CHAPTER 58 Nuclear Medicine Imaging of Myocardial Viability
Coronary artery disease (CAD) remains the number one cause of mortality in the United States, responsible for nearly half of daily deaths.1 The lifetime risk of developing CAD after 40 years of age is 49% for men and 32% for women.2 In developed nations, the leading cause of left ventricular (LV) dysfunction (i.e., heart failure) is CAD.3 Retrospective analysis of 13 randomized multicenter heart failure trials showed that CAD was present in approximately 70% of the greater than 20,000 enrolled patients.4 Over the past decade, the number of patients presenting with heart failure has increased exponentially. It has been estimated that 4.7 million patients in the United States have chronic heart failure, with 550,000 new cases per year, resulting in 1 million hospitalizations.
Heart failure is the leading cause of morbidity, mortality, and hospitalization in patients older than 60 years and is the most common Medicare diagnosis-related group.5 The diagnostic and therapeutic costs associated with heart failure are estimated to be more than $29 billion per year.1 The long-term prognosis for patients with heart failure remains poor, despite advances in different therapies. More recent data from the Framingham Heart Study showed 5-year mortality rates of 59% for men and 45% for women with heart failure in the period from 1990-1999.6 Mortality rates increase in older patients with heart failure.
DEFINITION OF MYOCARDIAL VIABILITY
Before the 1980s, the conventional wisdom was that impaired LV function at rest in patients with CAD was an irreversible process. This clinical dogma was shown to be not always true when observational studies in patients with LV dysfunction undergoing coronary artery revascularization exhibited improvement in regional and global LV function.7 To explain the subsequent improvement in function, the concept of viability was introduced. Dysfunctional but viable myocardium has the potential to recover function after revascularization, whereas the revascularization of dysfunctional scar tissue does not result in improvement of function. There are several mechanisms of adaptation that the myocardium follows to maintain viability during temporary or sustained reductions in coronary blood flow.
Dysfunctional but viable myocardium has been broadly categorized as either stunned or hibernating myocardium. Stunned myocardium refers to the state of delayed recovery of regional ventricular contractile dysfunction after a transient period of ischemia that has been followed by restoration of perfusion (Fig. 58-1).8,9 Resulting dysfunction may persist for hours to days, but generally improves with time. Hibernating myocardium refers to an adaptive rather than an injurious response of the myocardium to impaired coronary flow reserve (repetitive ischemia and stunning) and reduced resting coronary blood flow (Fig. 58-2).7,9 Hibernation and stunning are categorized as different pathophysiologic states; however, these states most often exist concomitantly as a continuum in patients with dysfunctional but viable myocardium.
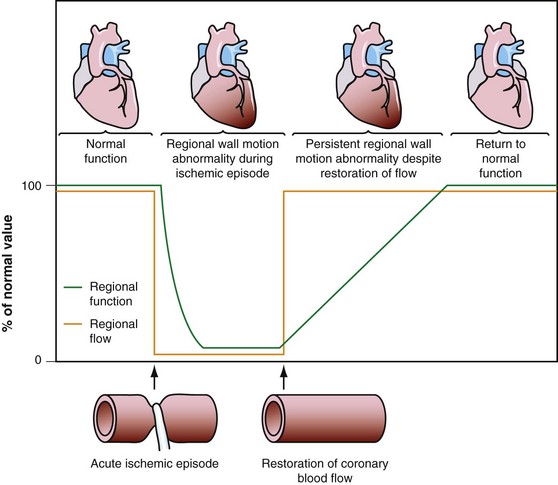
 FIGURE 58-1 Schematic diagram of stunned myocardium.
FIGURE 58-1 Schematic diagram of stunned myocardium.
(From Dilsizian V, Narula J. Nuclear investigation in heart failure and myocardial viability. In Dilsizian V, Narula J [eds]. Atlas of Nuclear Cardiology, 3rd ed. Philadelphia, Current Medicine, 2009, pp 201-224.)
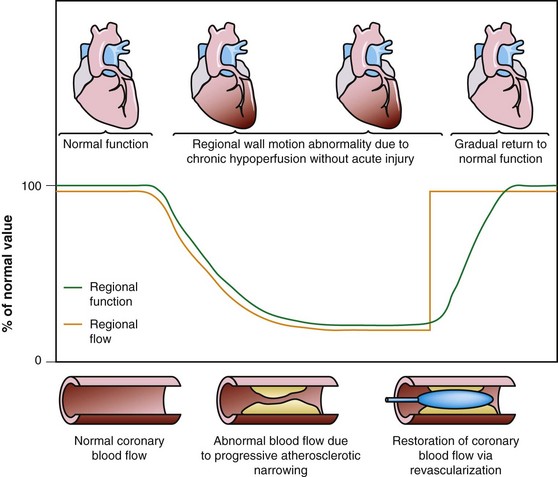
 FIGURE 58-2 Schematic diagram of hibernating myocardium.
FIGURE 58-2 Schematic diagram of hibernating myocardium.
(From Dilsizian V, Narula J. Nuclear investigation in heart failure and myocardial viability. In Dilsizian V, Narula J [eds]. Atlas of Nuclear Cardiology, 3rd ed. Philadelphia, Current Medicine, 2009, pp 201-224.)
There is extensive evidence delineating the adaptations of the myocardium to reduced blood flow. Signs of energy depletion and downregulation of energy turnover have been described in hibernating myocardium.10 These alterations of energy metabolism likely cause and perpetuate contractile dysfunction, continued tissue degeneration, and subsequent cardiomyocyte loss. These responses to regional hypoperfusion are thought to preserve the minimal amount of energy needed to protect the structural and functional integrity of the cardiac myocyte. Myocardial biopsy specimens have shown disorganization of the cytoskeletal proteins, dedifferentiation (expression of more fetal proteins), and changes in the extracellular matrix with evidence of reparative fibrosis with basement membrane thickening and increased collagen fibrils and fibroblasts. Hibernating myocardium showed a loss of contractile filaments (sarcomeres), an accumulation of glycogen in the spaces previously occupied by the myofilaments, nuclei with uniformly distributed chromatin, small mitochondria, and a nearly absent sarcoplasmic reticulum.11 Additional histologic studies analyzed myocardial biopsy specimens obtained during revascularization procedures, confirming that segments with recovery after revascularization contained viable myocytes compared with the large extent of fibrosis detected in irreversibly damaged myocardium.12
Myocardial Ischemia
Imbalance between oxygen supply and oxygen demand, which is determined by regional myocardial perfusion and the rate and force of myocardial contraction, is termed ischemic myocardium. Myocardial ischemia alters myocardial substrate metabolism. As blood flow and oxygen supply decline, oxidative metabolism decreases, and glycolysis increases. As ischemia continues, glycolytic metabolism becomes overwhelmed with excess production of lactate. During states of mild ischemia, lactate continues to be removed from the myocardium by the residual blood flow, but rapidly accumulates in tissue, with a further reduction in blood flow, during more severe states of ischemia. Subsequently, increased tissue concentrations of lactate and hydrogen ions impair glycolysis, leading to loss of transmembrane ion concentration gradients, disruption of cell membranes, and cell death.13
Cell death after myocardial ischemia and reperfusion can be the consequence of apoptosis and necrosis. The induction of either apoptosis or necrosis is regulated by similar biochemical intermediates, including alterations in high-energy phosphates, intracellular calcium accumulation, and reactive oxygen species. The reduction of contractile function associated with hibernation is thought to be a protective response of the myocardium to meet minimal metabolic requirements with the reduced supply of oxygen and substrates, leading to the situation of perfusion contraction matching, preventing apoptosis and cell death.14 Histologic analysis of hibernating myocardium shows myocytes in a stable noncontractile state with intact cell membranes and cellular metabolism with little or no evidence of apoptosis.15 In contrast to programmed cell death, or apoptosis, the term programmed cell survival has been used to describe the commonality between myocardial stunning, hibernation, and ischemic preconditioning, despite their distinct pathophysiology (Fig. 58-3).16
Injury as a result of myocardial ischemia can be categorized as a continuum from fully viable, through partially viable (admixture of scarred and viable tissue), to nonviable or scarred. Not all myocardium involved in an infarction is dead or irreversibly damaged. The process of infarction starts at the endocardium and spreads toward the epicardium. The extent of myocardial infarction (MI) can be reduced when the affected vascular territory is reperfused (spontaneously, pharmacologically, or mechanically), or when the area of MI is sufficiently collateralized. Even completed infarcts vary in their transmural extent, and the epicardium is usually the most likely site of viable myocardium. The likelihood of recovery of function after revascularization is related to the extent of myocyte injury and the amount of fibrosis (Fig. 58-4).17,18
Potential End Points Used in Myocardial Viability Studies
Patients with severe LV dysfunction who undergo CABG surgery or percutaneous coronary interventions can have a considerable risk of procedure-related morbidity and mortality.18,19 Identification of patients with the potential for improvement in LV ejection fraction and survival is needed to justify the higher risk of therapeutic intervention in these patients. Revascularization of viable myocardium has been shown to improve significantly regional and global contractile function.20–22 Improvement in regional contractile function is seen in approximately one third of dysfunctional segments, and an improvement in LV ejection fraction is seen in approximately 40% of patients.21,23 Revascularization of viable myocardium has also been shown to improve significantly symptoms and New York Heart Association functional class.24 The magnitude of improvement in heart failure symptoms after CABG surgery is linearly related to the preoperative extent of myocardial viability (Fig. 58-5).24
Revascularization may also be associated with many favorable clinical effects even in the absence of ventricular functional improvement. Benefits can include relief of chest pain or heart failure symptoms, improved exercise tolerance related to diminished inducible ischemia or improved diastolic function, stabilization (or reversal) of LV remodeling, stabilization of the electrophysiologic milieu, and prevention of MI.25–27 Besides improvement in LV ejection fraction, revascularization of viable myocardium may have a beneficial effect on the prevention of arrhythmias and sudden cardiac death, which may also improve longevity. One study showed that the long-term survival rates of patients who underwent CABG surgery were similar regardless of whether LV ejection fraction increased after revascularization.28 Chronic LV dysfunction can result from nontransmural necrosis in combination with viable (normal) myocardium. The subendocardial layer contributes significantly to contraction, and subendocardial necrosis of greater than 20% of the myocardial wall results frequently in akinesia.
Revascularization of these regions may not significantly improve contractile function. The prevention of remodeling and arrhythmias may be of critical clinical relevance, however. In such patients, preserved viability in the outer layers of the myocardium could prevent progressive LV dilation, despite the lack of any improvement in resting function after revascularization. The prevention or reversal of adverse LV remodeling seems to be an important determinant of long-term natural history outcomes, and strategies that prevent or reverse remodeling generally have very favorable effects on that natural history.29
A meta-analysis of the prognostic value of viability testing and the impact of therapeutic choice on survival showed a significant association between revascularization and improved survival rate in patients with LV dysfunction and evidence of myocardial viability independent of the imaging technique used.30,31 Additionally, these studies identified a 3.2% annual death rate in patients who had viable myocardium and underwent revascularization compared with a 16% annual death rate in patients who had viable myocardium and were treated medically (Fig. 58-6). Medical treatment of heart failure has improved substantially with the introduction of angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, and spironolactone. Patients with chronic ischemic LV dysfunction with viable myocardium have a poor prognosis when treated with medical therapy alone, however.32
NUCLEAR IMAGING TECHNIQUES FOR IDENTIFYING MYOCARDIAL VIABILITY
Single Photon Emission Computed Tomography
Tc 99m Labeled Radiotracers
Tc 99m labeled flow tracers, such as Tc 99m sestamibi and Tc 99m tetrofosmin, are lipophilic cationic complexes, which are taken up by myocytes across mitochondrial membranes and at equilibrium are retained within the mitochondria because of a large negative transmembrane potential. The initial uptake and retention of sestamibi and tetrofosmin (whether injected at rest or during stress) are flow-dependent and reflect cell membrane integrity and mitochondrial function. Myocardial ischemia interferes with mitochondrial K+-ATP channel activation, changing its mitochondrial membrane potential, and finally reducing cellular radiotracer uptake.33
Experimental studies have shown that myocardial retention of Tc 99m sestamibi and Tc 99m tetrofosmin requires cellular viability.34 Histopathologic studies have shown that tissue viability is a continuum, and that there is a nearly linear relationship with sestamibi uptake and the percentage of normal (viable) myocardium in dysfunctional regions.35 The most commonly used clinical viability criterion is the percentage of tracer uptake (frequently 50% to 60%) in dysfunctional segments. Most frequently, Tc 99m labeled tracers are injected under resting conditions, and the dysfunctional segments with tracer uptake of 50% to 60% are considered viable.36 Regional Tc 99m sestamibi or Tc 99m tetrofosmin activity has been shown to be closely correlated with the redistribution phase of rest-redistribution thallium 201 study, indicating myocardial viability (Fig. 58-7).37,38
Studies have suggested that sestamibi may underestimate myocardial viability, particularly in patients with severe LV dysfunction.39–41 Despite using quantitative techniques, studies have reported that rest sestamibi imaging underestimates myocardial viability compared with 18FDG-PET. Potential factors affecting the accuracy of sestamibi in assessing viability were determined by comparing the results of sestamibi SPECT and 18FDG-PET in patients with varying degrees of LV dysfunction. The concordance between sestamibi and 18FDG was 64% in patients with severe LV dysfunction and 78% in patients with mild to moderate LV dysfunction.42 In particular, only 42% of regions with blood flow–metabolism mismatch patterns by PET (the pattern most predictive of functional recovery after revascularization) were identified as viable by quantitative sestamibi SPECT.
Tc 99m Labeled Radiotracers with Nitrate Administration
Nitrates (intravenous, oral, or sublingual) enhance collateral blood flow and radiotracer uptake in hypoperfused myocardial regions that are subtended by severely stenosed coronary arteries. In most nitrate-enhanced studies, two sets of images are obtained: a resting image and a nitrate-enhanced image. Defect reversibility after nitrate administration (i.e., a defect filling in) is considered to be indicative of viability. Nitrate-enhanced Tc 99m perfusion tracer uptake compared with baseline is thought to be a better estimate of coronary flow reserve. Several investigators have used improvement in the regional concentration of a flow tracer injected after nitrate administration as a marker of viability.43,44
A retrospective analysis of studies using Tc 99m labeled flow tracers to assess improvement in regional function after revascularization showed a mean overall sensitivity and specificity of 81% and 66%.45 Most of these studies used a resting image, and segments were classified as viable when activity exceeded a certain threshold. Nitrate-enhanced studies, when analyzed separately, showed a higher accuracy, with a sensitivity of 86% and a specificity of 83% (Fig. 58-8).46 Studies that investigated functional outcomes showed an improvement of LV ejection fraction from 47% to 53% in patients with viable myocardium, whereas the LV ejection fraction did not change in patients without viable myocardium (40% vs. 39%). When gated SPECT regional function was added to nitrate enhancement, the accuracy of the technique for predicting recovery of function after revascularization was even higher.47,48
In patients with chronic CAD and nitrate-enhanced Tc 99m perfusion studies, superior survival was shown in patients with complete revascularization compared with patients treated with medical therapy and patients who underwent incomplete revascularization. The most important prognostic predictor of future cardiac events was the number of nonrevascularized dysfunctional regions with viable tissue on sestamibi imaging. That is, patients who had viable myocardium but who did not receive adequate revascularization had poorer prognosis.49
Thallium 201
Experimental studies have shown that extraction of thallium 201 across the cell membrane is unaffected by hypoxia, chronic hypoperfusion (hibernation), or postischemic dysfunction (stunning), unless irreversible injury (scarred myocardium) is present. Necrotic myocardium cannot retain thallium 201, and, despite its initial uptake, thallium 201 washout is accelerated in necrotic tissue.50,51 Because thallium 201 is not actively taken up in regions of fibrotic or scarred myocardium, a defect on rest images that persists on redistribution images (termed irreversible or fixed defect) represents a pattern of scarred myocardium.
Thallium 201 has been used and investigated extensively for identifying myocardial viability and hibernation, and was the first radiotracer to be used for this purpose.52 Despite the excellent flow kinetics and biologic properties, however, the low-energy gamma ray emission and long half-life of thallium 201 are potential limitations, leading to attenuation of photons, particularly in patients with a large body habitus, and higher radiation exposure compared with Tc 99m labeled perfusion tracers.
Myocardial perfusion scan clinical protocols can be used with thallium 201 with an injection of a tracer either during stress (exercise or pharmacologic) or at rest with subsequent late imaging after the redistribution of the radiotracer. The initial acquisition soon after thallium 201 injection primarily reflects delivery of the tracer through blood flow. The delayed redistribution images acquired 4 to 24 hours after radiotracer injection are a marker of sarcolemmal integrity and myocardial K+ space and Na+,K+-ATPase function, which reflects tissue viability.50,53 Delayed thallium 201 imaging for assessment of myocardial viability relies on properties of thallium 201 that allow for redistribution. Thallium 201 redistribution images represent a balance between thallium 201 washout and continued thallium 201 uptake via the active Na+,K+-ATPase transport system over time, and redistribution images reflect the distribution volume of thallium 201 as representative of the myocardial K+ space. In normal myocardium, the rate of thallium 201 washout is greater than the rate of uptake, resulting in a net thallium washout, which is potentiated further in infarcted myocardium. Redistribution imaging allows time for thallium 201 washout from necrotic myocardium, and for thallium 201 wash-in or redistribution into viable tissue.
Redistribution of thallium 201 after stress depends partly on the blood levels of thallium 201. Some ischemic but viable myocardial regions may show no redistribution on either early (3- to 4-hour) or late (24-hour) redistribution imaging, unless blood levels of thallium 201 are increased. Reinjection of 1 mCi of thallium 201 at rest immediately after either stress early (3- to 4-hour) redistribution or stress late (24-hour) redistribution studies boosts the blood level of thallium 201, potentiating the differentiation of hypoperfused and scarred myocardium from hypoperfused but viable myocardium.54
Thallium 201 Protocols for Viability Assessment
Numerous thallium 201 protocols are used clinically for the detection of myocardial viability. The following two protocols are optimized for viability detection: (1) rest-redistribution (Fig. 58-9)46,55 and (2) stress–4-hour redistribution–reinjection imaging (Fig. 58-10).54 The former assesses myocardial viability alone,56 whereas the latter assesses myocardial ischemia and viability.54 A pooled analysis of rest-redistribution and stress-redistribution-reinjection thallium studies reported high sensitivity (80% to 90%) and modest specificity (54% to 80%) for the prediction of recovery of regional function after revascularization.45 These conclusions must be viewed, however, in the context of the limitations of pooled data analysis. When taking into consideration regions with reversible defects (ischemia) and success of revascularization (re-examining regional perfusion or vessel patency after revascularization), stress-redistribution-reinjection thallium 201 imaging yields excellent positive and negative predictive accuracy (both 80% to 90%) for recovery of function after revascularization (Fig. 58-11).56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


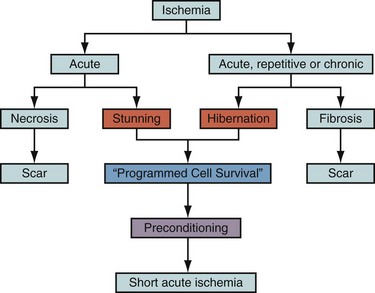
 FIGURE 58-3
FIGURE 58-3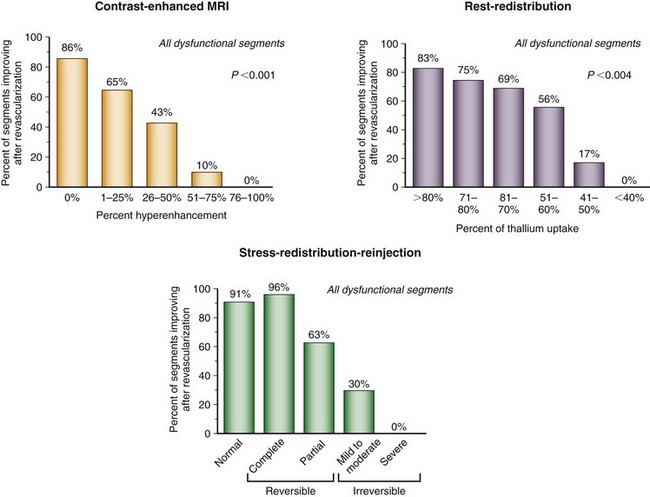
 FIGURE 58-4
FIGURE 58-4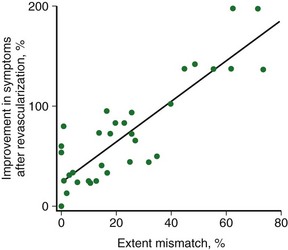
 FIGURE 58-5
FIGURE 58-5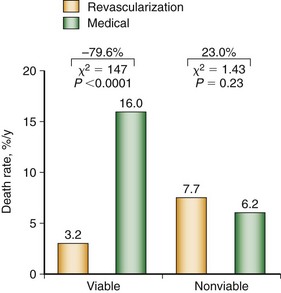
 FIGURE 58-6
FIGURE 58-6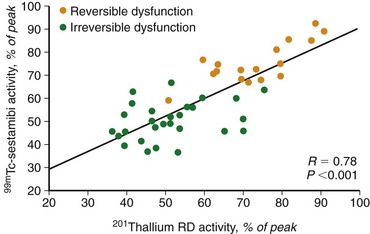
 FIGURE 58-7
FIGURE 58-7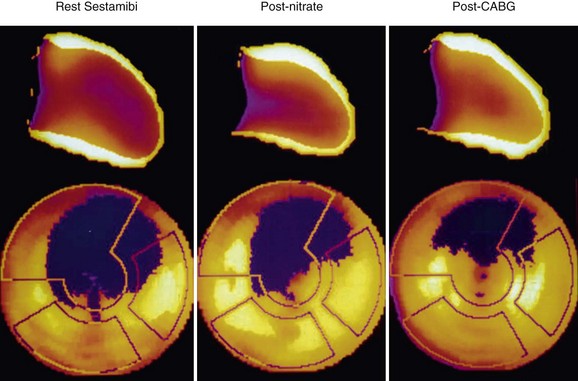
 FIGURE 58-8
FIGURE 58-8