Although significant progress has been made in the diagnosis and treatment of CCA in the last two decades, mortality continues to rise in low-prevalence areas of the United States, Europe, United Kingdom, and the non-high-risk areas of Asia and the Middle East [29, 30]. This chapter describes the incidence, risk factors, and mortality associated with cholangiocarcinoma.
1.1 Incidence and Mortality Trends: United States of America
In the US for the year 2012, there were 2,580 cases of ICC and 7,410 ‘other biliary,’ mostly ECC, cancers reported, amounting to approximately 3 % of total gastrointestinal malignancies using the US SEER program. SEER cancer statistics predict national incidence rates for cancer based on aggregate information from the North American Association of Cancer Registries (NAACR), which represents approximately 95 % of the US population. In 2013, it is estimated that there will be a total of 30,640 cases of ICC and primary liver cancer [3, 4]. The SEER Cancer Statistics Review (1975–2009) covers 18 designated areas and approximately 26 % of the US population, and reported a total age-adjusted incidence for ICC as 0.7 per 100,000 males and 0.6 per 100,000 females for 2005–2009. These latest incidence figures reflect a 1.5 % annual percentage change (APC) decline in males and a 0.6 % APC decrease in females between 2000 and 2009 [3, 4]. The overall trend for all US race and ethnicities (white, black, Hispanics, Native Americans, and Asian-Pacific Islanders) shows an increase in ICC from initial data collected in 1973–1975 (Table 1a and b). The age-adjusted incidence rates are highest in Hispanic Americans (1.22 per 100,000) and lowest in African-American males and females (0.3 per 100,000) [32].
Table 1
SEER incidence, US mortality and survival percent for all and selected races in (a) ICC, and (b) ECC
Incidence | Mortality | Survival (%) | |
|---|---|---|---|
(a) SEER incidence, US mortality and survival for ICC, 2005–2009 | |||
All races, total | 0.6 | 1.2 | 6.6 |
All races, male | 0.7 | 1.4 | 6.0 |
All race, female | 0.6 | 1.1 | 7.1 |
Whites, total | 0.6 | 1.2 | 6.4 |
Whites, male | 0.7 | 1.4 | 6.4 |
Whites, female | 0.5 | 1.1 | 6.3 |
Blacks, total | 0.5 | 1.1 | 7.9 |
Blacks, male | 0.5 | 1.3 | 0.0 |
Blacks, female | 0.5 | 1.0 | 9.7 |
(b) SEER incidence, US mortality and survival for ECC, 2005–2009 | |||
All races, total | 1.8 | 0.4 | 15.7 |
All races, male | 2.2 | 0.5 | 16.9 |
All race, female | 1.5 | 0.4 | 14.4 |
Whites, total | 1.8 | 0.5 | 15.6 |
Whites, male | 2.2 | 0.5 | 17.1 |
Whites, female | 1.5 | 0.4 | 14.4 |
Blacks, total | 1.7 | 0.4 | 13.3 |
Blacks, male | 2.1 | 0.4 | 14.1 |
Blacks, female | 1.5 | 0.3 | 12.7 |
Mortality rates have increased across all racial and ethnic groups by at least 3.5 % annual percentage per year, except in Asian/Pacific Islander women in whom mortality rates have decreased at a rate of 0.2 % per year [32]. The age-specific distribution of ICC and ECC peaks between the ages of 65–84 years and is relatively uncommon below age 45 and above age 85 [3, 4, 32]. The SEER-reported incidence of ECC, reported as ‘other biliary cancers,’ incorporates other non-epithelial tumors, though a similar age distribution and male preponderance for ICC is seen. Using cumulative SEER data from 13 sites registered from 1992 to 2009, there has been a 3.5 % APC increase in incidence of primary liver cancer and ICC. Recent United States data corroborate a continued trend of an increasing incidence of ICC from 0.13 per 100,000 in 1973 to 0.67 per 100,000 in 1997 [31].
In a review of the 30-year trend in SEER mortality since 1973, Nathan et al. demonstrated improved survival for both ICC and ECC. Five-year survival for resected ICC in the period 1973–1992 when compared to 1993–2002 demonstrated a significant improvement to 22.9 % from 16.5 % [29]. Their model reported yearly increased survival from 1992 onwards, resulting in a 34.4 % improvement through all three decades. Improved survival was more marked in ECC where multivariate modeling demonstrated a 23.3 % increase in adjusted survival per decade and a cumulative improved survival of 53.7 % from 1973 to 2002 [29].
Mortality from ECC is also steadily increasing from 0.07 per 100,000 in 1973 to 0.69 per 100,000 in 1997. The incidence of ECC has been otherwise static to slightly decreased in the last few decades. This may be a result of discrepant coding, or the impact of a decreasing incidence of gallbladder cancer [17]. The trend of increasing incidence of ICC and decreasing to static incidence of ECC is preserved even when making allowances for the differential coding that place Klatskin tumors with ICC with ICD-O-2 (1995–2001) and to either ICC or ECC with ICD-O-3 [15, 17, 18, 33].
Within a descriptive study of biliary tract cancers (gallbladder, extrahepatic, ampulla of Vater) from 1997 to 2002, cancers from 11,261 men and 15,722 women were identified from network or US population-based registries and normalized to the US census population in 2000 [7]. Populations of Native Alaskan/Native American and Asian-Pacific Islanders showed significantly higher rates of ECC compared with whites or blacks. Incidence rates for ECC were higher overall in men than women. Incidence rates were comparatively lower in all women with the highest rates in Native Alaskan/Native American and Asian-Pacific Islanders (Table 2).
Table 2
Incidence rates (per 100,00) of extrahepatic bile duct cancers, including ECC in the United States, 1997–2002 age-standardized to the US 2000 standard population
Age-standardized incidence rates of extrahepatic bile duct cancers, United States, 1997–2002 | ||
|---|---|---|
Male | Female | |
All races/ethnicities | 0.93 | 0.61 |
American Indian/Alaska Native | 0.90 | 0.76 |
White | 0.91 | 0.60 |
Black | 0.82 | 0.55 |
Asian-Pacific Islander | 1.50 | 0.92 |
Hispanic | 1.14 | 0.87 |
Non-hispanic | 0.92 | 0.60 |
Age-specific incidence rates in men showed a steady increase in incidence beginning in the early 20s during which the incidence curves for Asian-Pacific Islanders diverged and increased at a dramatically different rate than white or black males after age 50 [7]. Similar divergence was seen with Native Alaskan/Native Americans with the significant divergence occurring after age 75. A recent longitudinal view (1973–2007) of biliary tract cancers (ICC, ECC, and ampulla of Vater) in Native Alaskans established a rate of 2.6 per 100,000 compared to 1.2 per 100,000 and 1.0 per 100,000 in the US white and black populations, respectively, using the age-adjusted rates to the world standard million population. Remarkably, an increase in the rate of biliary tract cancer in Native Alaskan women from 0.9 per 100,000 (1973–1992) to 2.6 per 100,000 (1993-2007), without a similar increase in the males for the same interval periods (3.5 per 100,000 and 3.4 per 100,000), was noted [34].
Various regional US populations comprised of non-specific risk groups, such as Olmsted County, Minnesota, reported trend results that are consistent with US national incidence and mortality data for biliary tract cancers and CCA. Over the study period of 1976–2008, the age-sex-adjusted incidence of ICC in 116 patients increased from 0.3 to 2.1 per 100,000 person-years with the increased incidence in men accounting for the majority of the change [35]. During this time period, no overall increase in biliary tract cancer was seen, which was ascribed to concomitant decreases in gallbladder cancers, in women predominantly. Other variations within the US population may reflect socioeconomic status, underlying ethnicity, and other environmental factors. In general, Hispanics have a higher prevalence of hepatobiliary cancers and ICC than whites in the US, with a contrastingly higher predominance in females than males [32]. The prevalence of ICC in Asian-Pacific Islanders is not significantly different from other US population groups when reported for cancer cases 1990–2000 [32].
1.2 Incidence and Mortality Trends: United Kingdom, Europe, and Australia
In the United Kingdom, the Office of National Statistics (ONS) capture cancer data for England and Wales. The ONS noted an increase in the age-adjusted incidence and mortality from primary liver cancer, attributable primarily to a rise in HCC. The age-adjusted incidence for ICC (not histologically verified) was lowest in the period 1971–1973 at 0.11 per 100,000 males and 0.09 per 100,000 females, but steadily increased by 12-fold over the next three decades, to 1.33 per 100,000 and 1.06 per 100,000, respectively [36]. The age-adjusted incidence of ECC (separate from gallbladder cancers) declined between 1971–1973 and 1999–2001 in both males and females, although the rates rose in males in the decade between 1971–1973 and 1981–1983, before and then subsequently declining [36]. The mortality rate from primary liver tumors (including ICC) doubled between 1976 and 1994 [37]. Specific morphologic analysis attributed the rise to the increase in ICC rather than HCC [36, 38].
Age-adjusted mortality rates in England and Wales standardized to the European population increased from <0.02 per 100,000 in 1976 to just over 1 per 100,000 in 1994, accounting for differences in coding and uncertainties in death registration. Deaths from ICC reliably exceeded HCC as the most common cause related to a primary liver tumor in 1993 [38]. Gallbladder tumors and ECC were reported together and showed an overall decrease in age-adjusted mortality from 1974 to 1994. In absolute numbers, the total ECC mortality fell from 413 deaths in 1974 to 176 deaths in 1996. Data from Scotland (1968–1997) are consistent with the rest of the United Kingdom data, with a documented dramatic increase in incidence in ICC of 817 and 851 % in males and females, respectively [39]. The incidence of ICC increased to 1.53 per 100,000 in males and 1.24 per 100,000 in females from 1993 to 1997 compared with 0.12 in males and 0.17 in females (1968–1972). These data reflect small sample size with 9 diagnoses in 1968 and 68 diagnoses in 1997.
In a recent analysis of 12,638 biliary tract cancers, including ECC, diagnosed in England and Wales from 1998 to 2007, the median age at diagnosis was 75 years with an incidence of 2 per 100,000 adjusted to the European standard population [40]. The incidence was relatively static over time and between males and females. A further in-depth analysis of the incidence of ICC and ECC in England and Wales for the period of 1990–2008 reported an increase in age-adjusted incidence of ICC for males from 0.43 to 1.84 per 100,000 and 0.27 to 1.51 per 100,000 in females [33]. The trend in ECC declined to 0.51 from 0.78 per 100,000 in males and to 0.39 per 100,000 in females in the same time period. The noted trends in incidence were maintained in the England and Wales populations after addressing possible discrepancies in the coding of ‘Klatskin’ or ‘hilar’ tumors, by analyzing the data with Klatskin tumors in both the ICC and ECC groups [33] (Fig. 2).
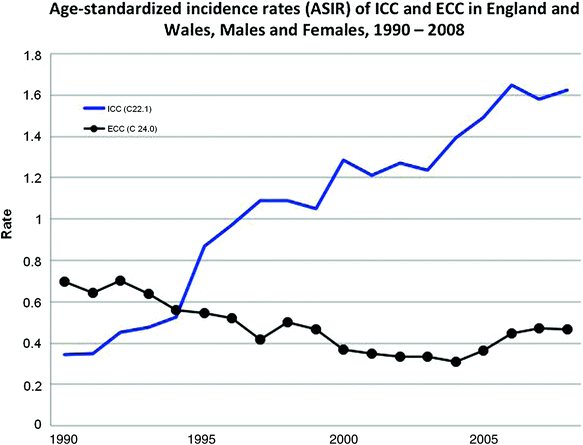

Fig. 2
Age-standardized incidence rates (ASIR) per 100,000 of ICC and ECC in England and Wales, 1990–2008, Males and Females combined. ICC (C22.1), excludes Klatskin tumors (M8162/3). ECC (C24.0), includes Klatskin tumors (M8162.3). Modified and reprinted with permission from Khan et al. 2012 [33]
Comparative incidence reporting in a variety of populations worldwide, using the official WHO-validated mortality database, showed steady age-adjusted increases related to ICC from 1979 to 1997 in the United States, Japan, Australia, England and Wales, and Scotland [41]. The opposite trend was seen for ECC and gallbladder cancers in the same group of nations. Of the countries with complete WHO mortality data that were studied, France displayed no change in age-adjusted mortality for ICC and ECC. Japan and Italy both reported increased mortality for ECC and gallbladder cancers [41] (Fig. 3).
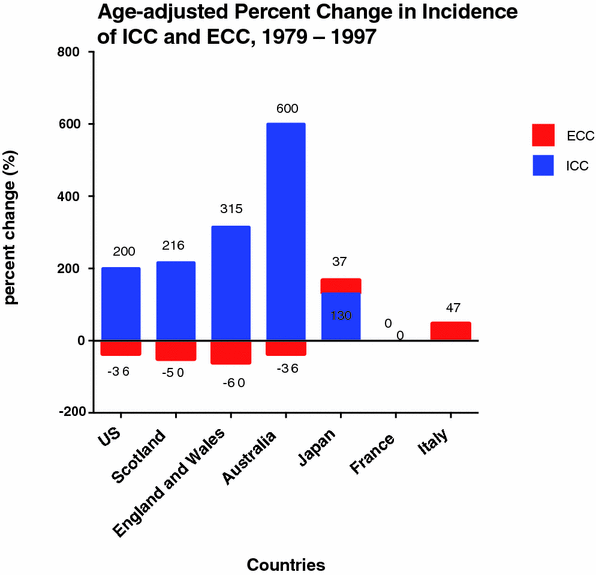

Fig. 3
Age-adjusted percentage change in incidence rate (per 100,000) of ICC and ECC in Select Countries, 1979–1997 [41]. Percent change is shown on y-axis and select countries on the x-axis. Numerical values for absolute percentage change are shown with the corresponding column
Reports of cancer incidence and survival data from Norway, Sweden, Denmark, Iceland, and Finland is made possible through the NORDCAN collaboration network. Primary liver (including ICC) and gallbladder (including ECC) each represent 1–2 % of the cancer burden within these countries [42, 43]. Specific data extracted from the Danish Cancer Registry shows a decrease in age-adjusted incidence of ICC (1.5 to 0.62 per 100,000) and ECC (1.05 to 0.65 per 100,000) from 1978 to 2002 in both men and women. The highest incidence of ICC and ECC was found in diagnosis groups aged 60–79 and aged 80 and older. The overall temporal incidence of ECC was 3.67 per 100,000 and 5.32 per 100,000 in the age division 60–79 and 80 and older, respectively [44] (Fig. 4).
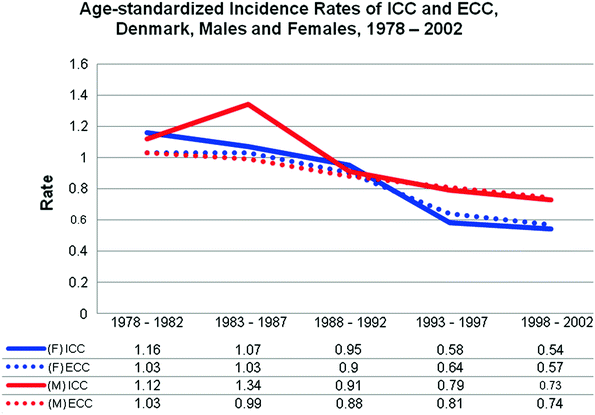

Fig. 4
Age-standardized (US 2000) incidence rates (per 100,000) of intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) for Denmark, Males (M) and Females (F), 1978–2002 [44]. Incidence rate is shown on the y-axis and grouped time intervals in years are shown on the x-axis with the graphed incidence data shown below in tabular format
In the Netherlands, a different trend was seen than that in Scandinavia. ICC diagnoses corresponding to ICD-O codes C22.1 from 1989 to 2009 were analyzed from the Netherlands Cancer Registry (NCR) data [45]. For both males and females, the three-year moving averages showed a 5 % annual percentage decline in age-adjusted incidence from 1989 to 1998 and a subsequent 9.4 % annual percentage increase from 1998 to 2009. Most marked was a 3 % annual percentage increase in ICC incidence within the group aged 45–49 years, where the increases in age-specific incidence are seen in later years in other low-endemic area studies [3, 34, 46]. Epidemiologic data from Italy reflect a trend most consistent with the UK data for 55,000 patients diagnosed in the period 1998–2005. Similarly, a French population-based study from Burgundy reported stable rates of age-adjusted ICC incidence. The adjusted incidence rates for ICC in 1976–1980 and 2001–2005 were 0.3 per 100,000 and 0.2 per 100,000, respectively, and 1.1 per 100,000 in 1976–1980 and 2001–2005 for ECC [47].
Regional-specific cancer incidence and mortality data across Europe are available in the WHO/IARC publications, Cancer Incidence in Five Continents, Vol. IX and Scientific Publication No. 159 [6, 48]. Determining the specific incidence and trend data for ECC and ICC in these aggregated publication is difficult due to the grouping of liver and intrahepatic bile duct tumors, and gallbladder cancers with other biliary tumors. Significant efforts have been made to understand the coding of tumors between ICD-8, 9, and 10 and ICD-O and O-2, in reporting data for ICC and ECC as biliary tract cancers. Notwithstanding this, the data bear out the worldwide trend of increased incidence of ICC and stable to declining incidence of ECC in the low- to normal-risk populations, and select high-risk groups within low-risk populations in North America, Europe, and the United Kingdom, with the exception of Denmark [17, 30, 31, 33, 36, 39, 47, 49].
1.3 Cholangiocarcinoma in Asia and Liver Fluke Infestation
The IARC Working Group on the evaluation of carcinogenic risks to humans formally addressed the association of cholangiocarcinoma to infections with the trematodes, Opisthorchis viverrini, and Clonorchis sinensis [20, 23, 50–52]. The liver fluke Opisthorchis viverrini is endemic to north and northeastern Thailand, western Malaysia, Cambodia, Vietnam, and Laos. Transmission to humans is via consumption of raw fish and water contaminated with sewage and agricultural pollutants [20, 50, 53, 54]. Infection with the trematode, Clonorchis sinensis, is the more common infectious agent related to cholangiocarcinoma in China, Korea, Taiwan, and Japan [50]. Cholangiocarcinoma in the Eastern European nations of Kazakhstan, Russia, Siberia, and Ukraine is likely related to transmission of O. felineus in freshwater fish and polluted water [50, 52]. The evidence supporting the association of Schistosomiasis japonicum with liver cancer and cholangiocarcinoma has been less convincing [20, 55].
The highest national prevalence data for O. viverrini infections are found in Laos (37 %) and Thailand (9.4 %) and for C. sinensis infections in Hong Kong (5.6 %) by most recent estimation [56]. Of the 35 million people infected with C. sinensis worldwide, an estimated 10–15 million live in China [57]. While public health efforts aimed at prevention and treatment are most advanced in these endemic trematode infection areas, the population-at-risk for infection is in excess of 67 million for O. viverrini alone [50]. Indeed, despite educational efforts, biliary tract complications related to liver fluke infection include cholestatic damage, inflammatory lesions, cirrhosis, and cholangiocarcinoma. Carcinogenesis is thought to be related to the chronic inflammation and attempts at cholangiocyte repair incited by trematode infection [58–60]. Cholangiocarcinoma related to food-borne trematodiasis is the most common cause of death. The calculated odds ratio (OR) for developing CCA following C. sinensis infection is 6.1 and with O. viverrini infection is 4.4 [56]. A recent meta-analysis of CCA risk factors in Asian countries calculated an OR of liver fluke infestation (both C. sinensis and O. viverrini) of 4.8 [61].
The trends in primary liver cell cancer, including ICC, are detailed in Table 3 [5, 6]. While these data do not specifically detail the trends in ICC alone in areas of high-risk and low-risk populations and in endemic areas of cholangiocarcinoma, the data reflect the demonstrated trends in ICC seen in population-based studies given a rather static incidence in HCC [5, 6].
Table 3
Age-standardized world (ASRW) incidence rates (per 100,000) showing trends in incidence of primary liver cancer (C22) including intrahepatic cholangiocarcinoma for high-risk, low-risk and endemic population areas
Age-standardized incidence rates in primary liver ICC in high-risk, low-risk and endemic population areas | ||||
|---|---|---|---|---|
1993–1997 | 1998–2002 | Location | ||
Male | Female | Male | Female | |
88.0 | 35.4 | – | – | Thailand (Khon Kaen) |
35.0 | 9.7 | 29.5 | 7.3 | China (Hong/Kong) |
30.7 | 8.4 | 26.8 | 7.3 | Japan (Nagasaki Prefecture) |
59.4 | 17.1 | 49.8 | 14.9 | Korea (Busan) |
4.6 | 2.0 | 4.8 | 2.1 | Russia (St. Petersburg) |
3.8 | 1.4 | 6.2 | 2.2 | USA (White SEER) |
20.7 | 10.4 | 27.5 | 10.2 | USA (Los Angeles, Korean) |
3.1 | 1.7 | 3.7 | 1.7 | UK, England (North Western) |
3.6 | 1.5 | 3.9 | 1.7 | UK, Scotland |
3.7 | 1.0 | 4.3 | 1.4 | Australia (New South Wales) |
3.7 | 1.8 | 4.0 | 1.9 | Denmark |
6.5 | 6.0 | 8.7 | 5.8 | Uganda (Kyadondo County) |
The region of Busan (Pusan) in South Korea continues to have one of the highest areas of primary liver cell cancer worldwide with 1990 incidence rates as high as 74.8 per 100,000 and 15.6 per 100,000 in males and females, respectively, for the age group of 35–64 years [62]. Intrahepatic cholangiocarcinoma is estimated to account for 20 % of total cases. High relative risk (RR) values for C. sinensis infection and alcohol consumption were associated with CCA in this population, whereas hepatitis B and C infections were more strongly associated with HCC. Additionally, case-matched studies from a tertiary referral practice demonstrated a high OR of ICC (OR 16.0) and ECC (OR 7.0) with associated C. sinensis exposure in cases with at least one of six parameters—history of ingesting raw freshwater fish or evidence of C. sinensis infection on stool microscopy, serology, pathology examination, or skin testing [63]. Again, an increased risk of CCA was not seen with concurrent hepatitis B or C infections [63].
The major risk factors for CCA in Asia are male gender, excess alcohol consumption, C. sinensis infection, and consumption of raw fresh water fish based on the report published in 2010 from the Korean Multicenter Cancer Cohort [64]. The study, based on 2000–2004 data, demonstrated that incidence and mortality rates closely aligned with the varying incidence rates of C. sinensis infection in the studied areas. The low and moderate endemic C. sinensis infections areas of Chungju (7.8 %) and Haman (31.3 %) showed age-adjusted incidence rates of 1.8 per 100,000 and 5.5 per 100,000 with corresponding age-adjusted mortality of 1.1 and 2.6 per 100,000, respectively. A further study using the Korean National Cancer Incidence Database (KNCID) from 1995 to 2004 attributes 9.5 % of CCA to liver fluke infection in low- to moderate-risk areas and in up to 22.6 % of CCA cases in high-endemic areas of Korea [65]. Incidence rates in Korean males were more than twice those of females [65]. Incidence rates of 4.1 per 100,000 in males and 1.8 per 100,000 in females were roughly twice those in the United States (1996–2000) and four times those in England and Wales (1991–2001). As outlined in Table 4, the rates of primary liver cancer (HCC and CCA) are decreasing and have been since 1985 [65, 66].
Table 4
Age-standardized incidence rates of intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) for selected regions in Asia, males and females, 1998–2002
Age-standardized incidence rates of intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) for selected regions in Asia, males and females, 1998–2002 | ||||
|---|---|---|---|---|
ICC (C22.1) | ECC (C24) | |||
Male | Female | Male | Female | |
China, Qidong | 10.3 | 4.6 | 0.0 | – |
China, Guangzhou | 0.3 | 0.1 | 1.1 | 0.8 |
China, Hong Kong | 2.3 | 1.7 | 0.3 | 0.2 |
China, Shanghai | 7.4 | 4.9 | 1.4 | 1.4 |
Japan, Hiroshima (1996–2000) | 1.7 | 0.8 | 2.4 | 1.2 |
Japan, Osaka | 1.7 | 0.9 | 2.7 | 1.5 |
Korea KCCR (1999–2002) | 5.4 | 2.5 | 3.3 | 1.5 |
Korea, Busan | 5.8 | 3.4 | 4.2 | 2.3 |
Korea, Daegu | 5.7 | 2.6 | 4.1 | 2.2 |
Korea, Daejeon | 5.0 | 2.1 | 2.8 | 1.3 |
Philippines, Manila | 1.3 | 0.9 | 0.1 | 0.1 |
Singapore, Chinese | 1.1 | 1.1 | 0.4 | 0.3 |
Taiwan | 4.3 | 3.9 | 0.7 | 0.5 |
Thailand, Khon Kaen | 71.3 | 31.6 | 0.4 | 0.1 |
Thailand, Chiang Mai | 8.2 | 4.0 | 0.4 | 0.2 |
Thailand, Bangkok | 2.5 | 1.4 | 0.3 | 0.1 |
Thailand, Songkhla | 1.6 | 0.5 | 0.1 | 0.2 |
Viet Nam, Hanoi | 0.1 | 0.1 | 0.0 | 0.0 |
Age-adjusted rates of ICC and ECC in east and southeastern Asia, using data collected from 18 cancer registries in Asia together with data from US and other Western registries, corroborate the rise in CCA worldwide [66]. Among populations of China, Korea, Japan, and Thailand, regional variations in the incidence of CCA are seen, with the highest rates of ECC noted in Korea. The areas of highest prevalence are concentrated around the Nakdong River and its lower extents [61, 62]. Though the majority of CCA incidence variation is related to varying prevalence of liver fluke infection, there are additional, as yet unknown, environmental, and ethnic factors that may explain the differential presentation of ICC and ECC within a population.
The Khon Kaen region of Thailand accounts for 70–90 % of primary liver tumors, as compared to high-risk areas, like Busan, Korea (20 %), Japan (5 %), and worldwide (10–25 %) [54, 65–67]. The highest prevalence of liver fluke infections occurs in the north (19.3 %), north–east (15.8 %), and central (3.8 %) regions of Thailand [54, 61]. O. viverinni infection is the strongest risk factor for developing CCA, and all areas have shown appreciable decreases over the last three decades [61, 68]. The prevalence of O. viverinni declined to an overall 9.6 % (6 million people) in 2001 from 14 % (7 million people) in the early 1980s [54, 61]. Average rates of CCA in the Khon Kaen Province area were 118 per 100,000 with an average prevalence of 24.5 % using data from 1990 to 2001 [54].
For the rest of Thailand, data in 2002 demonstrated an incidence of 71.4 per 100,000 for ICC in males and 31.6 per 100,000 in females. Corresponding rates of ECC in Korea are dramatically less at 0.4 per 100,000 in males and 0.1 per 100,000 in females [66] (Fig. 5a, b). Recent results documenting the outcomes of resected ECC in a cohort of 58 patients in Khon Kaen, Thailand, reported a 10.8 % 5-year survival rate that is somewhat reflective of the outcomes worldwide [69]. Survival remains poor despite public health education regarding the consumption of raw fish, more rigorous surveillance of at-risk populations, and increased efforts to treat the trematode infections that precede development of cholangiocarcinoma. Studies show similar poor long-term outcomes following surgery in patients resected for cholangiocarcinoma with and without an underlying trematode infection [53, 69, 70].
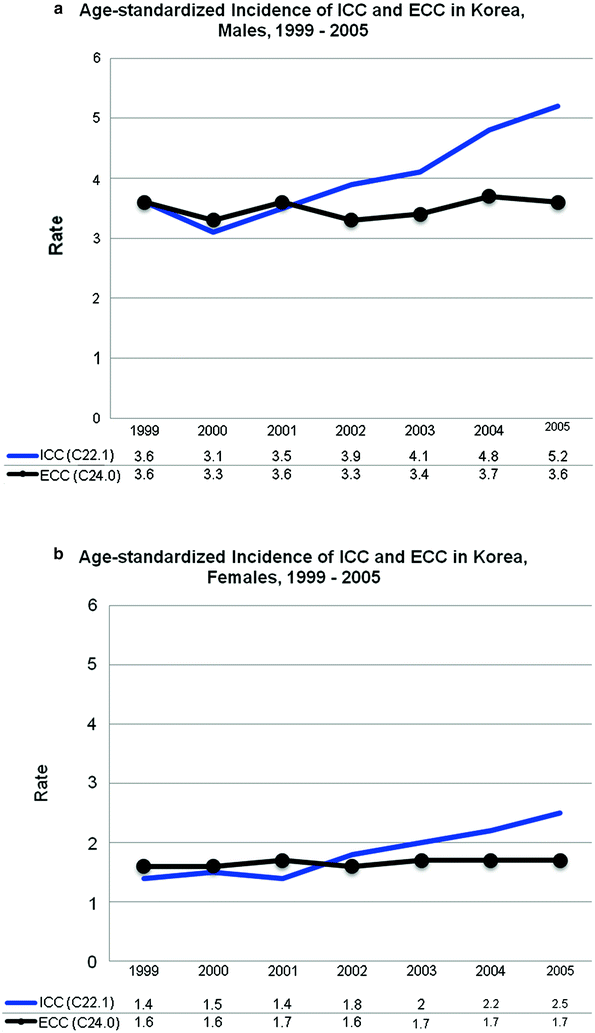

Fig. 5
Age-standardized incidence rates (ASIR) of intrahepatic cholangiocarcinoma (C22.1) and extrahepatic cholangiocarcinoma (C24.0) in Korean, a males and b females. Rates shown on x-axis are per 100,000 person-years. Overall rate for ICC is 4.1 males, 1.8 females with a +7.9 % and +10.6 % annual percentage change (APC), respectively, for time period. Overall rate for ECC is 3.5 males, 1.7 females with a +0.3 % and +1.3 % APC, respectively, for time period. Source Shin et al. 2010 [65]
The island of Japan has a higher risk than the rest of world for CCA, but a lower risk than Korea, China, Japan, and Thailand (Table 1) [5, 6, 41]. Bile duct cancers account for over 45 % of biliary tract cancers studied through the Japanese Biliary Tract Cancer Statistics Registry [71, 72]. The Japan Public Health Center Prospective Study reported outcome data on patients with ECC collected between 1990 and 1994 and found an association of cholelithiasis with the development of ECC in a study limited to gallbladder cancer and ECC [73]. Improvements in diagnostic techniques, patient selection for operation, and safety of extensive resections have improved over the last decade. Even though rates of CCA have declined overall in Asian populations, the overall survival still remains poor for this cancer once it develops.
1.4 Choledochal Cysts, Caroli’s Disease, and the Abnormal Pancreatobiliary Junction
Choledochal cysts (Types I–IV) involve the extrahepatic biliary tree, while Caroli’s disease (Type V) is defined as cystic dilation of the intrahepatic biliary tree [74]. Choledochal cysts are most common in Asia, particularly in Japan, and account for one-half to two-thirds of reported cases [74–76]. Type 1 choledochal cysts are most common, and there is a 3–4:1 preponderance in females [77–80]. The frequency of choledochal cysts is estimated between 1:13,000 and 1:2 million live births [78, 81].
From very early reports, an association of cancer concurrent with choledochal cysts was noted [74, 75, 82]. In a large study of 1,433 Japanese patients across all ages, the incidence of cancer related to a choledochal cyst was 3.2 % and predominantly adenocarcinoma, though cases of squamous carcinoma and mucinous adenocarcinoma have been reported. Of all patients in the series, 45 % were 10 years of age or less and the choledochal cyst-related cancers occurred in patients with a mean age of 32 (range, 15 to 66 years) [75]. The prevalence of cancer is <1 % in the first decade of life, increasing to 6.8 % in the second decade of life, and rising to >14.3 % in those over the age of 20 [82, 83]. Adenocarcinoma in patients as young as 12 and 15 years of age has been described [74, 84].
Cholangiocarcinoma is the most common cancer that occurs with choledochal cysts, and the patient risk is 20–30 times that of the normal population [79]. Gallbladder malignancy comprises 10 % of cancers associated with choledochal cysts, and CCA occurs in about 7 % of patients with Caroli’s disease [85, 86]. Synchronous or metachronous identification of CCA with treatment of choledochal cysts is reportedly as high as 20–28 % in Western literature and 8–30 % in reports from Asia [78, 81–83, 87]. More often, the literature describes adult patients who develop cholangiocarcinoma on long-term follow-up after a surgical intervention in infancy or adolescence [85, 88]. In the most recent two decades, published literature has shown a rate of 0–33 % CCA development following excision of choledochal cysts [79].
The theories of CCA pathogenesis in choledochal cysts include biliary stasis, exposure to infected bile and its carcinogenic byproducts, exposure to pancreatic amylase because of an abnormal pancreato-biliary junction (APBJ), and chronic inflammation related to biliary track stones [58, 59, 89, 90]. An APBJ is found concurrently with choledochal cysts in 39–92 % of cases [89]. Manometric studies have demonstrated high pancreatic amylase secretion with the cyst substance and noted pressure changes within the biliary tract system whereby reflux of pancreatic enzymes occurs [91]. The Japanese literature suggests that choledochal cysts do not occur without an APBJ [89]. The biliary tract epithelium may of itself be abnormal and more susceptible to the malignant degeneration that normally occurs in an age-dependent fashion. Mucosal adenomatous hyperplasia of cholangiocytes in response to chronic inflammation initiates the hyperplasia-metaplasia-carcinoma sequence that culminates in carcinogenesis [92, 93].
Other conditions that cause chronic inflammation of the biliary tree predispose patients to cholangiocarcinoma. Prior manipulation of the biliary tree for benign disease is an example of an acquired risk factor for CCA. A 5.5 % increased risk has been reported for patients that have undergone biliary-enteric bypass procedures, usually for choledocholithiasis [94]. The risk of CCA is highest following choledochoduodenostomy (7.6 %) and lowest following Roux-en-Y hepaticojejunostomy (1.9 %) [94].
Transduodenal sphincteroplasty for treatment for sphincter of Oddi dysfunction is also implicated as causative in the development of CCA (7.4 %) [95–97]. Other reported cases indicate that the risk continues to be present as many as 1–40 years following the initial intervention [95, 96]. The proposed mechanism for carcinogenesis is stasis of bile infected with intestinal contents secondary to the new biliary-enteric configuration. Conflicting data suggest that manipulation of the biliary tract for otherwise benign, or non-bile duct cyst disease, is itself a risk factor for cholangiocarcinoma [94, 98], while other reports do not support an increased cancer susceptibility [99]. Secondary stone formation caused by anastomotic stricturing and recurrent cholangitis after biliary-enteric anastomosis is also implicated in CCA metaplasia [100–102]. The constant irritation of the biliary tract by stones, which may be infected, also contribute to cholangiocyte metaplasia [100, 101].
Primary intrahepatic stones, or hepatolithiasis, are common in East Asia and a known risk factor for the development of CCA. Japan and Taiwan have the highest incidence of cholangiocarcinoma complicating long-standing hepatolithiasis at 1.5–9.4 % and 2.4–5.0 %, respectively [21, 103–106]. The overall incidence in the literature is estimated at 4–11 %, but has been reported to be as high as 20 % in a Taiwanese population [104]. Following hepatectomy for stone disease, one series of 29 patients from Japan documented a 17.1 % incidence of ICC. Stone disease predominantly affects the left lobe of the liver, though right-sided and bilateral stone disease is also described [105, 107, 108]. Tumors typically develop first within the intrahepatic ducts in close proximity to where stones are located though hilar tumors and ECC have been described [21, 104, 105, 108].
Survival following surgical resection in patients with hepatolithiasis and CCA is poor and is similar to patients with uncomplicated CCA. The cycle of biliary sepsis requiring drainage can delay and may prevent a patient from presenting for surgical resection. Diagnosing CCA is difficult in the face of hepatolithiasis; however, it should be suspected in patients with long-standing hepatolithiasis (>10 years), deranged liver function tests, age >40–50 years, and lobar atrophy, stricture, or obvious tumor mass on imaging [104, 108, 109]. As such, overall resectability rates may be lower due to advanced disease stage at time of presentation. Favorable long-term survival is associated with completeness of surgical resection rather than any specific impact of hepatolithiasis.
1.5 Primary Sclerosing Cholangitis and Cholangiocarcinoma
The development of CCA in patients with primary sclerosing cholangitis (PSC) is an important risk factor in Western populations. PSC is a chronic cholestatic disease with no optimal liver-directed therapy to appreciably treat disease. Use of ursodeoxycholic acid (UDCA) and immunosuppression with corticosteroids, tacrolimus, cyclosporine, and methotrexate may result in biochemical improvements of liver function tests in affected patients [110]. The natural history of the disease is an insidious onset of intra- and extrahepatic biliary strictures punctuated by bouts of treatment-refractory sepsis [110]. Stenting is used to alleviate biliary obstruction caused by dominant strictures. Cirrhosis, liver failure, CCA, and death are the most significant complications. In the absence of tumors, the median survival for patients with PSC is between 10 and 12 years, with asymptomatic patient survival of 70 % at 16 years [111–113].
PSC is closely associated with inflammatory bowel disease (IBD) (60–80 %), both ulcerative colitis (UC) (26–68 %) and, to a lesser extent, Crohn’s disease (13 %) [1, 22, 67, 114, 115]. The estimated prevalence of IBD with PSC ranges from 60 to 80 % in the United States, Nordic Countries, and Northern European populations with little variation over the last two decades [1, 22, 67, 112, 115]. The annual incidence risk of CCA with PSC ranges from 0.6 to 1.5 % [58, 116–118]. The prevalence of CCA complicating PSC is 7–18 % in the United States and has been reported as 8 and 14.3 % in Sweden and Germany, respectively [112, 114, 119, 120]. On average, patients develop CCA 30–63 months following the diagnosis of PSC [2, 22, 114] with up to 50 % diagnosed with CCA concurrent with their PSC diagnosis [114, 116, 121]. The primary tumor location in PSC-CCA is extrahepatic or hilar in 60–76 % of cases, 16–60 % intrahepatic, and indeterminate or both in 20–35 % [22, 111, 114, 117, 122].
The most complete datasets summarizing the known relationship of PSC with cholangiocarcinoma come from a large referral-based population in Minnesota (USA) and the Nordic Countries (Denmark, Finland, Sweden, Norway, Iceland) [22, 112, 122–125]. The cohort of 604 patients obtained from the Swedish Cancer and Deaths Registry (1970–1998) showed a 13.3 % incidence of patients with PSC developing a malignancy (any cholangiocarcinoma, gallbladder cancer, or HCC). The standardized incidence risk (SIR), adjusted for age and sex, compared to the general population of Sweden was 161 for developing any malignancy [118]. The updated data present information from a specific region of Sweden in patients with PSC who developed CCA between 1992 and 2005 [124]. The incidence of CCA in this cohort was 8.5 % with 5-year and 10-year cumulative incidences of 7 and 11 %, respectively. The SIR for all HPB malignancies was 177 and for CCA was 868 [124]. In the Swedish studies, no influence of duration of PSC to the development of CCA has been documented, in contrast to other Western studies [111, 118, 124]. The literature is relatively sparse on the interaction of PSC and CCA in Eastern populations. After documenting the pattern of PSC in the Japanese population, a further cross-sectional study reported a 3.4 % incidence of CCA in patients with PSC. Approximately 50 % of patients were diagnosed with CCA at the time of the PSC diagnosis. The time to CCA diagnosis was an average of 2.5 years later in the remainder of patients [126].
The gains in improved survival for patients with PSC and CCA have been seen with rapid treatment of sepsis, recognition of and stenting of dominant strictures, and liver transplantation. Using a neoadjuvant chemotherapeutic protocol followed by transplantation, actuarial 5-year survival rates of 82 % with transplantation compared to 21 % with resection alone have been reported [127]. Other series have seen modest survival rates of 35 % at 5 years with liver transplant for PSC without a chemotherapy protocol [117]. Recurrences in the transplant group are also demonstrably lower (13 vs. 27 %). Up to 20 % of patients with PSC on the waiting list may harbor an undiagnosed cholangiocarcinoma, as found on waiting list surveillance testing or in the liver explant [117, 128].
1.6 Hepatitis B, Hepatitis C and the Effect of Chronic Inflammation, Hepatocyte Injury, and Cholangitis
Historically, no association between chronic Hepatitis B (HBV) and Hepatitis C virus (HCV) infections in the pathogenesis of CCA was reported [62, 129], but this has changed in recent years [27]. The IARC linked, with limited supporting evidence, HBV and HCV infections to CCA through a causal mechanism of inflammation, chronic liver disease, cirrhosis, and fibrosis [51]. Chronic HBV infection is responsible for cirrhosis and the progression to HCC in sub-Saharan Africa and in East Asia. Chronic HCV infection is the predominant risk factor for HCC in Japan and Western countries [5, 6]. The influence of HBV and HCV on CCA is multifactorial. In conjunction with other probably contributory risk factors such as alcohol consumption, smoking, and consumption of nitrosamine-preserved foods, it has emerged as a more likely direct influence, though the exact mechanisms are unknown.
Studies from the United States, Japan, and Italy demonstrated an increased risk of ICC with HCV [130–133]. In direct contrast, other studies from the East, in Taiwan, China, and Korea, show a closer association of HBV infection and ICC [134–137]. In a series that published tumor location, the relationship of HBV or HCV infection is more commonly associated with ICC than ECC [130, 133, 135, 136]. The differential findings do correspond with population and geographic areas in which HBV and HCV are endemic. A recent publication from Taiwan, where both HBV and HCV are endemic, reported a significant association of ICC with HBV and HCV [135]. Additionally, co-infection with hepatitis surface antigen (HBsAg) and anti-HCV core antigen (anti-HCV) confers a higher risk of ICC formation than with either HBsAg or anti-HCV alone [135]. The findings of two separate meta-analyses support the view that HCV and HBV infection plays a significant role in the development of CCA, more often ICC than ECC [138, 139].
Although a new risk factor related to CCA has been described, the relative importance of this contribution for CCA pathogenesis is unclear. The HBV and HCV seropositivity rate in the patient populations from which results derive ranges from 1.6–49 to 1.2–36 %, respectively [131, 132, 137, 140]. In a 4:1 matched case-control study from Korea, an association of HBV (OR = 2.2) to ICC was found with more significant contributions from diabetes (OR = 3.2), hepatolithiasis (OR = 50), choledochal cysts (OR = 10.7), cirrhosis (OR = 13.6), and C. sinensis infection (OR = 13.6) [136]. The relationship to other risk factors is supported by other HCV studies with PSC, diabetes playing a stronger role in CCA pathogenesis than HBV or HCV [132, 141]. The RR of HBV pathogenesis in ICC is stronger in reports from Asian populations compared with other nationalities [138].
Molecular pathologic analysis of tissue samples has found HBV DNA and HCV RNA within the biliary epithelium and has been proposed this as a possible mechanistic theory for tumor growth [142]. The chronic inflammatory process incited by HCV core protein contributes to cellular proliferation of a damaged biliary epithelium [143]. The initiating events of cholangiocarcinoma involve cytokine activation of inflammatory cells and induce nitric oxide synthase (iNOS) within damaged biliary epithelia. DNA damage occurs in conjunction with nitrosylation of thiol and tyrosine residues to propagate further damage [2, 144]. iNOS-damaged DNA contributes to tumor suppressor p53 upregulation and may also induce damage that renders the p53-mediated DNA repair mechanism inactive [92]. This inflammatory cycle is better described in patients with PSC and CCA, and liver fluke infestation and CCA [2, 59]. However, HBV and HCV infection, as an example of a chronic inflammatory process, is a suitable mechanism for induction of the nitric oxide synthase process and its pleiotropic effects on biliary tract carcinogenesis [10]. While HBV and HCV primarily affect hepatocytes, research evidence supports a common hepatocyte progenitor cell origin for hepatocytes and cholangiocytes [11, 12, 145, 146]. Carcinogenic processes within the liver may thus affect susceptible cholangiocytes in close proximity, perhaps also explaining the increased association of HBV and HCV infection with ICC rather than ECC.
1.7 Environmental Factors
Thorotrast, discontinued as a colloidal intravenous contrast agent after 1955, derived from thorium-232, is recognized as a Class I carcinogen by the IARC [67]. Preferential deposition was seen within the substance of the liver (60–70 %), but its affinity was also for the reticuloendothelial system, the spleen and bone marrow. Historically, tumors of the liver presented as HCC or hepatic angiosarcoma, 10–12 years following the exposure [67, 147]. Dysplasia, or abnormal proliferation of bile ducts, was seen in Thorotrast-induced cases of liver cancer [67]. ECC was the more common of the biliary tract tumors. The IARC drew several conclusions regarding Thorotrast exposure and cholangiocarcinoma from initial data and follow-up of large cohort series from the United States, Germany, Denmark, Japan, and Sweden [148–153]. Thorotrast exposure caused an excess cancer (liver and bile duct) risk of 97 % persisting for up to 50 years, increasing risk and mortality from liver cancer with increasing amount of injected Thorotrast, and an increasing standardized mortality rate (SMR) and RR compared to controls as more time elapsed from first exposure to Thorotrast. One proposed mechanism of pathogenesis is alpha-ionizing radiation particles inducing instability in human mismatch repair genes [154].
1.8 Emerging and Other Risk Factors
Obesity and diabetes as elements of the metabolic syndrome are gaining increasing attention in the pathogenesis of CCA. Both are known risk factors for HCC in NAFLD and other gastrointestinal cancers (esophagus, pancreas, gallbladder, stomach) [155]. In one population case-control study from China, the risk of ECC and other biliary tract cancers increased with the increasing number of diagnostic components of the metabolic syndrome [156]. The increased risk profile persisted for patients with body mass index (BMI) <25 and non-diabetics with three other elements of the metabolic syndrome. Interestingly, high high-density lipoprotein (HDL) (OR = 8.17) and high triglyceride levels (OR = 5.28), additional elements of the metabolic syndrome, gave a higher OR for ECC than for metabolic syndrome as a single risk factor. In a low-risk population from Denmark (1978–1991), diabetes, but not obesity was significantly associated with ICC [157]. Data from the United Kingdom (1987–2002), another low-risk population, also showed a positive association with obesity (BMI ≥ 30) and conferred a 1.5-fold increased risk of developing CCA [158].
The relationship of obesity to CCA is not clearly defined and contradictory associations have emerged. Some reports postulate a carcinogenic mechanism based on the pro-inflammatory and carcinogenic components of bile when bile flow is impaired, as in obesity [73]. Still other less well-delineated risk factors for CCA may include heavy alcohol consumption, H. pylori infection, smoking, cholelithiasis, thyrotoxicosis, cirrhosis from all causes, and human immunodeficiency virus (HIV) [25–27, 132, 141, 157, 159].
1.9 Summary
Accurate morphologic and histologic tissue sub-typing will facilitate better classification of this disease and allow more precise calculations of incidence and risk factors. Strategies to attempt the cure of CCA include extensive surgical resection and or transplantation. However, the underlying liver disease, as in PSC, HBV, HCV, cirrhosis, and hepatolithiasis may complicate timely diagnosis of the tumor. Diagnostic tools and imaging have improved over the last few decades, though overall resectability remains low. Resection with negative margins remains the best predictor of long-term survival given the lack of suitable adjuvant therapeutic options. Major survival gains with CCA may be made by targeting prevention of the disease and modification of definite risk factors. Public health education to dissuade raw fish consumption and treatment of liver fluke infection in endemic areas are examples of what can be done to reduce the development of this tumor. Continued widespread HBV vaccination and HCV surveillance efforts should help address early CCA recognition in at risk population groups. Given the emerging risk factors for ICC and ECC, general lifestyle modification toward increased health and wellness may minimize the impact of obesity, diabetes and insulin resistance, smoking, and excess alcohol consumption. Further research into the molecular mechanisms of CCA development may be fruitful in developing tailored diagnostic, prognostic and treatment efforts [160].
2 Gallbladder Carcinoma
Gallbladder carcinoma (GBCA) is an epithelial tumor of the gallbladder, which is part of the extrahepatic biliary tract. The majority of tumors described in the literature are adenocarcinoma, but the category includes squamous, adenosquamous, neuroendocrine, and other mixed or undifferentiated malignant neoplasms of biliary, intestinal or foveolar features [8, 13]. The WHO and IARC assigns intrahepatic cholangiocarcinoma the ICD-O morphology code ICD-O 8140/3, 8144/3, 8310/3, 8480/3, 8490/3, 8560/3, 8503/3, 8470/3, 8070/3,8020/3, which corresponds topographically to gallbladder cancer (C23.0). [8–10]. ECC share the same morphologic codes as gallbladder carcinomas (see above), and are topographically described with gallbladder carcinomas (C23) and other tumors of the biliary tree (C24) [8, 9, 13]. In the reporting of outcomes, GBCA outcomes are sometimes grouped with other tumors of the extrahepatic bile duct (EHBD), such as ECC. Data trends, and risk factors for GBCA will be discussed in this chapter and where there is overlap with EHBD tumors or ECC, this will be specifically mentioned.
The incidence, prevalence, and geographic distribution GBCA vary widely with differential susceptibility based on gender, infectious exposure, ethnicity and genetics or family history, but correlate with the incidence and prevalence of cholelithiasis. Worldwide incidence ratios are difficult to parse out from ECC and other EHBD tumors; however, given the poor survival with this tumor, incidence rates generally follow mortality rates. Consistently high ASR-W incidence areas for GBCA are in India and Chile with rates in women of 8.6 and 27.3, respectively, and rates in men of 3.9 and 12.3, respectively, for ages 0–74 years [6]. Table 5 details the highest and lowest incidence rates of GBCA worldwide based on the most recent last IARC Cancer Incidence in Five Continents, Vol. IX publication covering data from 1998 to 2002 [6, 161]. Trends in GBCA show an overall declining mortality over the last 2 decades, although increases have been noted in Italy, Iceland, Korea and Costa Rica [5, 6, 41, 48, 162, 163]. Few reports regarding the prevalence and incidence of GBCA in Africa are published in the literature.
Table 5
Age-standardized world incidence rates (ASR-W) for GBCA, 1998–2002
ASR-W | Cases | |
|---|---|---|
(a) Age-standardized incidence rates of GBCA, World, Males, 1998–2002 | ||
Japan, Yamagata prefecture | 5.7 | 335 |
US, California, Los Angeles: Korean | 4.8 | 24 |
Ecuador, Quito | 3.4 | 78 |
Czech Republic | 3.2 | 1,072 |
Italy, Torino | 2.8 | 114 |
Slovakia | 2.7 | 389 |
Slovenia | 2.6 | 175 |
USA, Connecticut: Black | 2.5 | 15 |
Italy, Ragusa Province | 2.4 | 29 |
Brazil, Goiana | 2.4 | 37 |
China, Shanghai | 2.3 | 493 |
Poland, Warsaw City | 2.3 | 132 |
(b) Age-standardized incidence rates of GBCA, World, females, 1998–2002 | ||
Ecuador, Quito | 5.8 | 152 |
Slovakia | 4.5 | 364 |
Colombia, Cali | 4.4 | 164 |
Czech Republic | 4.3 | 1,821 |
Spain, Granada | 3.3 | 109 |
US, California, Los Angeles: Hispanic White | 3.2 | 218 |
Japan, 3 registries | 3.1 | 1,787 |
China, 2 registries | 2.9 | 1,279 |
Poland, 2 registries | 2.9 | 326 |
Italy, Torino | 2.5 | 121 |
US, California, Los Angeles: Korean | 2.4 | 16 |
Costa Rica | 2.4 | 167 |
Slovenia | 2.4 | 205 |
Individuals in their late fifth to seventh decades of life are more commonly afflicted with GBCA [5, 6, 164] (Fig. 6). The female to male ratio of GBCA is generally 3:1 in the United States, Australia, Europe, and Asia, and as high as 5:1 in India, Pakistan, Ecuador, and Israel [5, 6, 163–165]. Geographic areas of high prevalence include populations in India, Ecuador, Chile, Pakistan, and Japan [5, 6, 166]. High prevalence rates occur in specific populations in low-risk nations, like the Pima Indians, Hispanics, and Alaska Natives in the United States [3, 4]. The Mapuche Indians in the high-risk nation of Chile, and women in India and Pakistan, have some of the highest incidence of GBCA worldwide [5, 6, 167].
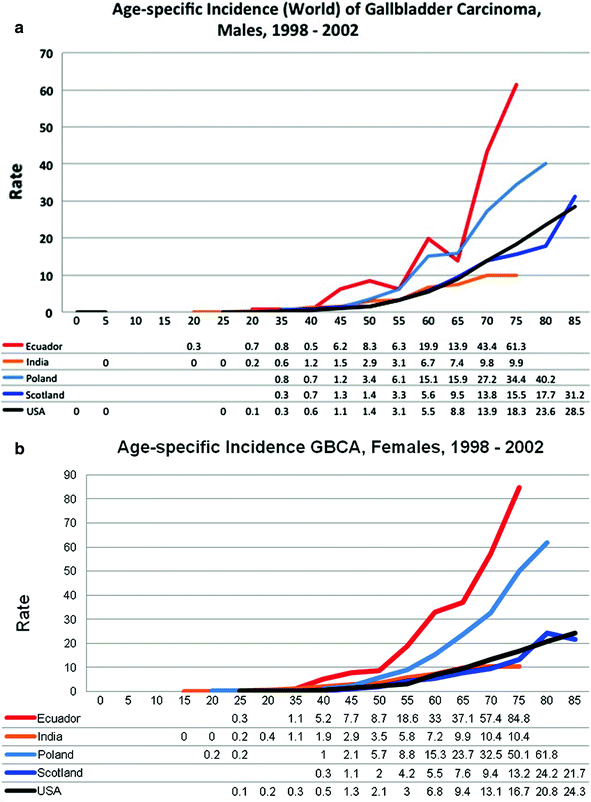

Fig. 6
Age-specific incidence world rates (ASRW) per 100,000 of GBCA in, a males and b females, 1992–2009 [195]. Rates shown are per 100,000 person-years on the y-axis. Patient age in years are shown on the x-axis
Risk factors for the development of GBCA include long-standing gallstones and the inflammatory changes that may impact the progression to carcinoma [168, 169]. The overall incidence of GBCA in the presence of cholelithiasis is <0.2 and greater than 80 % of patients with GBCA have gallstones [8, 168]. GBCA may also progress through an adenoma-carcinoma sequence or a dysplasia-carcinoma sequence [170]. In either case, the evidence supports an increased risk of GBCA with increasing number and size of gallstones, and other polypoid lesions of the gallbladder. Both theories of GBCA pathogenesis are plausible [170–173]. Additional risk factors for GBCA include multiparity, obesity, and biliary tract infections with S. typhi and Helicobacter spp. [174–180]. Many studies support an increased risk of GBCA in women with more childbirths (>3) and pregnancies [166, 178, 179]. The association of age at first birth, age at last birth, use of the oral contraceptive pill, menopausal status, and use of hormone-replacement therapy (HRT) with GBCA is less well-defined [164, 178, 179, 181].
Current data point to increasing cholecystectomy rates, especially since the introduction of laparoscopic cholecystectomy, are partially responsible for the improved incidence and survival trends in this rare and often deadly cancer [182–186]. Aggressive surgical resection remains the best option for cure [187–189




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree