Fig. 3.1
Image of a cyclotron, a particle accelerator in which charged particles accelerate from the centre outward, along a spiral path. These particles are held within an exact course by static magnetic fields and accelerated by rapidly alternating voltage. When the particles have developed the desired kinetic energy, they hit a target. This creates secondary particles (e.g. fluorine-18), which are guided outside the cyclotron and into instruments for radiopharmaceutical production
3.2.1.1 Decay Processes
There are several types of nuclear decay processes. Two of those are important for imaging purposes in sports medicine. The first one is called ‘metastable-state transitions’. A metastable state is an excited state of the nucleus that exists for a measurable lifetime. The atom in metastable state is the same as the atom in ground state, since they have the same Z and N. The only difference is the energy state. The decay of a metastable state towards the ground state occurs through de-excitation by means of γ-emission. An example is the decay of molybdenum-99 (99Mo) through technetium-99 m (99mTc) towards 99Tc. The prefix ‘m’ indicates the metastable state. 99mTc decays by emitting a γ-ray with a characteristic energy of 140 kilo-electronvolt (keV). 99Mo, on its turn, is a radionuclide which is produced by neutron bombardment of a target containing uranium-235. It disintegrates by emitting an electron (β−). 99Mo is commercially available in technetium-99 m generators, which is used to extract 99mTc in an on-site setting. Every nuclear medicine department has access to such a generator.
The second type of decay important in sports medicine is ‘positron decay’. In this decay process a positron is emitted, because of an excess of protons in the unstable nuclide. The decay process can be described by the conversion of a proton into a neutron, with the emission of a positron with a certain amount of kinetic energy. A positron (β+) is a positively charged electron. A positron cannot exist at rest in nature. As soon as it loses its kinetic energy, it immediately combines with an electron and undergoes a reaction called annihilation. The masses of the two particles are completely converted into energy: two annihilation γ-ray photons, each with energy of 511 keV, which leave their production site opposite to each other (Fig. 3.2). This is the basics of positron emission tomography (PET). The most important example of a positron emitting radionuclide is fluoride-18 (18 F).
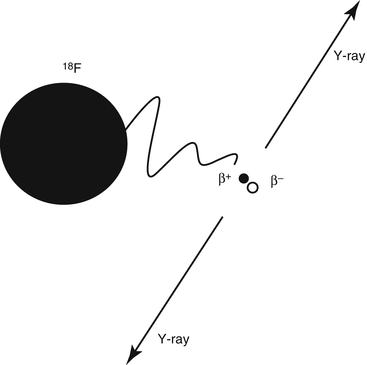

Fig. 3.2
Principle of annihilation. The radionuclide 18F emits a positron (β+), which finds an electron (β−) at the end of its course. Both particles annihilate and two γ-rays having 511 keV of energy are produced, 180° apart
3.2.1.2 Physical Half-Life
As mentioned before, the decay of each individual radionuclide is a random and spontaneous process. However, in a large group of the same radionuclides, the decay is rather constant. In fact, each radionuclide has its uniquely defined decay constant (λ). A term which is more commonly used for defining decay is ‘physical half-life’ (T 1/2). This is the time required for one-half of a group of radionuclides to decay. The half-lives of the most important radionuclides in sports medicine are 6.01 h for 99mTc and 1.83 h for 18F.
3.2.2 Camera Systems
Radiopharmaceuticals are usually administered intravenously. Therefore, the patient is the source of radioactivity. The cameras that are used in nuclear medicine to visualize the radiopharmaceuticals are the gamma camera and the PET camera. The detection of γ-rays emitted from the patient and transforming it into an image is the main principle of the camera systems used in nuclear medicine.
3.2.2.1 Gamma Camera
A γ-camera (or Anger scintillation cameras, named after its inventor) consists of the following components: a collimator, a scintillation crystal, a light guide, photomultiplier tubes and a positioning and energy discrimination system. The individual components are discussed in this section.
A collimator is a thick sheet of lead with multiple holes. The individual holes guide the individual γ-ray photons towards the scintillation crystal. Photons which do not travel in the right direction are absorbed in the septa between the holes. Different types of collimators are available, depending on the energy level of the emitted γ-rays from the radionuclide. For γ-ray photons of 99mTc, the parallel hole collimator is the collimator of choice. Only those photons which pass the collimator in a perpendicular course are transferred. Sometimes a pinhole collimator is used, especially for imaging small structures or organs.
After passing through the collimator holes, the γ-ray photons encounter the scintillation crystal. The individual photons are absorbed in the crystal (usually sodium iodide (NaI)) and converted into a small flash of light. As the energy of the photon increases, the flash of light becomes brighter. This flash of light is transferred through a silicon light guide to minimize the loss of intensity.
Next, the light reaches the photomultiplier tubes (PMTs). In fact, one flash of light is detected by multiple PMTs. The light interacts with photocathodes and is transformed into a photoelectron. This signal is amplified by electrodes or dynodes at increasing voltages in the PMTs, but also in external electronic preamplifiers. These signals are combined in a positioning system, which gives each signal from the individual PMTs different weights to derive the positioning information of γ-ray photons in x– and y-directions.
Finally, the energy pulse is examined (z-direction), to ensure that only photons falling within the photopeak are accepted. Usually, the same pulse as for positioning is used.
The technique as described above forms the basis of conventional planar nuclear medicine images. However, it has many limitations. Image quality is rather poor, due to low contrast, limited spatial resolution and poor statistics of detected γ-ray photons. In the last decennia, the image quality has been improved by the introduction of high-resolution collimators. At this moment, the spatial resolution of the gamma camera is around 8 mm.
3.2.2.2 Single-Photon Emission Computed Tomography (SPECT)
Image contrast of conventional planar imaging is rather low due to the presence of overlying structures that interfere with the region of interest. The effect of this superposition can be overcome by collecting images from different angles around the patient, thereby creating a 3D image. Dedicated gamma cameras have the opportunity to use this technique, called single-photon emission computed tomography (SPECT), to improve the sensitivity. This collection of images can be reconstructed into different slices and visualized in transverse, sagittal and coronal views. These tomographic images have higher contrast, because of the elimination of overlying activity.
Initially SPECT was performed with a single-head gamma camera system, rotating in a 360° orbit. This was a time-consuming method, not always well tolerated by patients. Nowadays, dedicated gamma cameras are supplied with a dual-head or (in less extant) triple-head configuration, thereby making SPECT possible in approximately 20 min.
3.2.2.3 Positron Emission Tomography (PET) Camera
Positron emission tomography (PET) is a unique imaging tool to visualize various physiological processes in the body. As mentioned in the section on decay processes (Sect. 3.2.1.1), positrons cannot exist freely. At the end of its kinetic energy (or at the end of its range), a positron meets his antimatter and annihilates into two 511 keV γ-ray photons, each in opposite directions. The detection of both photons is needed to determine the location of the annihilation in the field of interest (e.g. the thorax). Both these two photons have to be detected within a certain time window, to consider these two as one pair from the same annihilation process. A ring-shaped detector system is needed for this method of photon detection. Until recently, this time window had a predefined value. However, new developments in software lead to a correction method for the time a photon needs to travel from its origin to the detector, the so-called ‘time of flight’, which has advantages for spatial resolution. This, for example, makes PET systems different form SPECT systems.
A second difference from SPECT is that PET does not need the use of a collimator. Therefore, PET is able to provide images with better spatial resolution (approximately 4 mm) than planar images and SPECT (both approximately 8 mm). Third, the scintillation crystal in PET scanners consists of lutetium oxyorthosilicate (LSO) instead of NaI, which has much greater efficiency in detecting high-energy γ-rays (Melcher 2000).
Another difference between PET and SPECT is the correction for attenuation, i.e. correction for decrease in photon energy due to absorption in the body. In stand-alone PET systems, attenuation correction occurs with a transmission scan of a positron-emitting radionuclide (usually germanium-68 (68Ge)) which travels along the ring system.
Originally, PET was introduced for visualization of metabolic processes in the brain. However, nowadays it is used heavily in clinical oncology (primary tumour and metastases), cardiology (myocardial perfusion) and infection detection. In sports medicine, PET can play a role in detecting metabolic activity at the site of fractures, infections, joint problems, enthesopathies and stress-related problems, secondary to sports activity.
3.2.2.4 Hybrid Systems
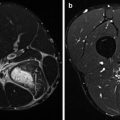
Developments in both soft- and hardware led to the implementation of hybrid systems, combining SPECT and PET with multi-detector computed tomography (CT). In these SPECT/CT (Fig. 3.3) and PET/CT cameras (Fig. 3.4), SPECT or PET and CT are performed in an immediate sequential setting, without changing the position of the patient. This co-registration provides physiological information, which can be perfectly correlated with anatomical information.
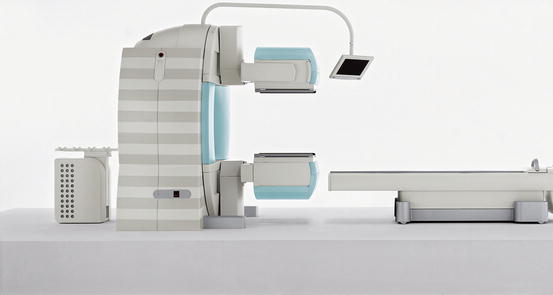
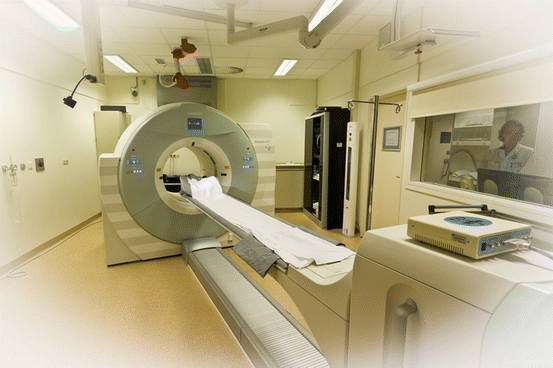

Fig. 3.3
Hybrid camera system, combining single-photon emission computed tomography with conventional computed tomography (SPECT/CT). These camera systems are at present the state of the art. They can be used for several nuclear medicine techniques. In sports medicine, they can be used for bone and leukocyte scintigraphy. The two opposing heads in the centre of the image are the gamma cameras, making this a dual-head system. The CT is incorporated in the gantry, to which the camera heads are attached (Courtesy of Siemens Medical Systems)

Fig. 3.4
Hybrid camera system, combining positron emission tomography with conventional computed tomography (PET/CT). These camera systems are at present the state of the art, as well. In sports medicine, they can be used for fluorine-18 (18F)-labelled fluorodeoxyglucose PET and 18F sodium fluoride PET (Image courtesy: Jan Pruim)
An additional advantage of these systems is that CT can also be used for attenuation correction. Therefore, the need of an additional 68Ge source has become obsolete in these hybrid camera systems. This leads to a more exact correction (based on Hounsfield units of different anatomic structures) and scanning time reduction. Furthermore, costs are reduced (one imaging modality), and the one-stop-shop principle (one scan instead of two separate scans) reduces waiting time for the patient.
Very recently, hybrid camera systems in which PET is combined with magnetic resonance imaging (PET/MRI) were introduced, especially for oncologic and cardiologic indications (Drzezga et al. 2012; Nekolla et al. 2009). In these PET/MRI systems, the different modalities can be used in a simultaneous setting, instead of a sequential matter. Furthermore, the attenuation correction is different in MRI compared to CT. The value of these PET/MRI systems in sports medicine is not yet determined.
3.3 Bringing Nuclear Medicine and Sports Medicine Together
Imaging in nuclear medicine depends on both the radionuclide and the chemical or pharmaceutical compound that is used in the administered radiopharmaceutical. In fact, the non-radioactive compound directs the radionuclide to the desired target. The choice of radiopharmaceutical most suitable for the individual patient depends on the complaints of the patient, the findings on physical and laboratory examination and the question and differential diagnosis the referring clinician has. In conventional nuclear medicine and SPECT, 99mTc is the most common radionuclide used for labelling. In PET, 18F is most commonly used.
This section discusses the use of different radiopharmaceuticals in nuclear medicine imaging in sports injuries. Also, the radiation dose of these different radiopharmaceuticals is discussed.
3.4 SPECT Techniques
3.4.1 Bone Scintigraphy
Bone scintigraphy is a widely available and well-established modality within nuclear medicine. It is one of the oldest techniques within this field of imaging, with a clinical experience for almost 50 years. It still remains the cornerstone of modern nuclear medicine practice. Its sensitivity is very high; however, its specificity is rather limited.
Amongst the early radionuclides were phosphorous-32, strontium-85 (85Sr), 87mSr and 18F. Despite the fact that 18F is superior to 85Sr, it is not widely available since it is produced in a cyclotron. The introduction of 99mTc made any imaging technique within nuclear medicine easier, including bone scintigraphy. In fact, the labelling of sodium triphosphate with 99mTc was the basis of bone scintigraphy as we know it today (Subramanian and McAfee 1971). Phosphates are ubiquitously present in nature and predominantly in the bone. Several chemical compounds containing phosphates have been evaluated for bone imaging, including pyrophosphate (PP), methylene diphosphonate (MDP), hydroxymethane diphosphonate (HDP, Fig. 3.5) and dicarboxypropane diphosphonate (DPD). A disadvantage of PP is the fact that it is prone to enzymatic hydrolysis. Diphosphonates are very stable and resistant to hydrolysis. DPD demonstrates the fastest blood clearance (Vorne et al. 1983); however, this advantage is not significantly different from other diphosphonates as long as scanning is performed 2–3 h after administration. HDP has the highest initial localization in the normal bone and the highest bone-to-soft-tissue ratio, compared to DPD and MDP, the latter showing the lowest localization (Godart et al. 1986). Therefore, the radiation dose to normal tissue is slightly lower in MDP than DPD and HDP. Eventually, each of the three agents has a high lesion-to-normal-bone ratio, and therefore HDP, MDP and DPD can all be used for bone scintigraphy.
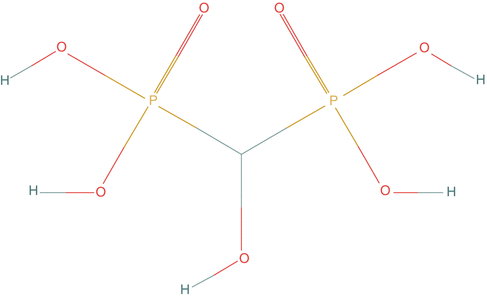

Fig. 3.5
Chemical structure of hydroxymethane diphosphonate (P2O7H5)
To understand the mechanism of skeletal uptake of the bone-seeking radiopharmaceuticals, basic knowledge of bone anatomy and physiology is mandatory.
3.4.1.1 Bone Anatomy
The bone is build of an outer surface (cortical bone) and an inner spongy structure (trabecular or cancellous bone). The trabeculae are oriented along lines of stress to provide maximum strength. It responds to physical stress by growing in length. Bone is surrounded by the periosteum, a fibrous sheath. Arterioles and capillaries which originate here penetrate the bone and enter the Haversian system and medullary space. In infants and children, the periosteum is thick and participates in bone formation. As bone matures, the periosteum becomes thinner and more adherent to the bone.
There are five types of bones in the human body: long, short, flat, sesamoid and irregular. Long bones are characterized by a diaphysis (consisting of the compact bone at the surface, the trabecular bone in the deeper layer containing red marrow, and a medullary cavity containing yellow marrow) and an epiphysis at both ends. Between the diaphysis and the epiphyses, the metaphysis is located. This is the location of the epiphyseal plates and thus the part of the bone that grows during childhood. At the end of its growth, it ossifies into bone. The epiphysis is covered with hyaline cartilage. The femora, tibiae, fibulae as well as the humeri, ulnae and radii are examples of long bones.
Short bones have only a thin layer of compact bone surrounding the trabecular interior. The carpalia and tarsalia are short bones. Flat bones consist of two parallel layers of compact bone with trabecular bone inside, for example, the sternum and the cranium. Sesamoid bones are embedded in tendons, like the patellae. Irregular bones do not fit the classification, as a consequence of their shape and name. Bones of the spine and pelvis are irregular bones.
3.4.1.2 Physiology of Bone Formation
The skeleton is a dynamically active organ system that undergoes continuous remodelling: it continuously repairs and strengthens itself. Bone remodelling is distinct from bone formation. Bone formation during embryonic development occurs through endochondral and intramembranous ossification. During intramembranous ossification, bone is formed directly from connective tissue. In endochondral ossification, the cartilage precedes the eventual bone. Thus, growth in length is a result of endochondral ossification of proliferating cartilage in epiphyseal plates.
Bone is a heterogeneous calcified connective tissue consisting of 65 % mineral and 35 % organic matrix. This organic matrix consists of approximately 90 % of collagen fibres. The mineral part is made up of calcium carbonate, calcium phosphates and crystalline hydroxyapatite. This part exists in a state of equilibrium with plasma, exchanging freely and rapidly between compartments. It contains 99 % of the body’s calcium and has therefore a critical function in the normal calcium balance.
On a cellular level there are three major types of bone cells: osteoblasts (bone-forming cells), osteocytes (mature osteoblasts surrounded by matrix) and osteoclasts (bone-resorbing cells). Osteoblasts are primarily responsible for the synthesis of extracellular matrix: they lay down the collagen, which becomes mineralized during bone formation. In disease state the function of these osteoblasts changes. There is an uncontrolled change in mineralization activity. This change forms the basis of bone scintigraphy. 99mTc-labelled diphosphonate complexes are absorbed on calcium phosphate in the new formed hydroxyapatite matrix. Therefore, uptake on bone scintigraphy represents local osteoblast activity.
As bone expands, osteoblasts become trapped in the matrix they produce and develop into osteocytes. Therefore, osteocytes are mature osteoblasts which have lost their ability to form bone, but rather play a role in maintenance activities. Osteoclasts are giant cells that resorb bone. They also secrete lytic enzymes, which dissolve inorganic calcium salts.
In a normal situation bone metabolism and remodelling is a balanced process. The process of resorption and formation of new bone occurs on the surface of bone. Collagen fibres are organized in patterns that create holes in the matrix. These holes are the site of hydroxyapatite crystal deposition. The osteoblast is the primary cell that promotes this deposition by increasing the phosphate ion concentration. It reduces the solubility of calcium and favours crystallization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree