid cyst
(b) submandibular (inferior to mylohyoid muscle) = external surgical approach
Type: frequently dermoid cyst
Size: few mm – up to 12 cm
√ thin-walled unilocular mass
CT:
√ homogeneous fluid material of 0–18 HU
√ heterogeneous mass ← various germinal components
√ fluid-fluid level ← supernatant lipid
√ rim enhancement frequent
MR:
√ hypointense / hyperintense (sebaceous fluid) / isointense relative to muscle on T1WI
√ hyperintense on T2WI + internally heterogeneous
Prognosis: malignant degeneration into squamous cell carcinoma in 5%
DDx: ranula
DISSECTION OF CERVICOCEPHALIC ARTERIES
= CRANIOCERVICAL ARTERIAL DISSECTION
= hematoma within media splitting off vessel wall → false lumen within media
Incidence: 5÷100,000 per year; responsible for 5–20% of strokes in young and middle-aged adult
Etiology:
A. SPONTANEOUS
(1) trivial trauma (frequent): nonrecalled minor trauma like coughing, vomiting, sports (bowling, tennis, archery)
(2) primary arteriopathy (rare): fibromuscular dysplasia (in 15%), Marfan syndrome, Ehlers-Danlos syndrome type IV, autosomal dominant polycystic kidney disease, osteogenesis imperfecta type I, cystic medial necrosis, collagen vascular disease, homocystinuria
Indirect evidence of arteriopathy:
intracranial aneurysm, widened aortic root, arterial redundancy
Associated with: hypertension (36%), smoking (47%), migraine (11%)
B. TRAUMATIC (rare)
severe blunt head and neck trauma / penetrating trauma (automobile accident, boxing, accidental hanging, diagnostic carotid compression, chiropractic cervical manipulation)
Pathophysiology:
primary intramural hematoma / penetration of blood into arterial wall via primary intimal tear → dissection usually extends cranially (same direction as blood stream) → narrowing of vessel lumen + enlargement of external diameter → pseudoaneurysm = dissecting aneurysm (with extension of hematoma into adventitia) → nidus for distal thromboembolism
Location: cervical ICA (68%), vertebral artery (27%), both ICA + vertebral artery (5%); multiple simultaneous dissections (28%)
Site: (a) subintimal dissection = close to intima
(b) subadventitial dissection = close to adventitia
√ arterial narrowing / occlusion
√ intimal flap
√ pseudoaneurysm
√ embolic distal branch occlusion of intracranial artery
US (low sensitivity due to location, 50% accuracy):
√ wall hematoma = thickened hypoechoic vessel wall
(DDx: intraluminal thrombus)
√ echogenic intimal flap floating in lumen (rarely depicted)
√ echogenic thrombus
Color Doppler (71–95% sensitive):
√ separation of 2 lumina with different Doppler signals
√ dampened / high-resistance Doppler waveform
DDx of increased ICA flow:
ICA redundancy, fibromuscular dysplasia, vasospasm, brain AVM, carotid cavernous sinus fistula, persistent trigeminal artery, anemia, hyperthyroidism
DDx of decreased ICA flow:
ipsilateral severe stenosis / occlusion of carotid siphon or intracranial ICA or M1 segment
NECT:
√ crescent-shaped hyperattenuating acute wall hematoma
CECT (50–100% sensitive, 67–100% specific):
√ target picture = narrow eccentric lumen surrounded by crescent-shaped mural thickening + peripheral thin annular enhancement (enhancing vasa vasorum)
√ increase in external diameter
√ intramural hematoma becomes isoattenuating to muscle with correct window settings (DDx: atherosclerotic plaque, thrombus)
√ intimal flap
√ dissecting aneurysm
√ arterial occlusion
MR (50–100% sensitive, 58–99% specific):
√ cross-sectional T1WI imaging ± fat saturation:
√ increase in external diameter of artery
√ narrowing of lumen
√ intramural hematoma:
√ isointense to surrounding structures during early / chronic stage
√ crescent-shaped hyperintense hematoma around eccentric flow void between 7 days and 2 months
√ iso- to hyperintense on T2WI around flow void of artery
3-D TOF MR angiography:
√ pseudoenlargement of lumen in subacute dissection = flow-simulating intramural hematoma
DDx: pseudodissection (= high SI of turbulent flow in aneurysmal dilatation)
Rx: best therapy not clear; anticoagulation (primary treatment) in absence of subarachnoid hemorrhage / dissecting aneurysm; surgery; endovascular stent placement
Carotid Artery Dissection
◊ Twice as common as vertebral artery dissection!
Frequency: 2–5–20% of strokes in persons aged 40–60 years
Age: 18–76 years (66% between 35 and 50 years)
• unremitting unilateral anterior headache (47–86%), neck pain (25%), facial pain
• TIA / stroke (49–82%), amaurosis fugax (12%):
• completed stroke usually during first 7 days after onset of symptoms, may occur up to 1 month later
• oculosympathetic paresis = Horner syndrome (52%)
• cranial nerve palsy, bruit (48%), pulsatile tinnitus
Location: cervical ICA usually at level of C1-2 (60%) within a few cm of carotid bifurcation > supraclinoid segment of ICA; bilateral carotid dissections (15%)
Length: a few centimeters
Angiography / DSA (gold standard):
√ “string” sign = long tapered usually eccentric irregular luminal stenosis beginning distal to carotid bulb + extending to base of skull (76%)
√ “string and pearl” sign = focal narrowing + distal site of dilatation
√ abrupt luminal reconstitution at level of bony carotid canal (42%)
√ fingerlike / saccular aneurysm (40%), often in upper cervical / subcranial region
√ intimal flap (29%)
√ double-barrel lumen similar to aortic dissection (rare)
√ slow ICA-MCA flow
√ tapered “flamelike” / “radish taillike” occlusion (17%) that spares the carotid bulb
| Cx: | (1) Thromboemboli due to stenosis |
(2) Subarachnoid hemorrhage (with intracranial location) | |
(3) Secondary aneurysm |
Prognosis: complete / excellent recovery (8%) with normalization in a few months; worsening in 10%
DDx:
(1) Fibromuscular dysplasia (“string of beads” sign, young woman, adjacent to C1-2, bilateral in 65%)
(2) Dysgenesis of ICA (hypoplastic / absent carotid canal)
(3) Atherosclerosis (carotid bifurcation + bulb)
(4) Radiation treatment (circumferential wall thickening in radiation field)
(5) Takayasu arteritis (long-segment wall thickening of aorta + side branches on both sides, young woman, common in Asia + Mexico)
(6) Giant cell (temporal) arteritis (extradural carotid siphon most severely affected, > 50 years of age)
(7) Behçet disease
Vertebral Artery Dissection
= hemorrhage into wall of vertebral artery
Prevalence: unknown; up to 15% of strokes in young adults
• headache: occipital (> 50%), frontal (20%), orbital (20%)
• neck pain (30%), ischemia of posterior circulation (57–84%)
• ischemia of cervical spinal cord, cervical root impairment
Location: in pars transversaria at level of C1/2 = V2 (35%); atlas loop = V3 (34%); bilateral vertebral artery dissections (5%); site of direct trauma
CT:
√ subarachnoid hemorrhage ← rupture of adventitia
MR (modality of choice):
√ decreased arterial lumen
√ diminished flow void
√ periarterial rim SI changes with time (hemoglobin)
Angio:
√ tapering of artery / intimal flap / complete occlusion
Cx: stroke (in up to 95%) after hours / weeks
Prognosis: full recovery with some residual deficit (88%); recurrence within 4–6 weeks involving multiple cervical arteries in sequence
EPIGLOTTITIS
= ACUTE BACTERIAL EPIGLOTTITIS= SUPRAGLOTTITIS
= life-threatening infection characterized by edema of supraglottic structures (= epiglottis + aryepiglottic folds)
[glottis, Greek = mouth of windpipe ↔ glotta / glossa = tongue]
Cause:
(a) infectious
Organism: Haemophilus influenzae type B, Pneumococcus, Streptococcus group A
◊ Less prevalent since introduction of vaccine against H. influenzae!
(b) noninfectious: angioedema, trauma, ingestion of caustic agent, anaphylaxis
Peak age: formerly 6 (range, 1–5) years; now more prevalent among adults + children of > 15 years
• toxic appearance, high fever, posturing with neck extension
• abrupt onset of inspiratory stridor + respiratory distress
• sore throat, drooling, severe dysphagia
Location: purely supraglottic lesion; associated subglottic edema in 25%
Lateral radiograph (preferred modality, frontal view irrelevant):
◊ Radiograph should be taken in erect position only!
√ “thumb” sign = bulbous thickening of epiglottis
√ thickening of aryepiglottic folds
√ circumferential narrowing of subglottic portion of trachea during inspiration
√ ± supraglottitis = additional swelling of supraglottic larynx
√ ballooning of hypopharynx + pyriform sinuses
√ cervical kyphosis
CT (NOT recommended in acute setting):
√ enlarged edematous epiglottis with mucosal enhancement
√ edema of entire supraglottic larynx + tongue base + tonsils
√ phlegmonous collection in adjacent soft tissues
Cx: necrotizing epiglottitis, deep neck abscess (4–25%)
Prognosis: in children mortal danger of suffocation ← hazard of complete airway closure; patient needs to be accompanied by physician experienced in endotracheal intubation
Rx: broad-spectrum antibiotics, steroids, direct laryngoscopy, emergent intubation (in children) may be required
EXTERNAL AUDITORY CANAL DYSPLASIA
Frequency: 1÷10,000 births; family history in 14%
Etiology:
(a) isolated
(b) Trisomy 13, 18, 21
(d) maternal rubella
(e) craniofacial dysostosis
(f) mandibulofacial dysostosis
Spectrum:
1. Stenosis of EAC
2. Fibrous atresia of EAC
3. Bony atresia (in position of tympanic membrane)
4. Decreased pneumatization of mastoid (mastoid cells begin to form in 7th fetal month)
5. Decreased size / absence of tympanic cavity
6. Ossicular changes (rotation, fusion, absence)
7. Ectopic facial nerve = anteriorly displaced vertical (mastoid) portion of facial nerve canal
8. Decrease in number of cochlear turns / absence of cochlea
9. Dilatation of lateral semicircular canal
Location: bilateral in 29%; M÷F = 6÷4
• pinna deformity; stenotic / absent auditory canal
Cx: congenital cholesteatoma (infrequent)
EXTRACARDIAC RHABDOMYOMA
= benign tumor consisting of immature striated muscle cells
Location: 70%–90% in head & neck
1. Adult rhabdomyoma
Age: 5th decade; M > F
Location: larynx, pharynx, oral cavity
Size: large ← slow rate of growth
2. Fetal rhabdomyoma
Median age: 4 years; M > F
Location: subcutaneous tissue with a particular predilection for postauricular region
√ signal intensity similar to muscle on T1WI and T2WI
√ usually enhancement after contrast administration
EXTRAMEDULLARY PLASMACYTOMA
= EXTRAOSSEOUS PLASMACYTOMA
Uncommon form of plasmacytoma (3–4%); questionable if precursor to multiple myeloma
Age: 40–60 years; M÷F = 2÷1
Location: air passages (50%) predominantly in upper nose and oral cavity; larynx; conjunctiva (37%); lymph nodes (3%); perirenal
• usually not associated with increased immunoglobulin titer or amyloid deposition
√ mass of one to several cm in size with well-defined lobulated border
Classification:
A. OSSEOUS
1. Medullary plasmacytoma
2. Multiple myeloma:
(a) scattered involvement of bone
(b) myelomatosis of bone
B. EXTRAOSSEOUS
3. Extramedullary plasmacytoma
Dx: requires exclusion of multiple myeloma by:
› bone marrow biopsy with normal results
› normal plasma electrophoresis
› negative skeletal survey
› negative bone marrow imaging with MR
Prognosis: relatively benign course (dissemination may be found months / years later or not at all); better than solitary bone plasmacytoma; progression to multiple myeloma in 50%
DDx:
(1) MULTIPLE MYELOMA
= malignant course with soft-tissue involvement in 50–73%:
(a) microscopic infiltration
(b) enlargement of organs
(c) formation of tumor mass (⅓)
• usually associated with protein abnormalities
• may have amyloid deposition
Age incidence: 50–85 years
◊ Extramedullary plasmacytoma tends to occur late in the course of multiple myeloma → indicates a poor prognosis (0–6% 5-year survival)
FIBROMATOSIS COLLI
= PSEUDOTUMOR OF INFANCY = STERNOCLEIDOMASTOID TUMOR OF INFANCY = CONGENITAL MUSCULAR TORTICOLLIS
= rare form of benign self-limiting benign fibrous mass within sternocleidomastoid muscle associated with torticollis in neonates and infants
Prevalence: 0.4% of live births
Cause: in > 60–90% associated with birth trauma during difficult breech / forceps delivery; positive family history (11%)
Path: compartment syndrome with pressure necrosis + secondary fibrosis of sternocleidomastoid m.
Histo: myoblasts + fibroblasts + myofibroblasts in various stages of differentiation
Associated congenital abnormalities:
developmental hip dysplasia, rib anomalies, talipes equinovarus (clubfoot), thoracic scoliosis, metatarsus adductus, mental retardation, seizure disorder
Associated skeletal abnormalities:
ipsilateral lateral mandibular asymmetry, cervicothoracic scoliosis, facial deformity, ipsilateral mastoid process hypertrophy, ipsilateral elevation of clavicle / shoulder
Age: 2nd to 4th week of life; M > F
• unilateral firm soft-tissue mass in mid to lower ⅓ of sternocleidomastoid muscle; typically unaffected at birth; enlarging neck mass over the course of 2–6 weeks
• torticollis (14–30%) tilting toward lesion ← muscle contraction
Location: lower ⅓ of sternocleidomastoid muscle affecting sternal + clavicular heads of the muscle; usually unilateral (R > L)
Average size: 1–3 cm
√ focal / diffuse enlargement of sternocleidomastoid muscle
√ NO extramuscular extension / associated soft tissue abnormality
√ mild mass effect on surrounding structures
X-ray (NOT indicated):
√ normal in 98%
√ lytic lesion in clavicular head at insertion of sternocleidomastoid muscle
US:
√ unilateral diffuse fusiform homogeneous expansion of sternocleidomastoid muscle
√ well- / ill-defined focal mass within muscle:
√ homogeneous echotexture (51%)
√ hypo- to iso- to hyperechoic depending on duration
√ mass moves synchronously with muscle at real-time
MR:
Indication: atypical sonographic features, NO resolution within 12 months
√ isointense lesion to normal muscle on T1WI
√ hyperintense lesion relative to normal muscle on T2WI
√ subtle patchy and linear areas of decreased signal intensity
√ heterogeneous enhancement
CT:
√ focal / diffuse homogeneous enlargement isoattenuating to other muscle
Prognosis: gradual spontaneous regression over 2–3 months; spontaneous resolution during next 4–8 months in 90% with / without treatment
| Rx: | (1) Muscle stretching exercise |
(2) Surgery in 10% | |
| DDx: | (1) Neuroblastoma (heterogeneous solid mass with calcifications) |
(2) Rhabdomyosarcoma | |
(3) Lymphoma (well-defined round /oval masses along cervical lymph node chain) | |
(4) Cystic hygroma (anechoic region with septations) | |
(5) Branchial cleft cyst | |
(6) Hematoma |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree