Fig. 1
(a, b) Contrast-enhanced angio-TC. (a) Diffuse calcifications of the abdominal aorta and iliofemoral arteries with bilateral obstruction of the distal tract of the superficial femoral artery in a patient undergoing chronic hemodialysis. (b) Magnification of the proximal tract of the superficial femoral artery showing diffuse atherosclerosis with calcific plaques
Patients with chronic renal failure may present several abnormal findings on chest radiography and CT, due to changes in the phosphorus and calcium metabolism, changes in hemostasis, arterial hypertension, fluid retention, or dialysis. The most frequent abnormalities include interstitial and alveolar edema and cardiomegaly, pleural effusion (Fig. 2), metastatic pulmonary calcifications, and calcifications of the bronchial walls, pleura, and chest wall vessels.
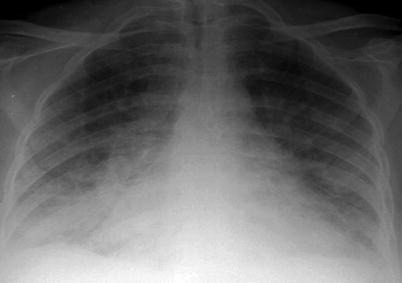

Fig. 2
Posteroanterior chest x-ray radiograph. Bilateral pleural effusion in a patient undergoing hemodialysis
Hematologic abnormalities are among the most consistent manifestations of uremia (Warnock 1992). These abnormalities include anemia, bleeding, and granulocyte and platelet dysfunction. The primary causes of anemia in chronic renal failure are a deficiency of erythropoietin, which is a glycoprotein normally produced by the kidney, and iron deficiency due to reduced iron intake and frequent blood sampling. Erythropoietin therapy has improved the general status of patients with chronic renal failure. In patients with uremic syndrome, the fat bone marrow is progressively replaced by hematopoietic marrow content (Ito et al. 1994) with prolonged T1 relaxation time in various anatomical regions (especially the spine and the upper and lower limb bones). A hemorrhagic diathesis is common in patients with chronic renal failure. Spontaneous nontraumatic bleeding may affect the perinephric and subcapsular spaces, the renal parenchyma, or the collecting system. Gastrointestinal bleeding is also common in uremic patients.
The most common neurologic complications in this patient group include focal white matter lesions, cerebral atrophy, osmotic demyelination syndrome, dialysis encephalopathy, hypertensive encephalopathy, intracranial hemorrhage, infarction, sinus thrombosis, and infection. Peripheral neuropathy is also common in patients with chronic renal failure.
Abnormalities involving the musculoskeletal system are numerous and frequent in patients with chronic renal insufficiency (Murphey et al. 1993). Chronic renal insufficiency, hemodialysis, peritoneal dialysis, renal transplantation, and administration of different medications provoke complex biochemical disturbances of the calcium-phosphate metabolism with wide spectrum of bone and soft tissue abnormalities termed renal osteodystrophy (Jevtic 2003). By the time dialysis is initiated, nearly all patients are affected. Renal osteodystrophy is a global term applied to all pathological features of bone in patients with chronic renal failure and it comprises osteomalacia or rickets according to patient age, secondary hyperparathyroidism with bone resorption (Figs. 3 and 4), pathologic fractures, (Fig. 5), periosteal reaction, osteosclerosis (Fig. 6), osteoporosis, soft tissue and vascular calcifications (Fig. 7), and brown tumors (Fig. 8). Bone resorption typically manifests along the radial aspects of the middle phalanges of the index and middle fingers (Fig. 3), beginning in the proximal metaphyseal region. Additional sites of subperiosteal bone resorption include the upper medial tibia, humerus, and femur; the superior and inferior ribs; and the lamina dura (Murphey et al. 1993). The irregular cortical surface may create a false appearance of periosteal reaction (pseudoperiostitis). Subchondral bone resorption affects the single distal interphalangeal joint (most frequently the fourth and fifth fingers); the metacarpophalangeal joint and the proximal interphalangeal joints; the distal clavicle; the acromioclavicular joint, sacroiliac joint, sternoclavicular joint (Fig. 4); the symphysis pubis, and the posterior patella (Murphey et al. 1993). Subligamentous bone resorption includes the inferior surface of the calcaneus and clavicle (coracoclavicular ligaments), greater and lesser femoral trochanters, anterior inferior iliac spine region, humerus near the insertion of the rotator cuff, ischial tuberosities, and elbow at the level of the extensor surface of the ulna and posterior olecranon (Murphey et al. 1993). Peridiscal destructive arthritis in patients on long-term hemodialysis, especially located on the cervical and dorsal spine, may mimic spondylodiscitis and corresponds to the hemodialysis spondyloarthropathy (Leone et al. 2001). Osteoclastomas or brown tumors (Fig. 8) are caused by localized replacement of bone by vascularized fibrous tissue (osteitis fibrosa cystica) resulting from PTH-stimulated osteoclastic activity. The fibrous tissue contains giant cells, and the lesions may become cystic following necrosis and liquefaction. Radiographically, brown tumors are well-defined lytic lesions, often eccentric or cortical, that may cause endosteal scalloping and osseous expansion (Murphey et al. 1993).
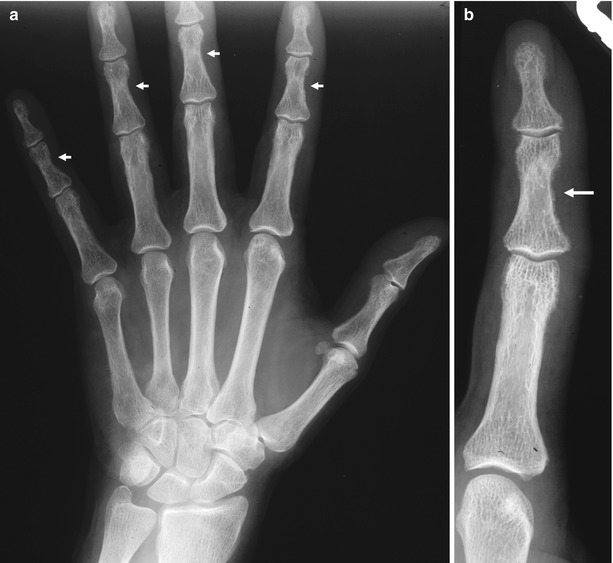
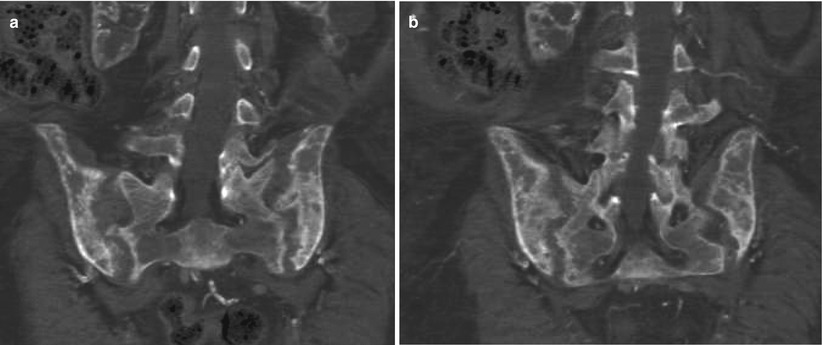
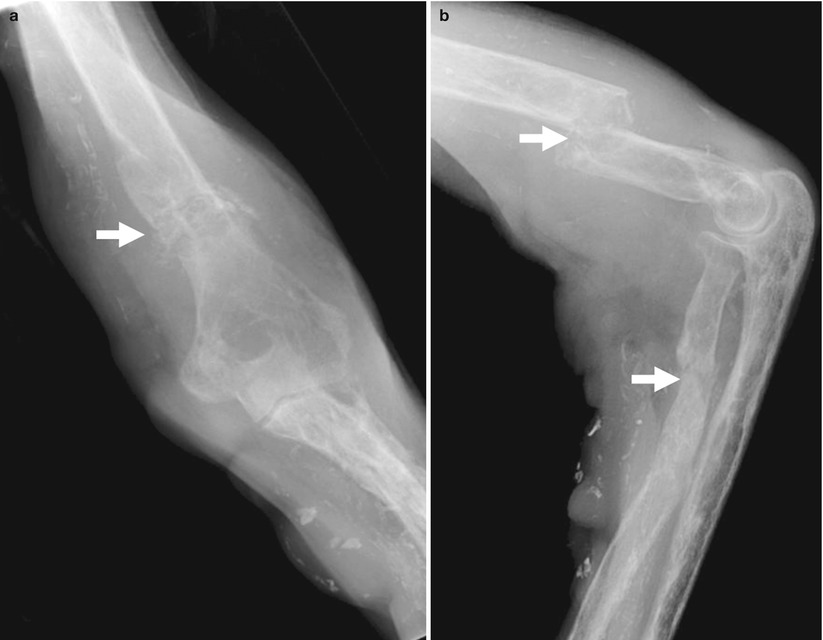
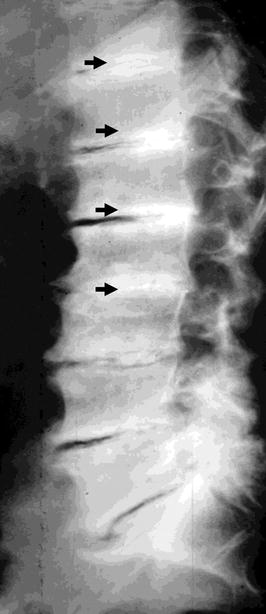
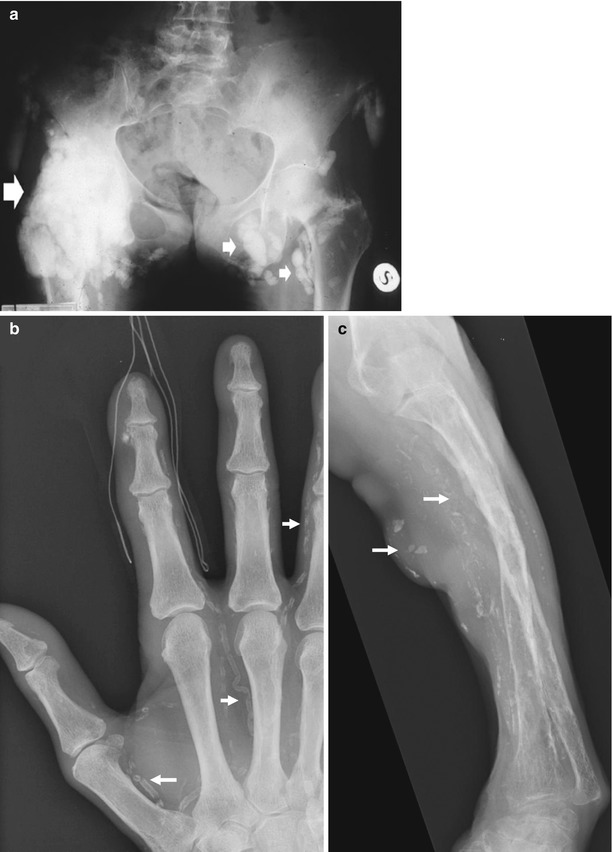
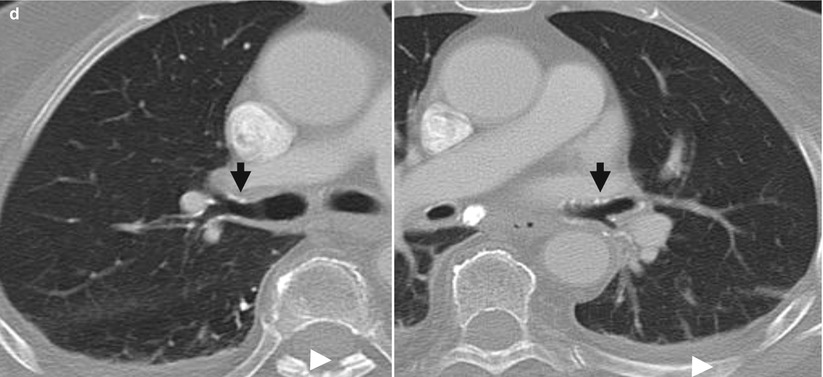
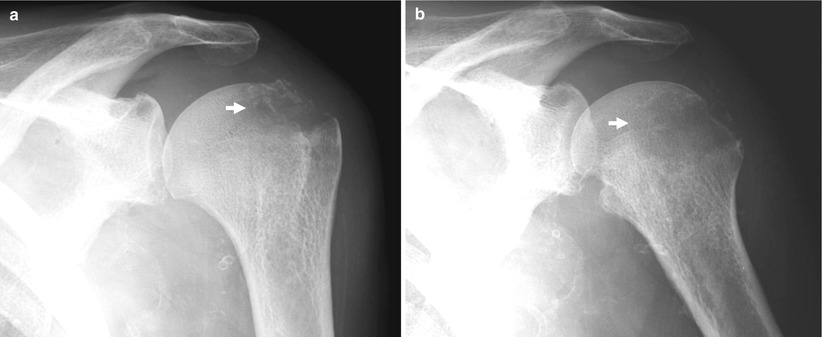

Fig. 3
(a, b) Plain hand radiograph of a male patient undergoing hemodialysis for 2 years. (a) Normal view; (b) magnification of the fifth finger. Secondary hyperparathyroidism with bone resorption along the radial aspects of the middle phalanges of the fingers (arrows)

Fig. 4
(a, b) Unenhanced CT. Bone window. Coronal reformations. Diffuse bone resorption at the level of the sacroiliac region in a patient undergoing hemodialysis with secondary hyperparathyroidism

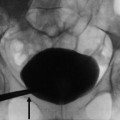
Fig. 5
Plain radiograph. Diffuse bone resorption at the level of the forearm bones with pathologic fractures (arrows)

Fig. 6
Plain radiography of the spine. Diffuse osteosclerosis of the spine in patient undergoing chronic hemodialysis. Diffuse sub-endplate densities at multiple contiguous levels, a pattern known as “rugger-jersey spine”


Fig. 7
(a) Renal osteodystrophy of a male patient undergoing hemodialysis for 2 years. Plain radiograph of the hip. Diffuse soft tissue calcifications (arrows). (b, c) Diffuse soft tissue and vascular calcifications (arrows). (d) Unenhanced CT. Calcifications at the level of the bronchial walls (arrows)

Fig. 8
(a, b) Plain radiography of the shoulder. Lytic area (arrow) due to a brown tumor, as proven by biopsy, in a patient undergoing hemodialysis
Other abnormalities related to biochemical disturbance of chronic renal failure include soft tissue/vascular or bronchial calcifications (Fig. 7) and crystalline arthropathies. Musculoskeletal sequelae predominantly related to dialysis include aluminum toxicity manifesting as osteomalacia from ingestion of aluminum salts in phosphate-binding antacids used to control hyperphosphatemia, amyloidosis with carpal tunnel syndrome, and destructive spondyloarthropathy (Murphey et al. 1993). Avascular necrosis, osteomyelitis, septic arthritis, tendinosis/tendon rupture, and bursitis/synovitis are caused by a combination of chronic renal failure, steroid/immunosuppressants, and dialysis (Lim and Ong 2013).
2 Acquired (Dialysis-Associated) Cystic Kidney Disease
Acquired (dialysis-associated) cystic kidney disease (ACKD) was first described by Dunnill et al. (1977) and is characterized by the development of numerous fluid-filled cysts in patients with end-stage renal disease who have undergone prolonged dialysis, but without a history of hereditary cystic disease (Fig. 9). The cysts measure 0.5–2 cm in diameter, contain clear fluid, are lined by either hyperplastic or flattened tubular epithelium, and often contain calcium oxalate crystals (Fig. 9). They probably form as a result of obstruction of tubules by interstitial fibrosis or by oxalate crystals (Alpers 2005). Frequently, some cysts may develop a hemorrhagic pattern (Fig. 10) causing hematuria. The likelihood of the development of ACKD increases with the duration of hemodialysis and is increased in men and older patients (Heinz-Peer et al. 1995). Other factors, such as the underlying renal insufficiency and serum creatinine level, have no significant influence on the development of ACKD (Heinz-Peer et al. 1995). After 1–3 years of hemodialysis, 10–20 % of patients have ACKD, 40–60 % at 3–5 years of hemodialysis, and more than 90 % after 5–10 years of hemodialysis. ACKD and neoplasia can develop in those patients who have undergone long-term hemodialysis (Scanlon and Karasick 1983).
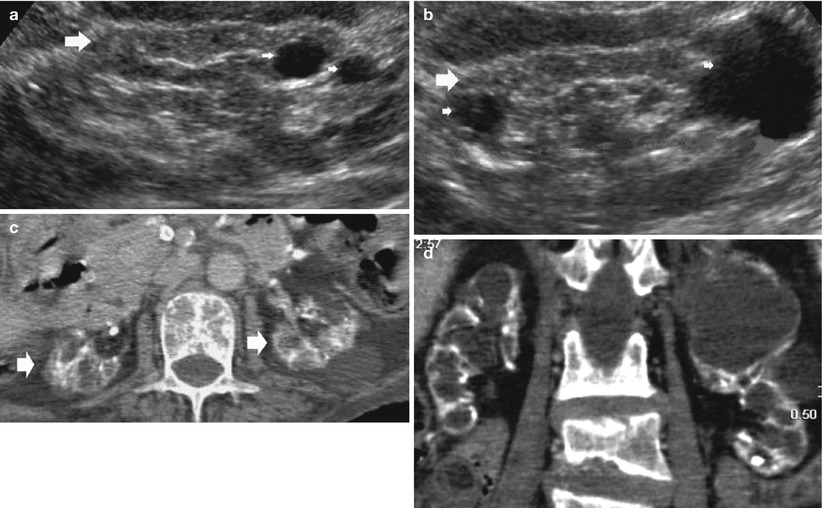
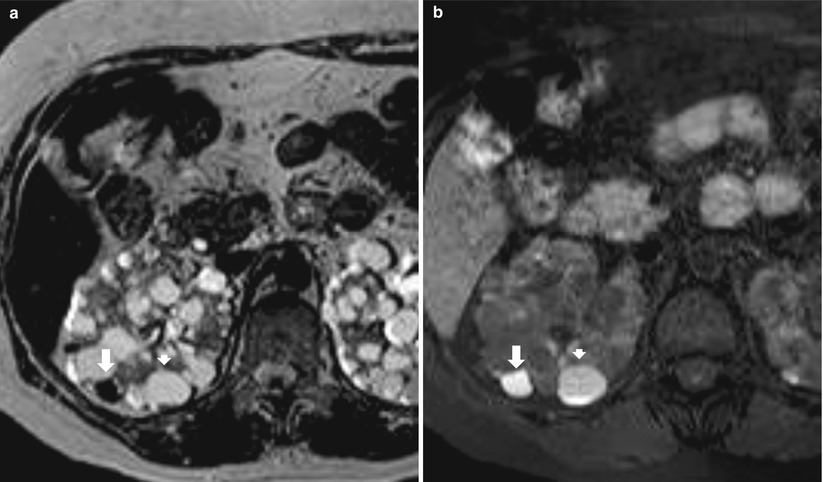

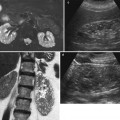
Fig. 9
(a, b) Baseline grayscale ultrasound. Fifty-seven-year-old male patient with acquired cystic kidney disease under dialysis treatment for 5 years. Diffuse reduction of the renal cortex thickness (large arrows) with evidence and multiple parenchymal cysts (arrows). (c, d) Seventy-one-year-old female patient with acquired cystic kidney disease under dialysis treatment for 10 years. Diffuse calcifications at the level of intrarenal arteries and multiple parenchymal cysts with diffuse reduction of the renal cortical thickness

Fig. 10
Acquired cystic kidney disease, hemorrhagic renal cyst. (a, b) Hemorrhagic renal cyst (long arrow) in the right kidney in a patient with acquired cystic kidney disease, appearing hypointense on T2-weighted MR sequence (a) and hyperintense on fat-suppressed T1-weighted MR sequence (b). A cyst with a proteic content is also identified (small arrow) appearing hyperintense both on T2-weighted (a) and T1-weighted sequence (b)
The development of renal cell carcinoma (Figs. 11, 12, and 13) in the wall of the cysts is the most serious complication of ACKD (Truong et al. 1995). In patients with dialysis-associated ACKD undergoing long-term hemodialysis, the prevalence of renal cell carcinoma is increased being in the range of 3–6 % (Gardner 1984). The prevalence is similar in patients with ACKD undergoing renal transplantation (Heinz-Peer et al. 1995). A comprehensive review of the pertinent literature shows that there is up to 50-fold increased risk of renal cell carcinoma in ACKD compared to the general population (Truong et al. 1995). ACKD-associated renal cell carcinoma accounts for approximately 2 % of deaths in renal transplant patients. A median length of survival of approximately 14 months and a 5-year survival rate of 35 % are comparable to the same data for renal cell carcinoma in the general population. Successful renal transplant probably decreases the risk of renal cell carcinoma in ACKD patients, but this preliminary observation needs confirmation. The development of ACKD-associated renal carcinoma is a continuous process with evolving phenotypic expression, including damaged renal tubule, simple cyst, cyst with atypical lining, adenoma, and, finally, carcinoma. The pathogenesis of this continuous process is not entirely known, but growth factor-induced compensatory growth of tubular epithelium initiated by the changes of end-stage kidney disease, and probably perpetuated by activation of proto-oncogenes, seems to be the most significant factor. The ACKD-associated renal cell carcinoma is seen predominantly in males, occurs approximately 20 years earlier than in the general population, and is frequently bilateral (9 %) and multicentric (50 %). ACKD-associated renal cell carcinoma is frequently asymptomatic (86 %) but may be associated with bleeding, abrupt changes in hematocrit, fever, and flank pain or rarely with hypoglycemia, hypercalcemia, or metastases at presentation (Truong et al. 1995).
Get Clinical Tree app for offline access

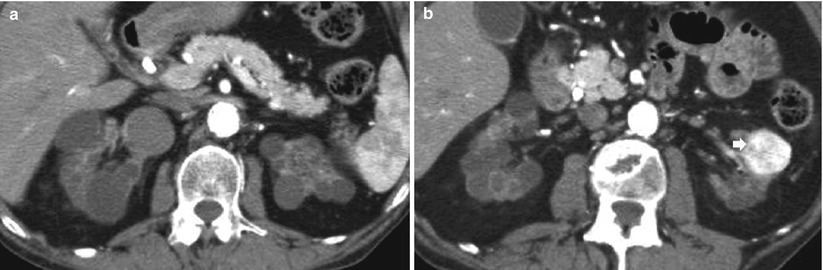
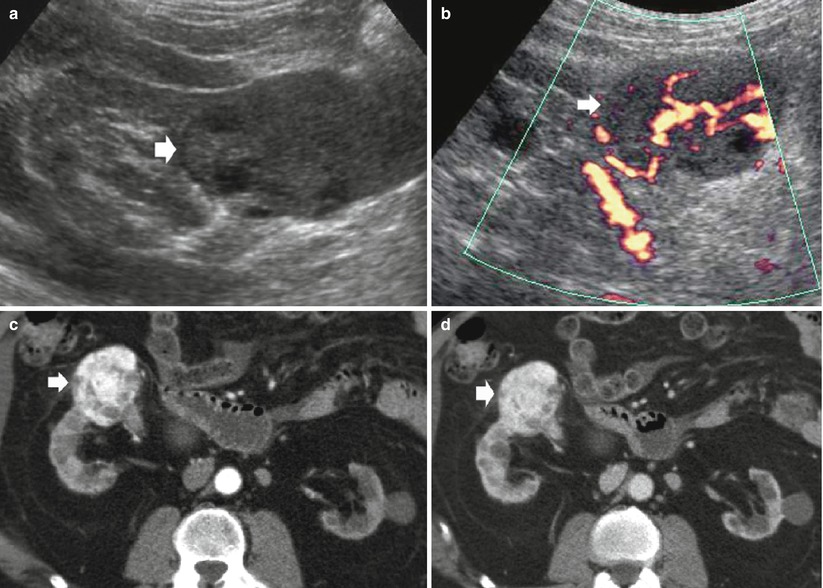
Fig. 11
(a–d) Clear cell renal cell carcinoma in a patient undergoing hemodialysis for 2 years and evidence of initial acquired cystic kidney disease. (a) Grayscale ultrasound. (b) Power Doppler ultrasound. Contrast-enhanced CT during corticomedullary (c) and nephrographic phase(d). Both kidneys present reduced dimensions and diffuse marginal scars. The left kidney presents some parenchymal cysts. (a, b) Evidence of a solid renal tumor (arrow), with some cystic intratumoral areas on the right kidney. The renal tumor presents intratumoral vessels on power Doppler ultrasound(b). After iodinated contrast agent administration, (c, d) the solid renal tumor (arrow) presents diffuse contrast enhancement and reveals infiltration of the anterior renal fascia