Chapter Outline
First Trimester, 584
General, 584
Nasal Bone, 586
First-Trimester Imaging, 586
Normal Pregnancy, 587
Gestational Sac, 587
Threatened Abortion, 588
Ectopic Pregnancy, 589
Multifetal Pregnancy, 591
Second and Third Trimesters, 593
General, 593
Fetal Neural Axis, 595
Face and Neck, 600
Heart, 600
Thorax, 601
Abdomen, 603
Adrenal Gland, 604
Abdominal Wall, 604
Urinary Tract, 606
Hydrops Fetalis, 608
Extremities, 609
Measurements and Growth, 612
Fetomaternal Structures, 615
General, 615
Placenta, 615
Gestational Trophoblastic Disease, 618
Amnion, 619
Umbilical Cord, 620
Uterus and Adnexa, 620
Pelvimetry, 621
Fetal MRI, 622
Differential Diagnosis, 622
First Trimester, 622
Second and Third Trimesters, 623
Fetal Head and Spine, 624
Fetal Chest, 625
Fetal Abdomen, 625
Fetal Extremities, 627
First Trimester
General
Reference
All ages in this section refer to the menstrual age or gestational age (GA) based on the last menstrual period (LMP) and not the embryonic age based on day of conception. A 4-week pregnancy by the LMP method thus corresponds to a 2-week pregnancy by the conception method. All measurements given in this section are for transvaginal sonography (TVS) unless otherwise stated.
Role of Imaging
First Trimester
- 1.
Confirm and date an intrauterine pregnancy (IUP).
- 2.
Determine fetal number and placentation.
- 3.
Evaluation for an ectopic pregnancy.
- 4.
Evaluation of first-trimester bleeding: assess viability
- 5.
Screening for chromosomal abnormalities.
- 6.
Screening for increased risk of preeclampsia
- •
Normal IUP
- •
Abortion: impending, in progress, incomplete, missed
- •
Ectopic pregnancy
- •
Subchorionic hemorrhage
- •
Second Trimester
- 1.
Determine fetal number and viability.
- 2.
Placental evaluation and location
- 3.
Estimate amount of amniotic fluid.
- 4.
Assess GA and growth
- 5.
Fetal survey
- 6.
Evaluate adnexa and cervix
Third Trimester
- 1.
Fetal presentation (vertex, breech) ( Fig. 10.1 )

FIG. 10.1
- 2.
Type of placenta
- 3.
Membranes
- 4.
Cervical os
- 5.
Biophysical profile (BPP), growth
Prenatal Screening
Screening Tests
- •
Several options exist for early prenatal screening with varying detection rates.
- •
Age and 15–18-week serum screen (“triple screen” or “quad screen”)—detection rate 50%–70%
- •
Age and fetal nuchal translucency (NT)—detection rate 70%–80%
- •
Age, NT, fetal nasal bone length (NB), maternal serum beta-human chorionic gonadotropin (β-HCG), and pregnancy-associated plasma protein A (PAPP-A)—detection rate 95%
Alpha-Fetoprotein (AFP) ( Fig. 10.2 )
- •
AFP is formed by the fetal liver, yolk sac, and gut and is found at different concentrations in fetal serum, amniotic fluid, and maternal serum (MSAFP).
- •
In the normal fetus, AFP originates from fetal serum and enters amniotic fluid through fetal urination, fetal gastrointestinal (GI) secretions, and transudation from membranes (amnion and placenta).
- •
Elevated MSAFP levels occur if there is transudation of AFP into the maternal serum, such as in open neural tube defects (NTDs) (AFP screening has an 80%–90% sensitivity for detection) or in fetal swallowing problems (abdominal wall defect).
- •
MSAFP is best measured at 16 weeks.
- •
False-positive causes of elevated AFP include:
GA 2 weeks ≥ to that estimated clinically
Multiple gestations
Fetal death
- •
In cases of elevated AFP, test for acetylcholinesterase in amniotic fluid, which is present in NTDs.
- •

Beta-Human Chorionic Gonadotropin
Normal β-HCG levels correlate with the size of gestational sac until 8th–10th weeks. Thereafter, β-HCG levels decline. Initially, the doubling time for the β-HCG is 2–3 days. Third International Reference Preparation = 1.84 × Second International Reference Preparation.
| β-HCG (mIU/mL) a | US | Outcome |
|---|---|---|
| <1000 | Gestational sac present | Abortion likely |
| <1000 | Gestational sac absent | Not diagnostic, repeat |
| >1000–2000 | Gestational sac present | Normal pregnancy |
| >2000 | Gestational sac absent | Ectopic pregnancy likely |
a Second International Standard. As a general rule, values measured according to the Second International Standard are equivalent to half that of the Third International Standard (e.g., 500 mIU/mL [2IS] = 1000 mIU/mL [3IS]).
| Risk Category | AFP | β-HCG | Estriol | Inhibin A |
|---|---|---|---|---|
| NTD/abdominal wall defect | Increased | Normal | Normal | Normal |
| Trisomy 21 | Decreased | Increased | Decreased | Increased |
| Trisomy 18 | Decreased | Decreased | Decreased | Normal |
- •
Invasive diagnostic tests: offered in the context of a positive first- or second-trimester screen, major fetal anomalies, or the presence of multiple soft markers of chromosomal abnormality.
- •
Chorionic villus sampling
- •
Amniocentesis
- •
Amniocentesis
Performed at 15–16 weeks using a ultrasound (US)-guided transabdominal approach. Desquamated cells of amniotic fluid are cultured and then karyotyped. In twin pregnancies, indigo carmine is injected into the amniotic cavity, punctured first to ensure sampling of both cavities. The main complication is fetal loss (1%).
Chorionic Villus Sampling (CVS)
- •
Performed earlier than amniocentesis: 11–12 weeks
- •
Transcervical or transabdominal approach under US guidance
- •
Risk of fetal loss is 1%
- •
NT Measurement
- •
Can be measured between 11+0 and 13+6 GA
- •
Measured from inner margin to inner margin
- •
Neck must be in neutral position
- •
Image must be midline (hard palate, nasal bone and hypoechoic diencephalon in view, no zygoma in view)
- •
Fetal head and upper thorax must occupy entire field of view
- •
If nuchal cord is present, mean of measurements above and below are taken
- •
Measurement is used to assign a likelihood ratio (LR) for chromosomal abnormalities (in conjunction with the other aspects of the first-trimester screen). The higher the measurement, the higher the LR and vice versa.
- •
Higher values are associated with:
Chromosomal abnormalities (21, 18, 13), 20%
Cardiac anomalies
Skeletal dysplasia
- •
Nasal Bone
- •
Presence or absence (or length) may be used between 11+0 and 13+6 in conjunction with other components of first-trimester screen to assign LR for chromosomal abnormalities.
- •
Assessed at midline image as for NT
- •
Should be separate from and brighter than the skin surface echo at the nasal tip
- •
Nasal bone absent in 60%–70% of cases of trisomy 21 (T21) and only 2% of normal fetuses at this GA.
- •
First-Trimester Imaging
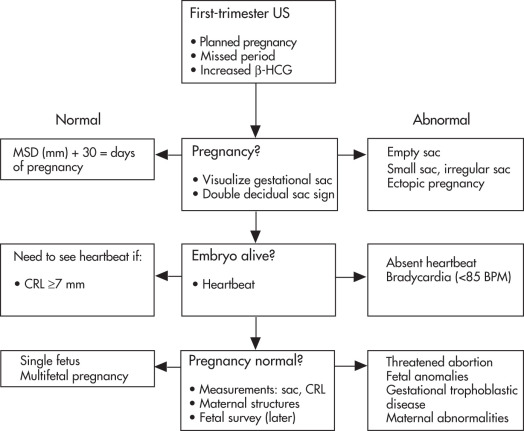
Approach to First-Trimester Sonogram ( Fig. 10.3 )
CRL = Crown-rump length
MSD = Mean sac diameter
Normal Pregnancy
Early Development ( Fig. 10.4 )
- •
Fertilization occurs in fallopian tubes.
- •
Oocyte + sperm cell = zygote.
- •
Cleavage occurs in the fallopian tube.
- •
Morula enters the uterine cavity.
- •
Blastocyst implants into the endometrial wall.
- •
Corpus luteum develops from ruptured graafian follicle (usually <2 cm), secretes progesterone, and induces decidual reaction.
- •
Corpus luteum regresses at 10 weeks, when its function is taken over by the placenta.
- •

Amniotic and Chorionic Membranes ( Fig. 10.5 )
- •
Amniotic cavity is initially small.
- •
Embryo lies within the amniotic cavity.
- •
Amniotic cavity enlarges and ultimately fuses with the chorion (14–16 weeks).
- •
Yolk sac is located outside the amniotic cavity but within the chorionic cavity; it is connected to primitive gut by the omphalomesenteric duct.
- •

Double Decidual Sac Sign ( Fig. 10.6 )
The double decidual sac sign is a useful early sign of IUP. It is based on the demonstration of three layers of different echogenicity:
- •
Decidua parietalis (hyperechoic)
- •
Fluid in the uterine cavity (hypoechoic)
- •
Decidua capsularis (hyperechoic)
- •
The double-bleb sign refers to the presence of an amnion and yolk sac at 5–6 weeks. The embryo lies between these two structures.

Yolk Sac
- •
Provides nutrients to the embryo until placental circulation is established.
- •
Angiogenesis and hematopoiesis occur in the wall of the yolk sac.
- •
Dorsal part is later incorporated into the primitive gut and remains connected by the omphalomesenteric (vitelline) duct.
- •
Fetal Heart
A fetal heartbeat should always be detectable if the CRL is ≥7 mm by TVS. Absence of cardiac activity in embryos >7 mm is indicative of fetal demise.
Pearls
- •
Occasionally, embryonic cardiac activity may be seen before a distinct embryo is visualized.
- •
In patients with threatened abortion, demonstration of cardiac activity is the single most important role of US.
- •
Gestational Sac
Normal Gestational Sac
The gestational sac is the implantation product that occurs in the uterus on approximately day 21. At that time the blastocyst is approximately 0.1 mm in size and cannot be seen by US. Normal sacs become visible when they reach 2–3 mm. Measurements:
MSD = ( Length + Width + Height ) 3
Normal MSD (mm) + 30 = Days of pregnancy
After the gestational sac has developed, a yolk sac, the fetal heartbeat, and the embryo will become visible.
| Age | β-HCG (mIU/mL) | Gestational Sac | Yolk Sac | Heartbeat | Embryo (Fetal Pole) |
|---|---|---|---|---|---|
| 5 wk | 500–1000 | + | – | – | – |
| 5.5 wk | >3600 | + | + | – | – |
| 6 wk | >5400 | + | + | + | – |
| >6 wk | + | + | + | + |
Order of appearance of structures: gestational sac → yolk sac → embryo (fetal pole) → amnion ( Fig. 10.7 )

Correlation of MSD and β-HCG Levels ( Fig. 10.8 )
- •
β-HCG and MSD increase proportionally until the 8th week (25-mm MSD).
- •
β-HCG doubles every 2–3 days.
- •
β-HCG levels decline after 8 weeks.
- •
Normal MSD growth: 1.1 mm/day
- •
Discordance between MSD and β-HCG indicates an increased probability of demise.
- •

Diagnosis of Pregnancy Failure
- •
US findings diagnostic of pregnancy failure:
CRL ≥7 mm and no cardiac activity
MSD ≥25 mm and no embryo
Absence of cardiac activity 14 days following previous US showing gestational sac without yolk sac.
Absence of cardiac activity 11 days following previous US showing gestational sac with yolk sac.
- •
US findings suspicious for, though not diagnostic of, pregnancy failure:
CRL <7 mm and no cardiac activity
MSD 16–24 mm and no embryo
Absence of cardiac activity 7–13 days following previous US showing gestational sac without yolk sac.
Absence of cardiac activity 7–10 days following previous US showing gestational sac with yolk sac.
Morphologic abnormalities
- •
Morphologic abnormalities suspicious for pregnancy failure:
Small gestational sac (MSD – CRL >5 mm)
Irregular shape of gestational sac or embryo
Large yolk sac (>7 mm)
Empty amnion
Threatened Abortion
Threatened abortion is a clinical term encompassing a broad spectrum of disease that occurs in 25% of pregnancies and results in true abortion in 50%. It includes:
- •
Blighted ovum
- •
Ectopic pregnancy
- •
Inevitable abortion
- •
Incomplete abortion
- •
Missed abortion
- •
Signs and symptoms of threatened abortion include bleeding, pain, contractions, and open cervix. If a live embryo is identified, predictors of poor outcome are:
- •
Bradycardia (<85 beats per minute [BPM])
- •
Small gestational sac size (if MSD – CRL <5 mm)
- •
Subchorionic hemorrhage (large, > two-thirds of the circumference)
- •
Large yolk sac (>6 mm)
- •
Irregular, crenated, or calcified yolk sac
- •
Abnormal gestational sac location
- •
Irregular sac shape
- •
Absence or thinning of the decidual reaction surrounding the sac
- •
Terminology of Abortion
Threatened abortion
- •
Vaginal bleeding with closed cervical os during the first 20 weeks of pregnancy
- •
Occurs in 25% of first trimester pregnancies
- •
50% survival
- •
Inevitable abortion
- •
Vaginal bleeding with open cervical os; an abortion in progress
- •
Incomplete abortion
- •
Retained products of conception causing continued bleeding
- •
Spontaneous abortion
- •
Vaginal bleeding, passage of tissue
- •
Most common in first trimester
- •
No US evidence of viable IUP; ectopic pregnancy must be excluded.
- •
High percentage have chromosomal abnormalities.
- •
Missed abortion
- •
Retention of a dead pregnancy for at least 2 months
- •
| Time | Mean (beats per min) |
|---|---|
| 5–6 weeks | 101 |
| 8–9 weeks | 143 |
| >10 weeks | 140 |
The presence of cardiac activity indicates a good but not a 100% chance that a pregnancy will progress to term. There is still a 20% chance of pregnancy loss during the first 8 weeks even if a positive heartbeat is present. During the 9th–12th weeks, the chance of fetal loss decreases to 1%–2% in the presence of a positive heartbeat.
Subchorionic Hemorrhage
Venous bleeding causing marginal abruption with separation of the chorion from the endometrial lining extending to the margin of the placenta. Usually (80%) occurs in the late first trimester and presents as vaginal bleeding. Prognosis: generally good if there is a fetal heartbeat and bleeding is minimal. Hemorrhage greater than two-thirds of the chorionic sac circumference is associated with more than a two-fold increase in risk of pregnancy loss.
| Week | Bleeding (%) | No Bleeding (%) |
|---|---|---|
| <6 | 35 | 20 |
| 7–8 | 20 | 5 |
| 9–11 | 5 | 1-2 |
US Features ( Fig. 10.9 )
- •
Associated with marginal separation of placenta
- •
Hypoechoic or hyperechoic blood separates chorion from endometrium
- •
Distinguish from retroplacental bleed and abruption by location and extent of placental involvement

Ectopic Pregnancy
General
Location ( Fig. 10.10 )
- •
Tubal, 97%
Ampullary (most common)
Isthmus
- •
- •
Interstitial (cornual), 3%
- •
Ovarian, 1%
- •
Cervical (very rare)
- •
Fimbria (very rare)

Clinical Findings
Incidence: 0.5%–1% of all pregnancies. Triad:
- •
Pain, 95%
- •
Hemorrhage, 85%
- •
Palpable adnexal mass, 40%
- •
Risk Factors
- •
Previous ectopic pregnancy
- •
History of pelvic inflammatory disease (PID)
- •
Tubal surgery or other tubal abnormalities
- •
Endometriosis
- •
Previous pelvic surgery
- •
Infertility and infertility treatments
- •
Uterotubal anomalies
- •
History of in utero exposure to diethylstilbestrol (DES)
- •
Cigarette smoking
- •
Intrauterine device (IUD) is not a risk factor but has been associated with ectopic pregnancy because IUD prevents IUP but not ectopic pregnancy.
- •
Diagnosis
Diagnostic Tests
Serum markers
- •
Normal β-HCG doubling time depends on the age of the pregnancy but on average is 2 days.
- •
Ectopic pregnancies usually have a slower increase in β-HCG than normal pregnancies.
- •
Low levels of β-HCG suggest ectopic pregnancy.
- •
Reduced levels of progesterone P4 suggest ectopic pregnancy.
- •
Culdocentesis
- •
Considered to be positive if >5 mL of nonclotted blood is aspirated; clotted blood indicates that a vessel has been entered; dry tap is nondiagnostic.
- •
Culdocentesis is preferred to detect ectopic pregnancy of <6 weeks; at later time points, US is preferred.
- •
US
- •
Always use TVS. If negative, also perform transabdominal ultrasound (TAS).
- •
Doppler US can be used to detect peritrophoblastic flow.
- •
US Features
Uterus
- •
May be normal
- •
Thick decidual cast with no gestational sac ( Fig. 10.11 )

FIG. 10.11
- •
Pseudogestational sac ( Fig. 10.12 )

FIG. 10.12
- •
Fills endometrial cavity symmetrically
- •
May be large
- •
No double decidual sign but rather a single rim of echoes around pseudogestational sac
- •
Extrauterine structures ( Fig. 10.13 )
- •
Free fluid in cul-de-sac (bleeding); may be anechoic or echogenic
- •
Simple adnexal cyst (10% chance of pregnancy)
- •
Complex adnexal mass (95% chance of ectopic)
- •
Tubal ring sign (95% chance of ectopic): echogenic rim surrounding an unruptured ectopic pregnancy
- •
Live embryo outside uterus; 100% specific but only seen in 25%

FIG. 10.13
- •
Pearls
- •
A normal TVS does not exclude an ectopic pregnancy.
- •
Accepted terminology for positive β-HCG without visualization at US is pregnancy of unknown location
- •
A normal IUP virtually excludes the presence of an ectopic pregnancy. The likelihood of a coexistent ectopic pregnancy is 1 : 7000 in pregnancies with risk factors or 1 : 30,000 in pregnancies with no risk factor.
- •
Heterotopic pregnancy: Presence of an intrauterine and ectopic pregnancy. Associated with assisted reproduction, PID.
- •
Cornual ectopic pregnancy
Symptoms occur later than with ectopic pregnancies in other locations.
Hemorrhage is more severe (uterus more hypervascular, erosion of uterine artery).
Higher morbidity and mortality. Look for complete rim of myometrium around gestational sac.
<5 mm myometrium between sac and external uterine margin
Interstitial line sign: hyperechoic endometrial line abuts but does not surround sac
- •
Cervical ectopic pregnancy
Requires evacuation
- •
Treatment
- •
Surgery if ruptured or >2.5 cm
- •
Methotrexate
- •
Direct instillation of potassium chloride
- •
If dilatation and curettage planned, uterine artery embolization may be helpful to reduce the risk of bleeding.
- •
| Class | Finding | Likelihood of Ectopic Pregnancy (%) |
|---|---|---|
| 1 | Normal IUP | Virtually none a |
| 2 | Normal or single ovarian cysts | 5 |
| 3 | Complex adnexal mass, free pelvic fluid, tubal ring | 95 |
| 4 | Live extrauterine embryo | 100 |
a Likelihood of a coexistent ectopic pregnancy is 1 in 7000 pregnancies; more common with ovulatory induction and in vitro fertilization.
Multifetal Pregnancy
General
Incidence: 1% of live births.
Types
Dizygotic twins (fraternal), 70%
- •
Independent fertilization of two ova
- •
Always dichorionic, diamniotic; each ovum has its own placenta and amnion. Overall, 80% of twins are dichorionic, diamniotic.
- •
Risk factors:
Advanced maternal age
Family history of twins
Ethnicity (e.g., Nigerian)
- •
Monozygotic twins (identical), 30%
- •
Duplication of single fertilized ovum
- •
May be monochorionic or dichorionic
- •
Independent of maternal age, heredity, and race
- •
Placental Unit ( Fig. 10.14 )
- •
Amnionicity: number of amniotic sacs
- •
Chorionicity: number of placentas
- •
Dizygotic twins are always diamniotic, dichorionic (i.e., have two sacs and two placentas). The two placentas may fuse but do not have vascular connections.
- •
Monozygotic twins have different amnionicity and chorionicity depending on the stage of cleavage of the single fertilized ovum.
- •
The amnionicity/chorionicity determines the risk of complications:
Monoamniotic: > monochorionic, diamniotic > dichorionic
Monoamniotic: cord entanglement
Monochorionic: twin-twin transfusion syndrome, twin anemia–polycythemia sequence
- •

US Imaging
Approach
- 1.
Define the presence and number of twins.
- 2.
Determine amnionicity and chorionicity.
- 3.
Growth estimation: determine the fetal weight for each twin.
- 4.
Are there complications or anomalies?
US Features
Findings definitely indicating dichorionicity:
- •
Separate placentas
- •
Different fetal sex
- •
Thick (≥2 mm) membrane separating twins in first trimester
- •
Lambda sign: chorion extending into intertwin membrane
- •
Findings indicative of diamnionicity:
- •
Thin membrane in first trimester
- •
Two yolk sacs
- •
In the second trimester, the sensitivity for finding an amnion is only 30%. In 70% of cases, an amnion is present but not visible.
Pearls
- •
Dichorionicity is easiest to establish in first trimester
- •
Different genders of fetuses always indicates dichorionicity
- •
Failure to identify a separating amnion is not a reliable sign to diagnose monoamnionicity.
- •
Twin peak sign
- •
Complications
Overview of Complications in Twin Pregnancies ( Fig. 10.15 )
All twins
- •
Increased incidence of premature labor
- •
Fetal mortality three times higher than for single pregnancy
- •
Neonatal mortality seven times higher than that for single pregnancy
- •
Dichorionic, diamniotic twins
- •
Perinatal mortality, 10%
- •
Monochorionic, diamniotic (MD) twins
- •
Perinatal mortality, 20%
- •
Twin-twin transfusion
- •
Twin anemia–polycythemia sequence
- •
Acardia
- •
Demise of cotwin
- •
Twin embolization syndrome
- •
Structural abnormalities
- •
Monochorionic, monoamniotic (MM) twins
- •
Perinatal mortality, 50%
- •
Entangled cords
- •
Conjoined twins
- •
All the MD complications as well
- •

Twin-Twin Transfusion Syndrome ( Fig. 10.16 )
Only occurs in monochorionic twins (25%). Results from arteriovenous communications in placenta. Very poor prognosis.

US Features
Recipient twin
- •
Large twin (increased estimated fetal weight [EFW])
- •
Polyhydramnios
- •
Polycythemia
- •
Fetal hydrops
- •
Donor twin (pump twin)
- •
Small twin pinned to side of gestational sac (decreased EFW) “stuck twin”
- •
Oligohydramnios
- •
Conditions Associated With Demise of a Twin
Vanishing Twin (“Blighted Twin”)
Demise of a twin in the early first trimester (<15 weeks) and subsequent resorption of the dead fetus. Risk to the surviving twin is minimal, especially if dichorionic.
Fetus Papyraceus
Demise of a twin in the second or third trimester and persistence of the dead fetus as an amorphous mass or flattened structure along the uterine margin. Complications: premature labor, obstruction at labor, embolization.
Twin-Twin Embolization Syndrome
Occurs only in monochorionic twins because they share a common placenta. Demise of one twin leads to passage of thromboplastic material into the circulation of the live twin. Results in thrombosis and multiorgan failure in the live twin and maternal disseminated intravascular coagulation (DIC).
Acardiac Parabiotic Twin
- •
Twin reversal arterial perfusion sequence (TRAPS)
- •
Most extreme manifestation of twin transfusion syndrome
- •
Occurs in monochorionic pregnancy
- •
Reversal of flow in umbilical artery (UA) of the acardiac twin with blood entering via the vein and leaving via the artery
- •
Poor development of acardiac twin above thorax
- •
Fetal Structural Abnormalities
All fetal structural abnormalities occur with higher frequency in twins (monozygotic > dizygotic). Most defects are not concordant and occur in only one twin. Some abnormalities are secondary to in utero crowding.
Conjoined Twins
Only occurs in MM twins. 75% are females. Prognosis is related to degree of joining and associated anomalies:
- •
Thoracopagus (most common, 70%): thorax is fused
- •
Omphalopagus, xiphopagus: anterior abdomen is fused
- •
Pygopagus: sacrococcygeal fusion
- •
Craniopagus: cranium is fused
- •
Ectopic Twin Pregnancy
There may be an increase in this condition because of more widespread use of ovulation induction and in vitro fertilization techniques. Incidence: 1 in 7000; consider if the patient has previously mentioned risk factors.
Second and Third Trimesters
General
Some pathologic entities in this section are described in more detail in Chapter 11 .
Pearls
- •
A normal cavum septum pellucidum, ventricular atrium (<10 mm), and cisterna magna (2–10 mm) virtually exclude all neural axis abnormalities.
- •
The two most common forms of NTDs are:
Anencephaly (missing cranial vault)
Myelomeningocele (most are associated with Chiari malformation). A normal cisterna magna excludes nearly all cases of myelomeningocele.
- •
Risk reduction of NTDs with supplemental maternal folic acid intake.
- •
Always obtain a four-chamber view and two views of cardiac outflow tracts (left ventricle [LV] → aorta, right ventricle [RV] → pulmonary artery [PA]).
- •
In the four-chamber view, chamber closest to anterior wall is RV.
- •
Bladder and stomach should be visualized by 14 weeks. If they are not seen, rescan the patient in 2 hours: bladder should fill within this time frame. Doppler shows iliac arteries “splayed” by bladder.
- •
Any structure or mass that touches the fetal spine most likely originates from the genitourinary (GU) tract.
- •
Fetal Neural Axis
Anatomy
Normal Central Nervous System (CNS) Structures ( Fig. 10.17 )
- •
Ventricles: <10 mm at atrium
Choroid plexus in atria of lateral ventricles should occupy 60%–90% of atrium. It is easier to see the far field atrium as reverberation artifacts that may obscure near field atrium.
Choroid absent in anterior or occipital horns
- •
Cisterna magna: 4–10 mm
- •
Thalami are in midline
- •
Cavum septum pellucidum
- •


Signal Intensities ( Fig. 10.18 )
Hyperechoic structures
- •
Choroid plexus
- •
Pia-arachnoid
- •
Dura
- •
Cerebellar vermis
- •
Specular reflections from ventricles
- •
Hypoechoic structures
- •
Brain white matter (WM)
- •
Cerebrospinal fluid (CSF)
- •

Spine ( Fig. 10.19 )
- •
Three hyperechoic ossification centers
Posterior ossification centers: two neural arches
Anterior ossification center: vertebral body
- •
Spinal cord is hypoechoic
- •

Holoprosencephaly
Failure of midline cleavage of the forebrain:
- •
Alobar form: no cleavage
- •
Semilobar form: partial cleavage
- •
Lobar form: almost complete cleavage
- •
Syntelencephaly or middle interhemispheric variant
- •
US Features ( Fig. 10.20 )
Alobar holoprosencephaly
- •
Monoventricle communicates with dorsal cyst
- •
Thin anterior mantle of brain tissue: “horseshoe” or “boomerang”
- •
Fused thalami
- •
No falx, corpus callosum, or septum pellucidum
- •
No brain tissue around dorsal cyst
- •
Semilobar holoprosencephaly
- •
Monoventricle with rudimentary occipital horns
- •
Posterior brain tissue is present (no dorsal cyst).
- •
Fused thalami
- •
Partial falx posteriorly
- •
No corpus callosum or septum pellucidum
- •
Lobar holoprosencephaly
- •
Similar to semilobar, though more formed falx and greater midline hemispheric division
- •
Very difficult to detect prenatally
- •
Syntelencephaly or middle interhemispheric variant
- •
Mild form with abnormal midline connection of cortex anterior to formed falx
- •
Not detectable prenatally
- •
All severe types have:
- •
Absent septum pellucidum and corpus callosum
- •
Thalamic fusion
- •
Associated midline facial anomalies: clefts, cyclopia, hypotelorism
- •

Pearls
- •
Identification of septum pellucidum essentially excludes holoprosencephaly.
- •
Fused thalami exclude severe hydrocephalus.
- •
Detectable cerebral mantle (horseshoe) excludes hydranencephaly.
- •
Look for midline facial abnormalities (clefts, hypotelorism, cyclopia, proboscis).
- •
Associated with trisomy 13
- •
Agenesis of Corpus Callosum (ACC)
The normal development of the corpus callosum begins anterior (genu) and progresses to posterior (splenium). Agenesis may be partial (affects dysgenesis posterior aspects) or complete.
US Features
- •
The corpus callosum is not visible in complete agenesis.
- •
Colpocephaly
- •
Lateral ventricles are displaced laterally (parallel lateral ventricles).
- •
Enlarged third ventricle expands superiorly (high riding third ventricle).
- •
Angulated frontal horns (coronal view)
- •
Abnormal (sunburst) gyral pattern in interhemispheric fissure is a late feature.
- •
The presence of a cavum septum pellucidum excludes complete ACC.
- •
Common associations include:
Dandy-Walker (DW) syndrome
Holoprosencephaly
Heterotopias
Chiari malformations
Trisomy 18 (T18)
- •
TVS scanning is often helpful for early diagnosis.
- •
Associated with pericallosal lipoma (hyperechoic)
- •
Hydranencephaly
- •
Near-total absence of cerebrum with intact cranial vault, thalamus, and brainstem
- •
Secondary to occlusion of internal carotid arteries (ICAs)
- •
Porencephaly
- •
Less severe vascular insult than in hydranencephaly
- •
Cystic lesions are often in free communication with the ventricle and/or subarachnoid cisterns.
- •
Ventriculomegaly ( Fig. 10.21 )
Refers to dilated (>10 mm) ventricles (measured at level of atrium). Associated with other anomalies in 75% of cases. Types:
- •
Hydrocephalus (noncommunicating, obstructive > communicating)
- •
Brain atrophy (enough brain tissue developed but it regresses later)
- •
Colpocephaly (not enough brain tissue developed)
- •

Hydrocephalus is the most common CNS abnormality. Causes include:
Obstructive (common)
- •
Spina bifida is the most common cause of hydrocephalus.
- •
Aqueductal stenosis
- •
DW syndrome
- •
Encephalocele
- •
Arnold-Chiari malformation
- •
Nonobstructive (uncommon)
- •
Hemorrhage
- •
Infection: cytomegalovirus (CMV), Toxoplasma (calcification)
- •
Chromosomal abnormality (T21)
- •
US Features
- •
Enlarged ventricles (>10 mm)
- •
Dangling choroid plexus in the lateral ventricle
- •
The presence of colpocephaly should prompt search for possible callosal agenesis.
- •
Cystic Structures
Cystic Teratoma
- •
Most common congenital intracranial tumor
- •
Solid and cystic components
- •
Choroid Plexus Cysts (CPCs)
- •
Very common between 12 and 24 weeks (second trimester); most resolve by third trimester
- •
Usually multilocular, 5–20 mm; may be bilateral
- •
Look for other markers of T18.
- •
If other markers are present, CPCs increase risk of T18 (consider amniocentesis).
- •
If no other markers present, no further action necessary in normal risk patient.
- •
Arachnoid Cysts
- •
Cystic space within the pia-arachnoid has a ball-valve communication with the subarachnoid space.
- •
Congenital or acquired (after hemorrhage, infection)
- •
No communication with ventricle.
- •
Must differentiate from cystic teratoma, porencephaly, and arteriovenous malformation (AVM)
- •
Hemorrhage
Similar imaging features and classification (Papile grades 1–4) to germinal matrix hemorrhage in fetuses born prematurely but different causes. In utero hemorrhage is very common. Causes:
- •
Maternal hypertension (HTN), eclampsia
- •
Isoimmune thrombocytopenia
- •
Maternal hemorrhage
- •
Nonimmune hydrops
- •
DW Syndrome ( Fig. 10.22 )
Abnormal development of posterior fossa structures characterized by:
- •
Posterior fossa cyst that communicates with the fourth ventricle
- •
Hypoplasia of the cerebellar vermis
- •
Variable hydrocephalus
- •
Variant form does not have enlargement of posterior fossa.
- •

US Features
- •
Posterior fossa cyst separates the cerebellar hemispheres and connects to the fourth ventricle.
- •
Absence or hypoplasia of vermis
- •
Other associations
Hydrocephalus
ACC
Congenital heart disease (CHD)
- •
Large Cisterna Magna ( Fig. 10.23 )
Diagnosis of exclusion; must exclude DW complex.


US Features
- •
Anteroposterior (AP) diameter >10 mm
- •
No communication with fourth ventricle
- •
Neural Tube Defect (NTD)
Incidence: 1 : 600 births in the United States. Increased risk (3%) in parents with previous NTD child. Screening: amniotic fluid and MSAFP are increased because of transudation of fetal serum AFP across the NTD. Spectrum of disease:
- •
Anencephaly (most common)
- •
Spina bifida and meningomyelocele
- •
Face and orbits usually intact
- •
Encephalocele (least common)
- •
Anencephaly ( Fig. 10.24 )
- •
Complete absence of cranial vault (acrania) and cerebral hemispheres; should be symmetric. Asymmetric absence should raise the suspicion of amniotic band syndrome (ABS).
- •
Angiomatous tissue covers base of the skull
- •
Some functioning neural tissue is nearly always present.
- •
Polyhydramnios, 50%
- •
Should not be diagnosed before 14 weeks of age (skull is not ossified)
- •

Encephalocele ( Fig. 10.25 )
Herniation of intracranial structures through a cranial defect. Cephalocele = meninges; encephalocele = brain and meninges. Most defects are covered by skin, and MSAFP levels thus are normal. Location: occipital, 70%; frontal, 10%. Lesions are typically midline. Asymmetric lesions should raise the suspicion of ABS. Prognosis depends on the amount of herniated brain. Mortality, 50%; intellectual impairment, 50%–90%.

Associations
- •
Other intracranial anomalies
- •
ABS
- •
Meckel-Gruber syndrome
- •
US Features
- •
Extracranial mass lesion (sac)
- •
The sac may contain solid (brain tissue), cystic (CSF space), or both components; absence of brain tissue in the sac is a favorable prognostic indicator.
- •
Bony defect
- •
Lemon sign (skull deformity)
- •
Spina Bifida and Myelomeningocele ( Fig. 10.26 )
Location: lumbosacral > thoracic, cervical spine. MSAFP is elevated unless the myelomeningocele is covered with skin. Incidence: 0.1% of pregnancies.
Associations (as a result of imbalanced muscular activity):
- •
Clubfoot
- •
Hip dislocations
- •