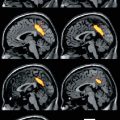
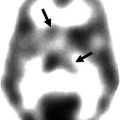
Fig. 40.1
Magnetic resonance imaging. (a) A 5-year-old female patient with right frontal lobe epilepsy treated in our center. T1- and T2-weighted sequences showed widespread bilateral cortical atrophy that did not fully explain EEG and clinical data of the patient. (b) Images from one of our patients (a 36-year-old man) with nonlesional left temporal lobe epilepsy and postoperative seizure-free outcome. T1- and T2-weighted sequences did not clearly reveal lateralized structural abnormalities explaining EEG and clinical data of the patient. Subsequent multimodal analyses showed an epileptogenic region in the right frontal lobe in the first patient and in the left temporal lobe in the second one
In this chapter we aim to review the value of nuclear medicine neuroimaging combined with source analysis based on electrophysiological information (EEG/MEG) to improve EZ localization in drug-resistant nonlesional focal epilepsy, with a significant positive impact on surgery outcome.
40.2 Nuclear Medicine Neuroimaging in Nonlesional Drug-Resistant Focal Epilepsy
Subtraction of the interictal SPECT from the ictal SPECT coregistered to MRI (SISCOM) improves the ability to detect epileptogenic lesions in patients with nonlesional MRI, especially in extratemporal lobe epilepsies (Jayalakshmi et al. 2011; la et al. 2009; O’Brien et al. 1998, 2000; Spanaki et al. 1999). In patients with extratemporal epilepsies, SISCOM may help to detect subtle focal cortical dysplasia (FCD) not detected by MRI (Dupont et al. 2006; Huberfeld et al. 2006; Stanley et al. 1998). SISCOM has also been described as particularly useful in identifying the seizure-onset zone in these lesions (Dupont et al. 2006). Figure 40.2 shows how SISCOM analysis could detect a subtle FCD in the right frontal lobe, in the patient previously presented in Fig. 40.1a, which corresponds to the seizure-onset zone in this case. SISCOM has also shown high localizing and predictive value for seizure-free outcome in extratemporal lobe epilepsy (Kim et al. 2009; Knowlton et al. 2008b). Kim et al. (2009) investigated the influence of various techniques, including SISCOM, in a pediatric epilepsy surgery group with excellent surgical outcome. SISCOM was capable of localizing in 66.7 % of temporal and 84.6 % of extratemporal cases. Similar results were found by Barba et al. (2012), which showed that SISCOM had a high localization value in this difficult patient group.
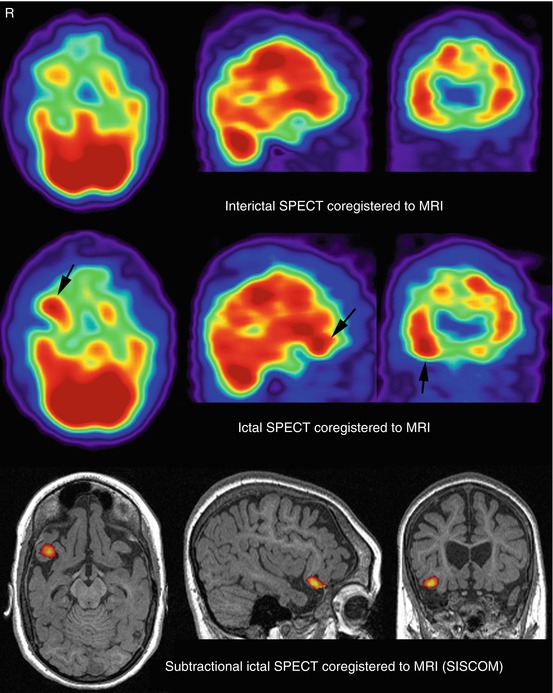

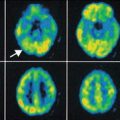
Fig. 40.2
SISCOM study (threshold, +2 standard deviations) in the patient presented in Fig. 40.1a. SISCOM allowed the detection of subtle focal cortical dysplasia in the right frontal lobe demonstrated by a focal hyperperfusion in this region during ictal SPECT
The paper published by O’Brien et al. (2000) reported an excellent outcome when SISCOM localization was concordant with surgical-resection site in patients with drug-resistant focal epilepsy and normal MRI. They observed that resection of the area of increased perfusion was associated with better surgical outcome. Figure 40.3 presents SISCOM analysis from one of our patients with a nonlesional left TLE and postoperative seizure-free outcome. In this patient, MRI did not clearly reveal lateralized structural abnormalities (Fig. 40.1b) explaining EEG and clinical data. Indications for SISCOM in patients undergoing presurgical evaluation also include nonsubstrate-directed partial epilepsy, multilobar pathology, and conflicting results in the noninvasive evaluation (Cascino 2004).
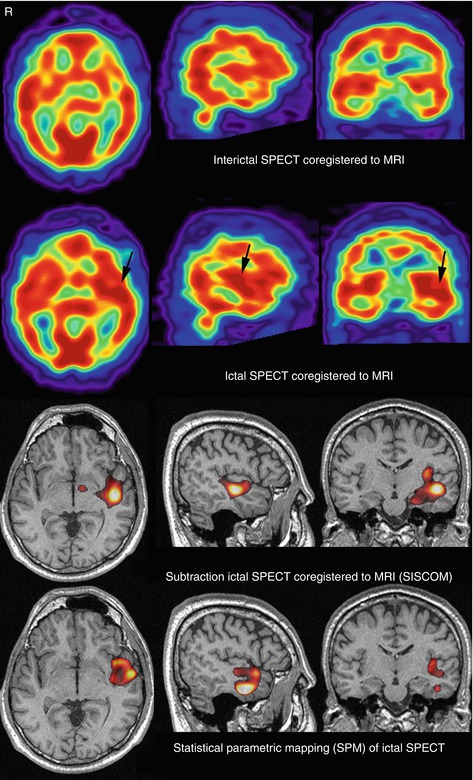

Fig. 40.3
SISCOM study (threshold, +2 standard deviations) in the patient presented in Fig. 40.1b. SISCOM showed a focal cerebral hyperperfusion in the left temporal lobe, concordant with EEG and clinical data, in a region apparently normal by MRI. Ictal SPECT compared with a control brain SPECT database by using SPM also showed a focal cerebral hyperperfusion partially overlapping the left temporal regions detected by SISCOM
The presence of a SISCOM alteration may obviate the need for intracranial EEG (iEEG) recordings in selected patients (Ahnlide et al. 2007; Hogan et al. 2006; von Oertzen et al. 2011). The prospective study of Ahnlide et al. (2007) in patients with nonlesional MRI (or widespread MRI lesion) and discordant data in the standard presurgical assessment is of special interest. They showed that SISCOM results altered the electrode implantation scheme in the majority of patients and – if concordant with other tests (semiology, EEG, MRI) – were predictive of good seizure outcome. Correlations with surgical outcome suggest that SISCOM also provides complementary information to MRI or neurophysiological findings (Ahnlide et al. 2007; Bouilleret et al. 2002; Brinkmann et al. 1999; Kaminska et al. 2003; Kim et al. 2001; Marks et al. 1992; von Oertzen et al. 2011). On the other hand, 18F-FDG PET has higher spatial resolution and lower background activity than perfusion SPECT. However, one of the limitations of 18F-FDG PET for the evaluation of epilepsy during the ictal phase is its low temporal resolution. This is due to the fact that 18F-FDG uptake period (30–45 min) is significantly longer than the average seizure duration (1–2 min) which leads to a mixture of interictal, ictal, and postictal phases. This makes only interictal 18F-FDG PET feasible (Kim et al. 2011). Nevertheless, interictal 18F-FDG PET has an established role in the noninvasive localization of EZ (Desai et al. 2012).
Interictal18F-FDG PET coregistered to MRI (18F-FDG PET/MRI) of the same patient provided more sensitivity than 18F-FDG PEt alone in the detection of cortical lesions (Lee et al. 2005a). Lee et al. (2005a), by using ictal SPECT and 18F-FDG PET, showed that seizure-free outcome could be achieved in 47 % of patients and that up to 90 % seizure reduction could be achieved in 80 % of the patients with drug-resistant epilepsy with normal MRI. In the last years, 18F-FDG PET/MRI has shown a high sensitivity (up to 98 %) for FCD, especially in patients with mild FCD type I and normal MRI (Blumcke and Hamer 2012).
Recently, Rubi et al. (2011) prospectively evaluated 18F-FDG PET and 18F-FDG PET/MRI results of 31 nonlesional pediatric patients. They demonstrated the ability of 18F-FDG PET/MRI to guide a second look at MRI studies previously reported as nonlesional, turning a meaningful percentage of these into subtle-lesional. Another two studies have validated 18F-FDG PET/MRI in nonlesional childhood epilepsy (Lerner et al. 2009; Ollenberger et al. 2005). Thus, 18F-FDG PET/MRI has become a useful tool for preoperative EZ detection in patients with drug-resistant epilepsy and normal or less specific findings on MRI. It has produced better surgery outcomes and longer seizure-free periods postoperatively.
Despite the demonstrated utility of nuclear medicine neuroimaging in nonlesional drug-resistant focal epilepsy, recent studies combining SPECT and/or PET with electromagnetic source localization data have shown incremental validity to determine EZ for surgical purpose without invasive electrodes or for planning intracranial electrode placement in patients with nonlesional drug-resistant focal epilepsy.
40.3 Nuclear Medicine Neuroimaging and Electromagnetic Source Localization
Source analysis based on electrophysiological information, using either EEG or MEG, and neuroanatomical data (e.g., MRI) allow revealing the source localization of interictal/ictal epileptic discharges (Bast et al. 2004; De et al. 2012; Heers et al. 2012; Kaiboriboon et al. 2012; Knowlton 2006; Morales-Chacon et al. 2003; Wheless et al. 2004). These methods include low-resolution electromagnetic tomography (LORETA), dipole brain electric source analysis (BESA), and brain distributed variable resolution electromagnetic tomography (VARETA). Recently, a Bayesian spatiotemporal model for source reconstruction of EEG/MEG data has been proposed (Trujillo-Barreto et al. 2008). Figures 40.4 and 40.5 show LORETA results of ictal scalp EEG of the two patients shown in Figs. 40.1, 40.2, and 40.3. Figure 40.6 shows the multimodal registration of both cases, including LORETA and SISCOM.
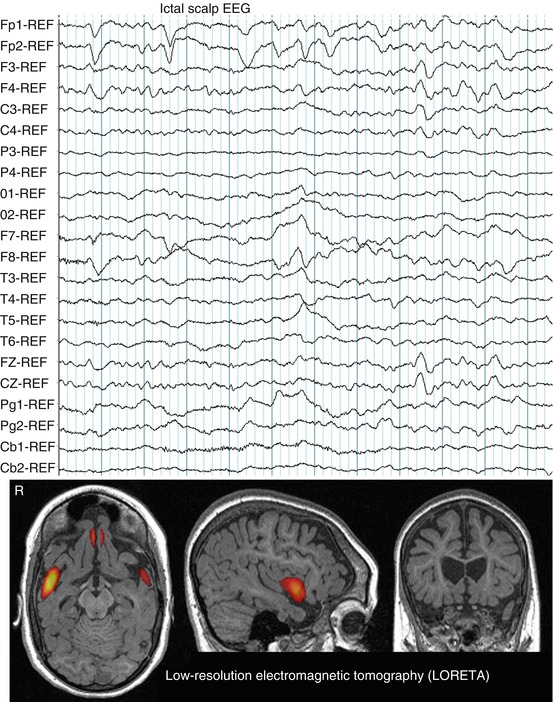
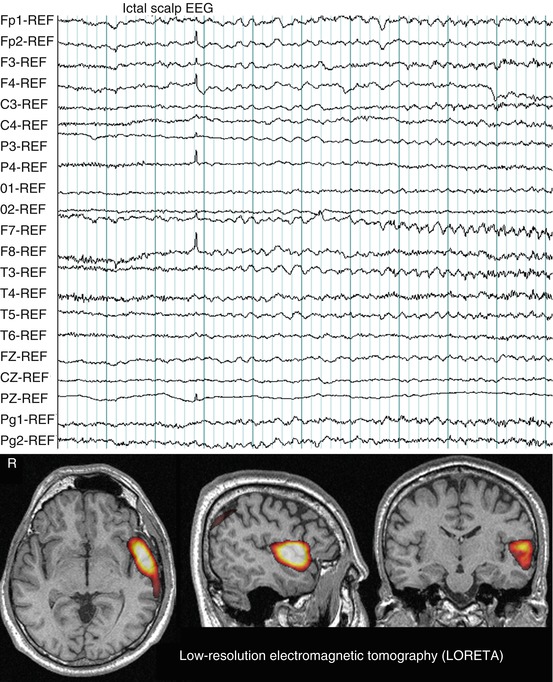
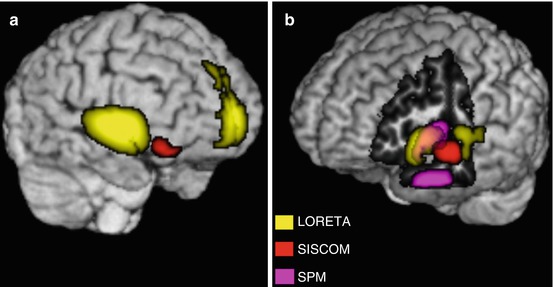

Fig. 40.4
Ictal scalp EEG pattern during a typical partial seizure of the patient presented in Fig. 40.1a. Note rhythmic activity at seizure onset in channels containing the frontal leads, Fp1-2, F3-4, Fz. LORETA solution (time-domain analysis of peak activity) showed bilateral frontal sources. Hot colors signify LORETA thresholded at 2 standard deviations above the mean value per voxel of the whole volume, after rescaling LORETA to the SISCOM study performed in the patient

Fig. 40.5
Ictal scalp EEG pattern during a typical complex partial seizure of the patient presented in Fig. 40.1b. Note rhythmic theta activity at seizure onset in F7, T3, and Pg1. LORETA demonstrated a left temporal source

Fig. 40.6
Multimodal registration. (a) Rendering of MRI (T1-weighted in the native space) with a right anterior brain view of the patient presented in Figs. 40.1a, 40.2, and 40.4. The image includes SISCOM and LORETA that helped to improve the detection of epileptogenic zone in this patient in which MRI findings did not fully explain EEG and clinical data. (b) Rendering of MRI with a left anterior view of the brain belonging to the patient presented in Figs. 40.1b, 40.3, and 40.5. The image was cut out to show medial aspect of left temporal lobe and includes multiples approaches to localize epileptogenic zone, which helped to improve surgical outcome in this patient with nonlesional left temporal lobe epilepsy
Several studies comparing perfusion SPECT with source analysis methods have been published (Knowlton 2006; Knowlton et al. 2008a, b; Morales-Chacon et al. 2009; Santiago-Rodriguez et al. 2006; Schneider et al. 2012).
The study of Santiago-Rodriguez et al. (2006) evaluated the concordance of hypoperfusion zones measured by interictal SPECT with BESA and VARETA in patients with complex partial seizures. They concluded that the concordance of hypoperfusion zones was better with BESA than with VARETA.
In an interesting study, Knowlton et al. (2008b) investigated the prediction of epilepsy surgery outcome regarding ictal SPECT, source analysis based on MEG, also defined as MSI, and 18F-FDG PET. Most of the 34 SISCOM patients in this study had extratemporal lobe epilepsy with nonlocalizing MRI or EEG. SISCOM had the highest predictive value (odds ratio = 9.9) for excellent surgical outcome. In another study, they observed that MSI had the highest concordance rate with iEEG compared to ictal SPECT and 18F-FDG PET (Knowlton et al. 2008a). Previous studies had found lower concordance rates of MSI, SISCOM, and iEEG in neocortical epilepsies compared to mesial TLE cases (Hwang et al. 2001; Won et al. 1999). Another study had demonstrated that positivity of all tests including MSI, 18F-FDG PET, and ictal SPECT predicted increased odds for seizure-free outcome after surgery (Stefan et al. 2000).
Our group have found in 18 TLE patients with normal appearing MRI that ictal video-EEG (V-EEG) has the highest percentage of correct lateralization (100 %) followed by ictal SPECT and choline/creatine and N acetyl aspartate/creatine metabolic ratios measured by magnetic resonance spectroscopy (Morales-Chacon et al. 2009). This study confirmed that localizing data provided by V-EEG and complemented by functional neuroimaging studies can be used to perform successful temporal lobectomies on patients with drug-resistant nonlesional TLE or showing bilateral structural abnormalities.
Jayakar et al. (2008) reported the outcome of a cohort predominantly of children with nonlesional drug-resistant partial epilepsy undergoing resective surgery. The epileptogenic region was identified by integrating clinical exam, V-EEG, ictal SPECT, extraoperative subdural monitoring, and electrocorticographic. They concluded that a multimodal integrative approach can minimize the size of resection and alleviate the need for invasive EEG monitoring.
More recently, Schneider et al. (2012) conducted the largest retrospective study to compare ictal iEEG with MSI and SISCOM in patients with nonlesional neocortical epilepsy. The most important finding from this study was that sublobar concordance of ictal iEEG with either MSI or SISCOM was superior to ictal iEEG alone in localizing the EZ. They also found that specificity and positive predictive value of ictal iEEG were higher combined with MSI and SISCOM. Interestingly, they observed that MSI was more advantageous compared to SISCOM in predicting seizure-free epilepsy surgery outcome, when sublobar concordance of MSI with ictal iEEG was present, whereas a positive SISCOM concordant with ictal iEEG and complete resection may have prognostic implications, forecasting a more advantageous epilepsy surgery outcome. Schneider’s study clearly shows that a multimodal approach can significantly contribute to predict surgical outcome.
Few studies have directly compared 18F-FDG PET, SISCOM, and MSI with iEEG in the same patients (Seo et al. 2009, 2011). Seo et al. (2011) demonstrated in children with nonlesional epilepsy that both SISCOM and MSI had better lobar concordance with iEEG than statistical parametric mapping (SPM) analysis of 18F-FDG PET. These findings suggest that nonlesional neocortical epilepsy with both positive MSI and SISCOM may indicate a higher chance of a localized iEEG result. Therefore, both diagnostic modalities provide additional and not redundant localizing information over the one provided by iEEG alone, even if iEEG is localizing (Fig. 40.7). In a previous study, this group had already suggested that multimodality approach may improve surgical outcome (Seo et al. 2009).
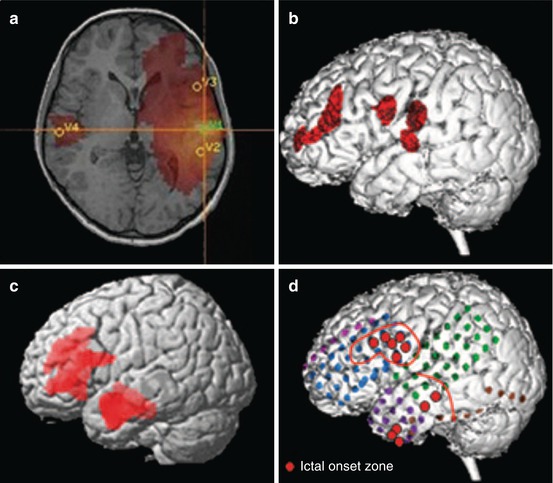

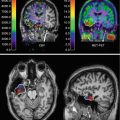
Fig. 40.7
A 14-year-old boy with focal epilepsy since age 4. Scalp EEG disclosed frequent interictal spikes in the left hemisphere, especially in left anterior temporal areas. MRI was normal. (a) Magnetoencephalography showed maximal source activity in left inferior frontal region. (b) Subtraction peri-ictal SPECT coregistered to MRI demonstrated increased perfusion in left frontal region. (c) Statistical parametric mapping–PET showed hypometabolism involving left frontal and temporal region. (d) Intracranial EEG revealed ictal onset in left lateral temporal and inferior frontal areas (red circles). The patient became seizure free following left frontal corticectomy and left anterior temporal lobectomy at 12 months of follow-up. Pathology revealed type IIA focal cortical dysplasia in both frontal and temporal lobes without hippocampal sclerosis (From Seo et al. (2011))
One interesting study conducted by Zumsteg et al. (2005) assessed the usefulness of PET with H2 15O or 13NH3 and LORETA in three patients with partial status epilepticus. They concluded that PET studies of cerebral blood flow and noninvasive source modeling with LORETA provided useful information for localizing the ictal activity in patients with partial status epilepticus.
In spite of the growing number of studies combining nuclear medicine neuroimaging and electromagnetic source localization in patients with nonlesional drug-resistant focal epilepsy, in which the usefulness of this multimodal approach has been shown, there is no meta-analysis clarifying the importance of this approach neither cost-effectiveness studies to identify the most cost-effective method. Several issues make it difficult to establish how useful the multimodal approach is, rather than single modality, and under what circumstances: (1) epilepsy surgical management and procedures are highly variable depending on the center’s experience and expertise; (2) difficulties for independently quantifying true clinical added value of a single test due to multidisciplinary factors in the surgical decision process; and (3) the absence of a reliable standard reference to assess the clinical relevance of the test under consideration.
Nevertheless, although appropriate clinical trials are still needed to provide more evidence, the multimodal approach seems to have higher clinical value in nonlesional drug-resistant neocortical focal epilepsy patients, especially in children. Moreover, the multimodal approach provides additional relevant information, not only to determine the EZ in patients initially rejected for surgery, but also to guide intracranial electrode implantation.
Finally, advances in neuroimaging by high-field MRI, diffusion tensor imaging, functional MRI, magnetic resonance spectroscopy, near-infrared spectroscopy, and optical imaging of intrinsic signals have also the potential to identify structural subtle lesions in drug-resistant epilepsy patients (Alarcon et al. 2006; Berg and Engel 2006; Blume et al. 2004; Chapman et al. 2005; Lee et al. 2005a; Siegel et al. 2001; Sylaja et al. 2004; Wu et al. 2013; Lenkov et al. 2012; Sgouros et al. 2001). These new techniques combined with recent advances in nuclear medicine neuroimaging are promising in order to noninvasively localize the EZ and to guide surgery in drug-resistant focal epilepsy patients that exhibit nonlesional MRI.
40.4 Conclusions
Multimodal imaging using nuclear medicine and electromagnetic source localization helps to improve the selection of surgical candidates, as well as surgical outcome in nonlesional drug-resistant focal epilepsy patients, particularly in children with neocortical epilepsy. In our view, the multimodal approach provides additional relevant information not only to determine the EZ in patients initially rejected for surgery but also to guide intracranial electrode implantation. Future evidence-based imaging studies are required to clarify to what extent is the multimodal approach useful, rather than a single modality, and under what circumstances. Cost-effectiveness studies are also necessary to identify the most cost-effective method.
Acknowledgments
We thank Odalys Morales Chacón and Eloisa Le Riverend Morales for their English assistance. We would also like to thank Maria Luisa Rodriguez and Abel Sanchez for their useful cooperation.
References
Alarcon G, Valentin A, Watt C, Selway RP, Lacruz ME, Elwes RD, Jarosz JM, Honavar M, Brunhuber F, Mullatti N, Bodi I, Salinas M, Binnie CD, Polkey CE (2006) Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry 77:474–480PubMedCentralPubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree