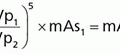Nuclear Tomographic Imaging—Single Photon and Positron Emission Tomography (SPECT and PET)
19.0 INTRODUCTION
The focus of this chapter is SPECT and PET imaging. Specifically, the chapter covers the principles of operation, image acquisition and reconstruction, data corrections, performance, and quality control for both of these nuclear tomographic imaging modalities. The chapter also covers dual modality imaging (SPECT/CT, PET/CT, and PET/MR) and other recent developments in SPECT and PET imaging. A section on PET image quantification and detrimental factors affecting the quantitative accuracy is also included. Finally, the chapter concludes with a comparison of these two imaging modalities.
19.1 FOCAL PLANE TOMOGRAPHY IN NUCLEAR MEDICINE
1. Focal plane tomography was the dominant nuclear medicine (NM) tomographic technique prior to the development of SPECT. Many such devices were developed:
a. Rectilinear scanner with focused collimators.
b. Anger tomoscanner (two opposed small cameras, converging collimators, raster scanning, multiple coronal whole-body tomographic images).
c. Anger camera with a seven-pinhole collimator (cardiac short-axis images).
d. Planar gamma camera imaging is weakly tomographic.
(i) Spatial resolution degrades with distance (closer structures sharper).
(ii) Photon attenuation increases with depth (closer structures more visible).
19.2 SINGLE-PHOTON EMISSION COMPUTED TOMOGRAPHY
1. SPECT is a rotational NM tomographic technique, invented in the early 1960s, but did not become routine until the 1980s. Examples of SPECT scanners are shown in Figure 19-1.
a. Planar gamma camera images are acquired over 180° (cardiac) or 360° around the patient.
b. Transverse images are formed by filtered backprojection (FBP) or iterative reconstruction.
2. Image acquisition consists of one or more SPECT camera heads revolving about the patient, and acquiring projections (Fig. 19-2):
a. Continuous mode: images acquired over angular intervals as the head moves continuously
b. “Step-and-shoot” mode: images acquired when the head stops at evenly spaced angles
c. Pixel format usually 642 or 1282, but large enough to not degrade resolution due to pixel size
d. Zoom factor typically approximately 1.5 for 642 cardiac, to reduce pixel size and, thus, improve resolution
e. Typically, 60-64 (642) or 120-128 (1282) projections, to reduce reconstruction streak artifacts
f. Circular orbit: brain imaging (on systems with a small camera head physical edge-to-useful field-of-view edge distance or on systems with a head holder beyond the patient table; resulting in higher resolution compared to body imaging, due to a small radius within the patient’s shoulders)
g. Noncircular (“body contouring”) orbit (NCO): body imaging (for a circular orbit, camera heads would be many centimeter from the body surface for anterior and posterior projections, resulting in a loss of spatial resolution). NCO manually specified on some older systems, but automatically determined on modern systems.
Ideally, a 180° arc of projections would be sufficient for reconstruction, as opposed projections would be mirror images. However, due to a large amount of attenuation of photons from activity in the half of the patient opposite the camera head and spatial resolution degradation with distance, projections over a complete revolution (360°) about the patient are required. An exception is cardiac SPECT, where a 180° arc symmetric about the heart, from right anterior oblique to left posterior oblique, is employed. Artifacts can be introduced, but resolution and contrast improve, as projections opposite the heart have poorer resolution and contrast due to greater distance and attenuation than those on the same side as the heart.
3. Transverse image reconstruction: is performed using either FBP or an iterative algorithm, after uniformity and center-of-rotation correction of projection images.
Filtered backprojection reconstruction (Fig. 19-3):
a. Projection images are mathematically filtered.
b. Each transverse slice is formed by backprojection of the corresponding row of projections.
c. The theoretical ideal kernel is a ramp filter in the spatial frequency domain.
d. Projections contain substantial statistical noise (radioactive decay process, low pixel counts).
e. High frequencies in the spatial frequency domain are dominated by statistical noise.
f. Gamma camera resolution reduces higher spatial frequency information of imaged structures, increasingly with distance.
g. Ideal ramp FBP results in unacceptable noise in transverse slices.
h. Projections are smoothed by ramp filter “roll-off” at higher spatial frequencies.
i. Filter kernel “roll-off” reduces noise at the expense of spatial resolution.
j. Filter kernel selection is a compromise between resolution and noise.
k. Different kernels are employed for different SPECT studies, based on the spatial resolution (due to collimator and detector-to-imaged organ distance) and amount of statistical noise (due to amount of activity injected, collimator and acquisition time per projection).
l. A filter with a higher spatial frequency cutoff is required for projections of better spatial resolution and less quantum mottle, to avoid unnecessary reconstruction resolution loss.
m. A filter with a lower spatial frequency cutoff is required for projections of poorer spatial resolution and greater quantum mottle, to avoid excessive reconstructed quantum mottle.
Iterative reconstruction (Fig. 19-4):
a. Filtered backprojection is computationally efficient but assumes perfect projection images of a three-dimensional object, which is not true, due to photon attenuation by the patient, the inclusion of Compton scatter, and collimator resolution degradation with distance.
b. The initial activity distribution assumption is uniform or the result of simple backprojection.
c. Projection images of the initial distribution are then calculated and compared to the actual (acquired) projection images.
d. The activity distribution is adjusted based upon the projection image comparison.
e. Distribution projection, projection comparison, and distribution adjustment are repeated until the calculated projection images are approximately equal to the actual projection images.
f. Projection calculation can incorporate and thus compensate for
(i) Decreasing system resolution point spread function with distance from the camera
(ii) Effects of attenuation using a map of attenuation coefficients (µ) in the patient
(iii) A model of photon scattering
a) Modified PSF
b) Secondary energy window images (dual- or triple-energy window), or
c) Photopeak transverse images and attenuation and material density maps (effective scatter source estimation)
g. Reconstructed images can be of either higher quality than FBP images or equivalent quality with less administered activity or reduced scan time.
h. Iterative reconstruction is computationally less efficient than FBP, but feasible due to increasing computer speed, the small SPECT matrix sizes, and computationally efficient algorithms such as ordered subset expectation maximization.
i. Three-dimensional spatial filtering is usually applied afterward, due to substantial noise in images reconstructed from projections with relatively poor counting statistics.
4. Attenuation correction in SPECT: Radioactivity whose photons traverse long paths through the patient produces fewer projection image counts than activity near the patient surface. This is exhibited as a gradual decrease in activity toward the center of reconstructed images of a uniform cylinder of radioactivity (Fig. 19-5, left). Many methods have been developed for attenuation correction (AC):
a. The Chang approximation method for FBP assumes a constant µ value; may be used for brain and abdominal studies where µ is relatively constant; cannot attenuation correct thorax studies, where µ is highly variable; and should be verified (including µ value) with a phantom before clinical use (Fig. 19-5, middle and right).
b. Sealed radioactive sources (commonly Gd-153, which emits 97- and 103-keV γ-rays) to measure attenuation were added by several SPECT camera manufacturers in the 1990s:
(i) Transmission projection images were acquired and reconstructed to generate transverse µ maps similar to x-ray CT images.
(ii) The µ maps were then used for iterative reconstruction AC.
(iii) Several transmission-sealed source configurations were developed.
a) Scanning collimated line used with parallel-hole collimators
b) Arrays of fixed line used with parallel-hole collimators (cardiac only)
c) A fixed line located at the focal point of a fan-beam collimator
(iv) Transmission data were usually acquired simultaneously with emission data (performing the two separately posed significant transmission-emission data spatial alignment problems and greatly increased total imaging time).
(v) The transmission radionuclide was one with primary γ-ray emissions significantly lower in energy than those of the radiopharmaceutical.
(vi) Separate transmission and emission photon energy window images were acquired.
(vii) Scattering of higher energy emission photons in the patient and detector produced cross-talk in the lower energy transmission window.
c. Hybrid SPECT/CT scanners, where x-ray CT image data can be used for radionuclide emission data AC, have supplanted radioactive transmission sources.
d. Extensive studies of radioactive source- and x-ray CT-based myocardial perfusion SPECT AC have demonstrated a reduction in attenuation artifacts and improved diagnostic performance.
e. Other studies have shown that emission data and µ map spatial misalignment can cause AC artifacts in myocardial perfusion SPECT images.
f. A period of transition is needed to retrain even experienced clinicians to interpret attenuation-corrected myocardial perfusion SPECT images.
5. Generation of coronal, sagittal, and oblique images:
a. Coronal and sagittal slices are commonly generated by simple reordering of the pixels from the transverse slices.
b. Oblique images parallel (vertical and horizontal long-axis images) and perpendicular (short-axis images) to the long axis of the left ventricle are standard for cardiac SPECT.
c. Due to considerable anatomic variation among patients, the long axis of the heart must be determined before the computer can create the oblique images.
d. The long axis is commonly defined manually by a technologist, although the software on most systems is now capable of correct automatic reorientation, with operator verification and override.
6. Collimators for SPECT are most commonly parallel-hole. Fan-beam collimators (converging and parallel-hole in the x– and y-directions, respectively, Fig. 19-6 left), which have better spatial resolution and efficiency, but a smaller transaxial FOV, than parallel-hole, have been designed for brain SPECT (FOV truncation artifacts would occur if used for body SPECT). A variable focal length converging collimator for cardiac imaging has been developed, where the focal length increases from the center outward, ending up parallel-hole at the edge. A heart-centric orbit is employed to achieve an approximately fourfold increase in sensitivity (Fig. 19-6 right).
7. Multihead SPECT cameras have been developed to reduce the limitations imposed on SPECT by collimation and limited time per view, and permit the use of higher resolution collimators than a single-head system for a given level of quantum mottle. Severe requirements upon the electrical and mechanical stability of the camera heads are imposed (X and Y offsets and X and Y magnification factors of all heads must be precisely matched throughout the rotation about the patient.), but current multihead systems are very stable and provide highquality tomographic images. Configurations (Fig. 19-1) are
a. Double-head, 180° opposed heads: head and body SPECT, whole-body (WB) planar
b. Triple-head, fixed-angle: head and body SPECT, but limited crystal width (no WB planar)
c. Double-head, variable-angle: head and body SPECT and WB planar (180° opposed), and cardiac SPECT (90° perpendicular)
8. Multielement detector SPECT cameras that employ a multitude of small or curved detectors and alternative scanning techniques are now commercially available. For example:
(i) Twelve detectors equally spaced over 360° around the patient
(ii) Detector: 16 (x) × 128 (y) 6-mm-thick CZT crystals; 2.46 × 2.46 mm elements
(iii) Integrated parallel-hole tungsten collimator
(iv) Each (31.5 cm axial) SPECT FOV scan: a combination of detector swivel, rotation, and auto-contouring
(v) Volume sensitivity approximately 3 times that of a dual-head NaI(Tl)/LEHR SPECT camera
(vi) 40 to 220 keV energy range
(i) Nine 8 × 8 cm, 5-mm-thick CZT crystals; 2.46 × 2.46 mm elements
(ii) Equally spaced along a stationary L-shaped gantry
(iii) Detector orientations and pinhole collimation allows all views to be acquired simultaneously without detector motion
(iv) Counting efficiency approximately 5 times that of a conventional NaI(Tl)/LEHR SPECT camera
(i) Nine 4 × 16 cm, 6-mm-thick CZT crystals, 2.46 × 2.46 mm elements
(ii) Parallel-hole tungsten collimation and an L-shaped gantry
(iii) Scanning of the heart achieved by translation and swivel of each detector
(iv) Approximately 8 times the sensitivity of a conventional NaI(Tl)/LEHR SPECT camera
d. CardiArc dedicated cardiac (CardiArc Inc.)
(i) Three adjacent curved NaI(Tl) crystals and an array of photomultiplier tubes
(ii) “Slit-hole” scanning
(iii) One stationary series of lead sheets with horizontal gaps
(iv) A second curved lead sheet with six vertical slits rotates back and forth in electronic synchrony with six corresponding regions of the crystals
(v) Sensitivity gain of approximately 4 compared to a conventional NaI(Tl)/LEHR SPECT camera
9. SPECT spatial resolution depends upon a number of factors, including the radionuclide, collimation, radius of rotation, orbit (circular or body-contouring), and reconstruction filter. Brain SPECT resolution is better than that for body SPECT, due to a smaller orbit radius. SPECT resolution can be measured by acquiring and reconstructing a line source, such as a capillary tube, placed along the axis of rotation, for which NEMA has developed a protocol (Fig. 19-8):
a. A 22-cm diameter, cylindrical plastic water-filled phantom.
b. Three Co-57 solid or Tc-99m liquid line inserts (one on the central axis, two offset by 7.5 cm).
c. A 15-cm radius circular orbit, 360° acquisition; and ramp FBP (a “roll-off” reduces resolution).
d. Typical reconstructed FWHMs (LEHR collimator) are 9.5 to 12 mm (central), 7 to 8 mm (tangential), and 9.4 to 12 mm (radial).
NEMA resolution, while a useful index, does not depict clinical resolution. Patient studies may involve larger radii of rotation, shorter acquisition times, and possibly a higher-efficiency, lower-resolution collimation than the NEMA protocol; and a ramp filter “roll-off” to reduce noise, which introduces more blurring (larger FWHM). Furthermore, the NEMA protocol is not applicable to iterative reconstruction with resolution, attenuation, and scatter compensations.
10. Comparison of SPECT to conventional planar gamma camera imaging: SPECT resolution should be equivalent to that of planar, but FBP clinical resolution is typically slightly worse. The camera may be placed closer to the patient when acquiring a planar image, clinical FBP SPECT requires smoothing for noise reduction and obtaining a sufficient number of SPECT counts may require lower-resolution collimation than for planar. However, iterative reconstruction SPECT with system resolution compensation can result in better resolution than planar. The main advantage of SPECT is the removal of overlapping structures of nonuniform activity, leading to improved contrast and reduced structural noise. Iterative reconstruction can partially compensate for patient scatter, collimator spatial resolution decrease versus distance and septal penetration, and patient photon attenuation using maps from radioactive source or x-ray CT.
11. Quality control in SPECT is critical to identify malfunctions or maladjustments that can result in a loss of resolution and significant artifacts that may mimic pathology. Following acceptance testing by a medical physicist, a QC program should be established that ensures system SPECT performance remains comparable to that at acceptance.
a. X and Y magnification factors (gains) relate distances in the object to numbers of pixels in the acquired image, determined by imaging two point sources a known distance apart, along a line parallel to the camera’s X and Y axes:

and should be equal to one another, as well as between detectors of a multihead system, to prevent projection, coronal, sagittal, and oblique image distortion.
b. Multienergy spatial registration is important in SPECT for both imaging radionuclides which emit photons of more than one energy (e.g., In-111, Ga-67), and for uniformity and axis-of-rotation (AOR) corrections determined with one radionuclide to be valid for others. Gain and multienergy registration errors are not applicable to multielement cameras, as detector elements are square and the number of element rows and columns is fixed.
c. Center-of-rotation (COR) calibration aligns projection images to the AOR (an imaginary line about which the SPECT camera revolves).
(i) Misalignment may be mechanical (e.g., camera head not centered in the gantry), electronic, or a digital setting.
(ii) Misalignment may be constant or vary versus camera head angle, and may vary along the AOR.
(iii) Four other possible misalignments are axial tilt, detector-to-detector axial shift, yoke swivel, and axial swivel with respect to the AOR.
(iv) If small, a loss of resolution will result, but if large enough, a reconstructed point or line source will appear as a “doughnut” (Fig. 19-9, first three images left to right).
(v) COR alignment is assessed by acquiring SPECT projection images of one or more point sources, or a line source (placed along the AOR) and analyzing them with the SPECT system’s computer.
(vi) COR misalignment is corrected by shifting each clinical projection image in the x-direction by the proper number of pixels prior to reconstruction.
(vii) When a line source or multiple points are used for calibration, a unique correction can be derived and applied for each transverse slice.
(viii) If misalignment varies with camera head angle, it can only be corrected if angle-by-angle correction is permitted by the system’s computer.
(ix) Separate COR corrections are required for different collimators and dual-head configurations (e.g., 180° and 90°) and, on some systems, for different camera zoom factors and image formats (e.g., 642 versus 1282).
d. Uniformity of a camera head is important, and more so for SPECT versus planar imaging.
(i) Slight nonuniformities in projection images are magnified by reconstruction.
(iii) Partial rings appear when multihead SPECT projections are not acquired by all heads over a 360° arc.
(iv) Ring artifacts are most apparent in high count density studies, such as liver scans, but may be most harmful in studies such as myocardial perfusion with poor counting statistics and large variations in count density, where they may be misinterpreted as physiologic variations.
(v) Modern gamma cameras have digital circuits for energy and linearity correction but not for local nonuniformity due to, for example, collimator dents or manufacturing defects.
(vi) Minor local nonuniformities are corrected by acquiring a very high count uniform flood image (at least 30 million counts for a 642 pixel format and 120 million for 1282) and computing each pixel correction factor as the ratio of the average pixel count in the image to the count in that pixel. Projection images are uniformity-corrected before COR correction and reconstruction.
(vii) Uniformity correction images for each camera head and collimator are typically updated weekly to monthly, and for some cameras, separate intrinsic correction images for each radionuclide are also required.
(viii) Uniformity correction effectiveness can be assessed by inspecting for ring artifacts, the transverse slices of a SPECT scan of a uniform cylinder, or SPECT performance phantom filled with Tc-99m in solution but is limited to the parts of the detector containing the projected image of the phantom.
e. Camera head tilt causes a loss of reconstructed spatial resolution and contrast (less toward the center and greatest toward the edge), as out-of-slice activity will be backprojected into each transverse slice (Fig. 19-10), as the camera head is assumed to be aligned with the AOR (exactly parallel for most collimators).
(i) Head tilt may be tested with a bubble level on a camera head with a flat surface parallel to the collimator face.
(ii) Some older cameras require manual head tilt adjustment; on others, it is automatic.
(iii) Head tilt accuracy should be periodically tested, with either a bubble level, or (better) by a SPECT acquisition of a point source in the camera FOV, centered in the axial (y) direction, but near the edge in the transverse (x) direction. The position of the point source will vary in the y direction from image to image, visualized in a cine of the projection images if there is head tilt.
12. SPECT quality control phantoms fillable with Tc-99m or other radionuclide solutions are commercially available (e.g., Jaszczak ACR SPECT phantom). Resolution, contrast, and uniformity (although only over a relatively small portion of the SPECT FOV) can be semiquantitatively assessed and are used for system acceptance testing and then periodic (e.g., quarterly) testing.
13. Dual modality imaging—SPECT/X-ray CT systems incorporate two camera heads capable of planar imaging and SPECT and an x-ray CT system, and a common patient bed; some have an x-ray CT system for attenuation coefficient (mu) map generation and image coregistration only, whereas others can produce diagnostic quality CT images. The advantages and disadvantages are the same as those for PET/CT.
a. Advantages:
(i) Acquisition is much faster than radioactive sealed source CT acquisition.
(ii) Attenuation information has less statistical noise than that for sealed source CT.
(iii) Nonuniform attenuation in myocardial perfusion imaging, particularly by the diaphragm and, in women, the breasts, can cause apparent perfusion defects. X-ray CT-based AC has been reported to improve diagnostic accuracy by compensating for these artifacts.
b. Disadvantages:
(i) γ-Ray energy linear attenuation coefficient must be estimated from CT HU number.
(ii) Artifacts can occur due to incorrect an incorrect mu values or high atomic number material (e.g., metal objects and concentrated contrast material).
(iii) SPECT and x-ray CT information are not acquired simultaneously, and interscan organ motion can result in SPECT-CT misregistration and, therefore, SPECT artifacts.
(iv) SPECT x-ray CT heart misregistration can cause myocardial perfusion artifacts.
SPECT/CT is supplanting SPECT-only imaging, as it has become mainstream and systems have proliferated. SPECT/CT does require the additional QC step of SPECT and CT alignment verification in every clinical examination for optimum visual and quantitative accuracy.
19.3 POSITRON EMISSION TOMOGRAPHY
PET generates images depicting the distribution of positron-emitting nuclides in patients. Nearly, all PET systems today are coupled to x-ray CT systems to create what is referred to as a PET/CT scanner (Fig. 19-11).
1. Design and principles of operation: In a typical PET system, several rings of detectors surround the patient. PET scanners use annihilation coincidence detection (ACD) instead of collimation to obtain projections of the activity distribution in the subject. The PET system’s computer then reconstructs the transverse images from the projection data, as does the computer of an x-ray CT or SPECT system. Modern PET scanners are multislice devices, permitting the simultaneous acquisition of many transverse images over a preset axial distance.
a. Annihilation Coincidence Detection: Positron emission results in two 511-keV photons that are emitted in nearly opposite directions (Fig. 19-12). If both photons from an annihilation are detected in the scanner at nearly the same time, a process called ACD, then the line in space connecting the locations of the two interactions, which is known as a line of response (LOR), establishes the trajectories of the detected photons.
(i) True coincidence is the nearly simultaneous interaction with the detectors of emissions resulting from a single nuclear transformation.
(ii) A random coincidence (also called an accidental or chance coincidence) occurs when emissions from different nuclear transformations interact nearly simultaneously with the detectors.
(iii) A scatter coincidence occurs when one or both of the photons from a single annihilation are scattered, and both are detected.
c. Detection of interactions: Scintillation crystals optically coupled to photomultiplier tubes (PMTs) or other light detectors are used. The signals from the PMTs or other light detectors are processed using pulse mode to create signals identifying the position in the detector, deposited energy, and time of each interaction. The energy signal is used for energy discrimination to reduce mispositioned events due to scattering and the time signal is used for coincidence detection. Original PET detector designs had one scintillator coupled to one PMT. However, due to cost and limitations on PMT size, current systems use a block of scintillation crystals coupled to a smaller number of PMTs (Fig. 19-14). The relative magnitudes of the signals from the PMTs coupled to the detector block are then used to identify the location of the crystal within the detector block that had a photon interaction, as in a scintillation camera. The ideal scintillation detector characteristics include prompt light output (short decay constant), high linear attenuation coefficient (stopping power), high conversion efficiency, and high energy resolution to discriminate against scattered events. Originally, BGO used to be the dominant scintillation crystal material in PET scanners. Recently, LSO (LYSO) has become the dominant crystal material.
d. Timing of interactions and detection of coincidences: The timing of the interaction of an annihilation photon with the scintillation detector is determined from the leading edge of the electrical signal from the PMTs. When the time signals from two detectors occur within a selected time interval called the time window, a coincidence is recorded. A typical time window for a system with BGO detectors is 12 ns. A typical time window for a system with LSO detectors, which emit light more promptly, is 4.5 ns.
e. True versus random coincidences: Randoms rate is defined by: Rrandom = τS1S2, where τ is the coincidence time window and S1 and S2 are the actual count rates of the detectors, often called singles rates. Randoms decrease linearly with decreasing timing window and increase proportionally with the square of the activity in the patient. This is contrasted with an approximate proportional increase in the true coincident rate.
f. Scatter coincidences: Scatter coincidences are dependent upon the amount of scattering material and thus occur less in head than in body imaging. Scatter coincidences in PET imaging can be very high (60% to 70% of the acquired coincidence data), particularly in larger cross-sections of the body and when data are acquired in three dimensions. The use of narrower energy discrimination window can reduce the number of scattered events but will also negatively affect the number of recorded true coincidences.
g. Two-dimensional and three-Dimensional data acquisition: PET coincidence data can be acquired in two-dimensional (slice) or three-dimensional (volume) mode (Fig. 19-15). In slice mode, coincidences are detected and recorded only within each ring of detector elements or within a few adjacent rings of detector elements. Septa (made from tungsten) prevents coincidences outside an axial slice from reaching the detector ring of that slice hence limiting the acquisition to two-dimensional mode. In volume mode, the absence of the septa allows coincidences to be recorded from the whole detector volume. In volume mode, the total number of recorded events is substantially increased (increase in scanner detection efficiency), but so is the number of recorded scattered and random events. In volume mode, the scanner sensitivity increases linearly from the edges of the scanner to the center of the scanner (Fig. 19-16). This variation in scanner sensitivity requires overlapping of bed positions in situations where patient imaging extends beyond the physical axial length of the scanner. Overlapping of bed position reduces the effect of this variability or the resultant images. Some PET systems are capable of acquiring data in two or three-dimensional mode using retractable septa, but most new PET systems can only acquire data in volume mode.
h. Transverse Image reconstruction: Following the acquisition of the LORs, the data are corrected for several confounding factors and reconstructed using iterative or analytical techniques. Image reconstruction in twodimensional mode follows similar approaches to SPECT, while three-dimensional mode requires techniques that capture the volumetric data.
2. Data Correction: Several data corrections are required to ensure that the resultant PET image truly depicts the radiopharmaceutical biodistribution in the body. These corrections are
a. Normalization correction: conducted to adjust for performance variability among the thousands of detectors in the scanner.
b. Randoms correction: conducted to subtract random events from the recorded total number of events. Two approaches are used: randoms assessment from delays or randoms assessment from singles with the latter resulting in a more accurate correction.
c. Scatter correction: conducted to subtract scatter events from the recorded total number of events. Two approaches are used: scatter is estimated from reconstructed PET and attenuation images while employing computer modeling of photon interactions or scatter is estimated from fitting smoothly varying continuous functions to the tails of the projection profile outside the imaged object. The first approach is mainly used on new hybrid PET/CT systems where the CT scan is used as the attenuation map.
d. Deadtime correction: conducted to account for pulse pileup and deadtime effects whereby various factors are applied to correct any deviation of the scanner count rate performance from a linear response with increasing activity concentrations.
e. Decay correction: conducted to correct for radioactivity decay during the imaging time (Eq. 19-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree