Clinical presentation
Imaging study
Stable with penetrating flank/back injury
Chest, abdomen, and pelvic CT using intravenous, oral, and rectal contrast. Consider angiography to exclude pseudoaneurysm
Gross hematuria – hemodynamically stable and/or adequately resuscitated
Abdominal and pelvic CT with oral and IV contrast
Persistent hemodynamic instability – requires emergency intervention (surgery or angiography)
Intraoperative IVP when stable or delayed abdomen and pelvic CT
Hemodynamically stable with microscopic hematuria without other indication for abdominal-pelvic CT
Observation until hematuria resolved
Hemodynamically stable with microscopic hematuria and with other clinical indication for abdominal-pelvic CT
Abdomen and pelvic CT with oral and IV contrast
Hemodynamically stable with or without microscopic hematuria, but findings of direct flank impact (lower rib fracture(s), lumbar transverse process fracture(s), flank ecchymosis, or pain on palpation)
Abdomen-pelvic CT with oral and IV contrast
3 Penetrating Renal Trauma
Penetrating trauma accounts for about 10 % of cases encountered in acute traumatic injuries of flank and back. Between 15 and 42 % of these injuries are accompanied by microscopic hematuria, but the absence of hematuria does not exclude major injury to the renal parenchyma, pedicle, and proximal collecting system. Federle et al. (1987) reported 13 patients with renal pedicle injuries among 41 sustaining penetrating renal injuries. Rather than surgical exploration, currently most hemodynamically stable patients with these injuries undergo contrast-enhanced MDCT to determine the location and extent of injury. In most cases, the injury can be managed nonoperatively as was true for 20 of 27 patients reported by Federle et al. (1987).
In a study of 143 patients with penetrating renal injuries, Armenakas et al. found the majority were minor parenchymal wounds with 54 % of all injury grades using the AAST classification managed conservatively with only three failures for delayed hemorrhage (1999). In this study, 61 % of patients had injuries involving adjacent structures including the liver, spleen, and diaphragm.
In the author’s practice, penetrating flank and back injuries are investigated as part of an abdominal-pelvic MDCT study. Arterial phase, portal venous phase, and 3–5 min delayed phase images are acquired. All patients with penetrating torso injury receive oral, rectal, and intravenous contrast. The arterial phase images are vital to demonstrate early contrast blushes that indicate bleeding or pseudoaneurysms in order to distinguish these from urine extravasation that may be seen on delayed images after opacification of the collecting system.
Penetrating flank and back injuries can be divided in three broad categories: (1) injury limited to retroperitoneum with no direct involvement of visceral structure, (2) injury involving visceral structure(s) but limited to retroperitoneum, and (3) injuries extending into the intraperitoneal compartment. It is not necessary to perform peritoneal lavage, laparoscopy, and laparotomy to assess the peritoneal compartment as this is reliably accomplished by high-quality MDCT. The presence of oral/colonic contrast is vital to utilize to maintain high accuracy. Patients with no visceral injury are managed conservatively, while those with intraperitoneal extension require laparotomy and potential retroperitoneal exploration. Patients with direct renal injury may or may not require surgery depending on the precise injury. To diagnose the full extent of penetrating injury, the course of the penetrating object needs to be followed. Ballistic objects may follow irregular paths due to deflection (as by bone) and also create a volume of destruction due to their cavitation wave that extends potentially well beyond their direct tract. Renal injury can certainly arise from nonflank and back penetrating torso injuries and is assessed as part of the total abdomen-pelvic study.
4 Renal Injury Grading
The American Association for the Surgery of Trauma (AAST) grading system is based on surgical findings, some of which can be diagnosed by MDCT (Table 2) (Moore et al. 1989). However, several types of renal injury are not included in the AAST that are also commonly encountered by MDCT. The following grading system was developed at the author’s trauma center to address these additional pathologic findings and how they potentially impact treatment (Table 3) (Mirvis et al. 2001).
Table 2
American Association for the Surgery of Trauma renal injury grading scale
AAST injury grade | Description |
|---|---|
I | Renal contusion or subcapsular hematoma with intact capsule |
II | Superficial cortex laceration that does not extend to deep medulla or collecting system or nonexpanding hematoma |
III | Deep laceration(s) with or without urine extravasation |
IV | Laceration(s) extending into collecting system with contained urine leak |
V | Shattered renal parenchyma, renal vascular pedicle injury, or devitalized kidney |
Table 3
MDCT grading of renal trauma (Maryland Shock Trauma Center)
Grade | Injury type |
|---|---|
I | Laceration(s) restricted to cortex |
Renal contusion | |
Less than 1 cm diameter subcapsular hematoma | |
Perinephric hematoma not filling Gerota’s space | |
No ongoing bleeding | |
Segmental renal infarction | |
II | Laceration(s) extends to medulla with intact collecting system |
Greater than 1 cm diameter subcapsular hematoma with intact renal function | |
Perinephric hematoma filling, but not distending Gerota’s fascia | |
No ongoing bleeding | |
III | Injury extends into renal collecting system with urine extravasation limited to retroperitoneum |
Perinephric hematoma that distends Gerota’s fascia but is confined to retroperitoneum | |
No ongoing bleeding | |
IV | Fragmented parenchyma (3 + fragments) – typically devitalized tissue and large perirenal hematoma |
Ongoing bleeding | |
Urine extravasation into peritoneal cavity or continually expanding collection | |
Subcapsular hematoma that compromises renal perfusion | |
Renal pelvic or complete ureteropelvic junction tear | |
Vascular pedicle injury | |
Intrarenal pseudoaneurysm or arteriovenous fistula |
5 Injury Grading and Intervention
The majority of renal injuries are minor constituting 74–98 % (Thomason et al. 1989). Most patients with grade I and II injury are managed successfully nonoperatively, while grades IV and V often require intervention (Cannon et al. 2008; Shariat et al. 2007; Shefler et al. 2007; Wright et al. 2006). In a study by Shariat et al. of 77 patients with grade IV injury (66 % blunt injury and 34 % penetrating), 32 had renal exploration with 63 % managed by renorrhaphy and 37 % with nephrectomy (2008). In a retrospective review of a database with 2,467 patients, Santucci et al. correlated need for renal surgery with injury grade as follows: grade I = 0 %, grade II = 0 %, grade III = 3 %, grade IV = 9 %, and grade V equals 86 % (2001). Nephrectomy is more commonly required for high-grade penetrating injury (Wright et al. 2006). Some predictive factors for nonconservative management of renal injury include separation of the renal poles, nonvisualization of the ipsilateral ureter, multiple points of urinary extravasation, high transfusion requirements, and surgery for concurrent nonrenal injuries (Cannon et al. 2008; Shariat et al. 2008).
Renal injuries typically requiring intervention include vascular injuries (active bleeding or pseudoaneurysm), collecting system disruption, major hematoma, and persistent or enlarging urinoma (Figs. 1, 2, 3, and 4). Often percutaneous interventions as angiographic embolization, catheter or ureteric stent placement, and intra-arterial stenting for main renal artery injury (Dowling et al. 2007) are successful (Figs. 5 and 6). Though less common than blunt renal injuries, penetrating trauma has a higher likelihood to produce vascular injury, and such injuries may manifest in the subacute or late period after injury. Delayed, focused renal CT-angiography may be prudent to perform in cases of deep or widespread parenchymal or hilar involvement.
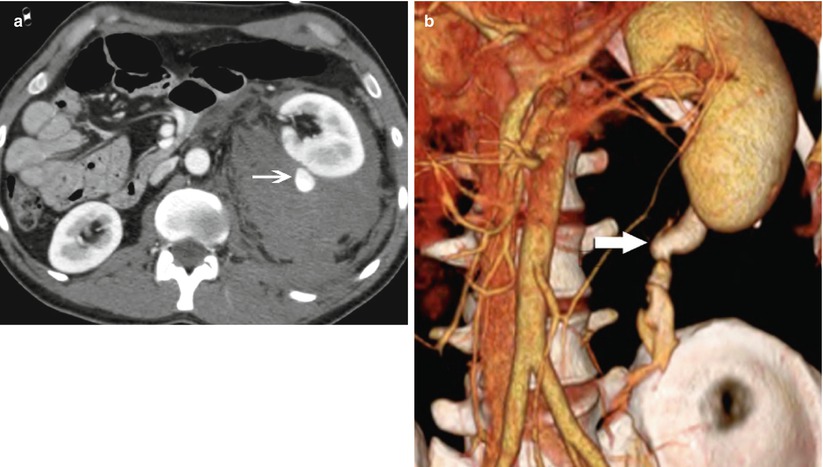
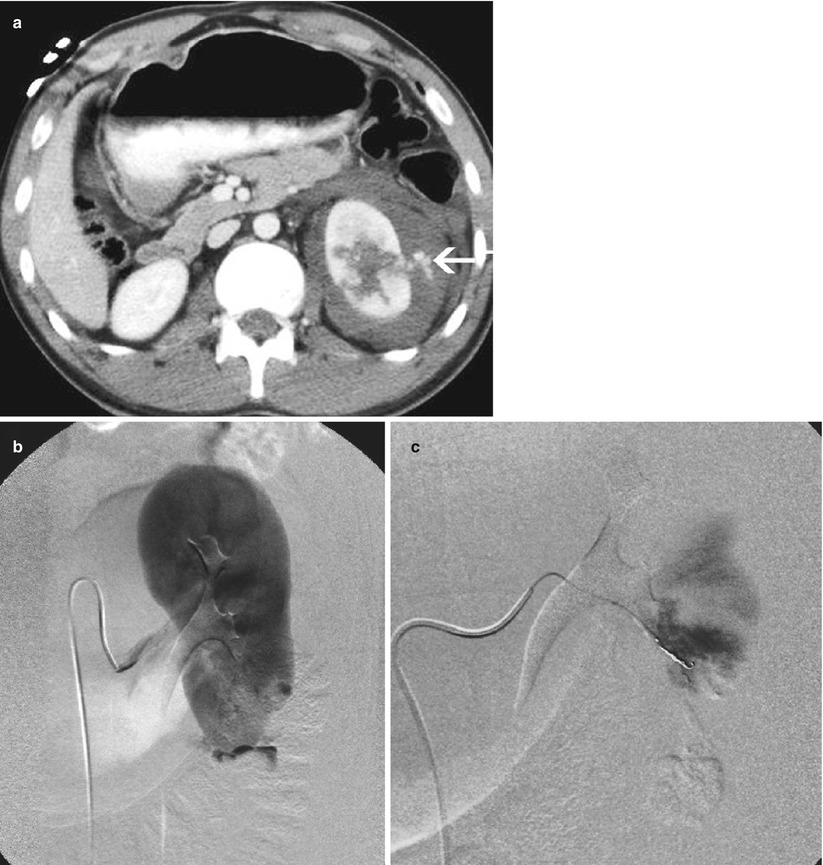
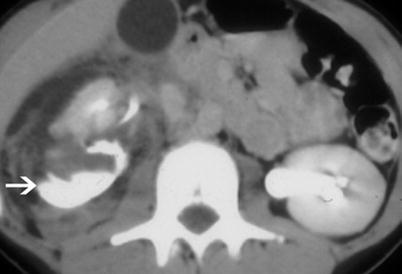
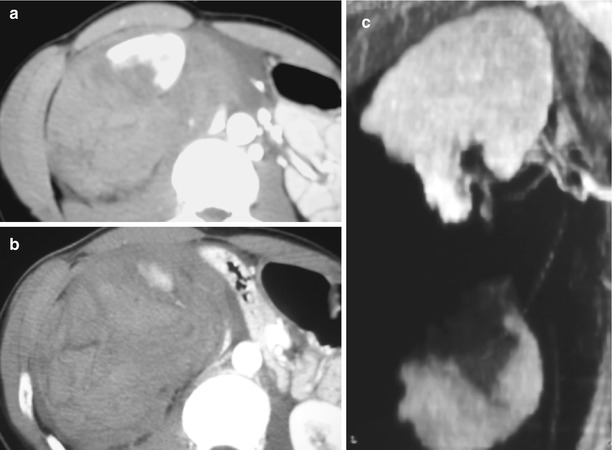
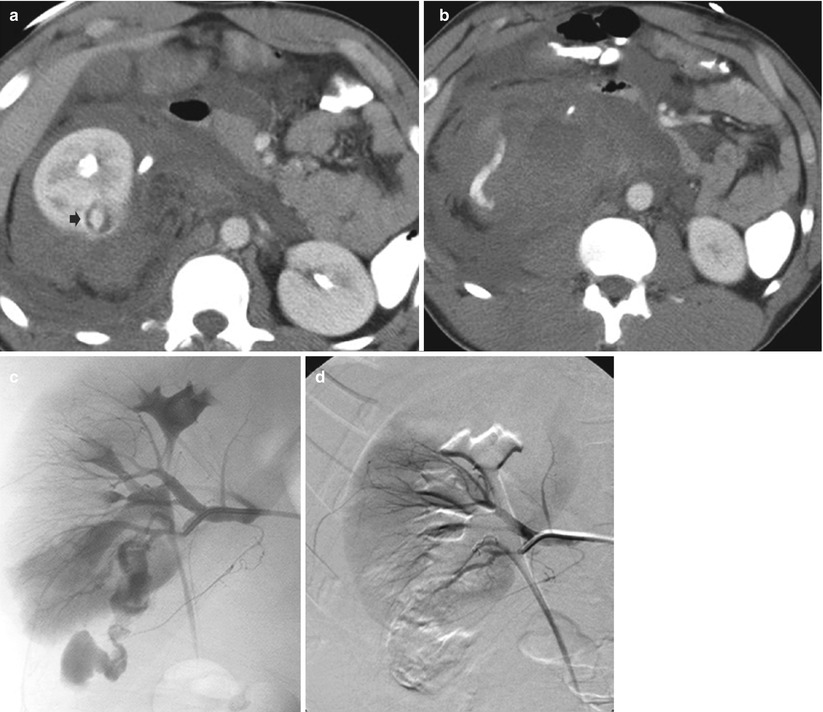
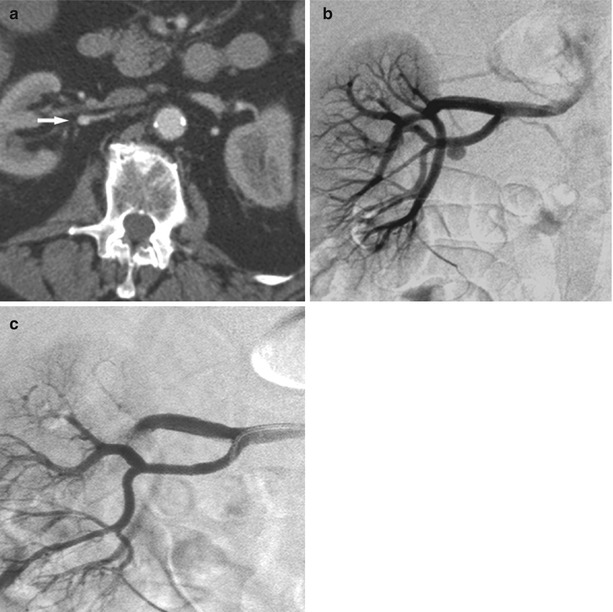

Fig. 1
High-grade renal injury. (a) CT image with intravenous contrast obtained in a 35-year-old man after 25-ft fall shows a large left perinephric hematoma displacing the kidney anteriorly. Active bleeding arises from the posterior aspect (arrow). (b) Volume 3D image demonstrates bleeding tracking inferior to the kidney (arrow). Note the collecting system has not yet opacified helping to distinguish bleeding from urine leak

Fig. 2
Active renal bleeding treated by coil embolization. (a) A 21–year–old man injured in fall sustaining deep lacerations through the mid–left kidney. There is ongoing bleeding into the perinephric hematoma (arrow). (b) Selective renal arteriogram verifies bleeding from lower pole branch. (c) The bleeding is occluded after superselective segmental branch coil embolization

Fig. 3
Urinoma. Urinoma (arrow) collects dependent to the right kidney with urine draining from the central collecting system. Antegrade flow through the ureter is intact

Fig. 4
Catastrophic renal injury – split. (a, b) There is a huge hematoma surrounding fragments of transected right kidney. (c) Coronal reformation shows renal split along the transverse plane

Fig. 5
Embolization for active renal bleeding in a 28-year-old male stabbing victim. (a) CT image with intravenous contrast shows large right perirenal hematoma with focus of active bleeding in posterior aspect (arrow). (b) Large hematoma and some iodinated recent bleeding lie caudal to kidney. (c) Image from renal arteriogram displays site of bleeding from lower pole segment branch tracking inferior to kidney. (d) After proximal coil placement, bleeding has been arrested

Fig. 6
Renal artery stenting. (a) CT image of blunt trauma patient shows a small pseudoaneurysm of the renal artery (arrow). (b) Selective right renal angiogram verifies aneurysm. (c) Pseudoaneurysm successfully covered with stent graft
6 CT Findings of Renal Injury
6.1 Minor: Contusion and Laceration
Contusions (Fig. 7) are seen as ill-defined low-attenuation regions with irregular margins. They may appear with “striated nephrograms” on contrast-enhanced CT patterns due to areas of edema that create differentiated flow through the parenchyma. On delayed noncontrast-enhanced CT, contusions may appear as areas of punctate contrast extravasation on the background of nonenhanced parenchyma (Lang et al. 2009; Mirvis 1996). Superficial laceration(s) is linear to irregular low-attenuation areas limited to the renal cortex and superficial medullary region and typically has a small amount of associated perinephric blood. It should be noted that both lacerations and contusions are best seen on the arterial phase scan when there is peak renal cortical enhancement and are typically less conspicuous on delayed portal and pyelogram phases.
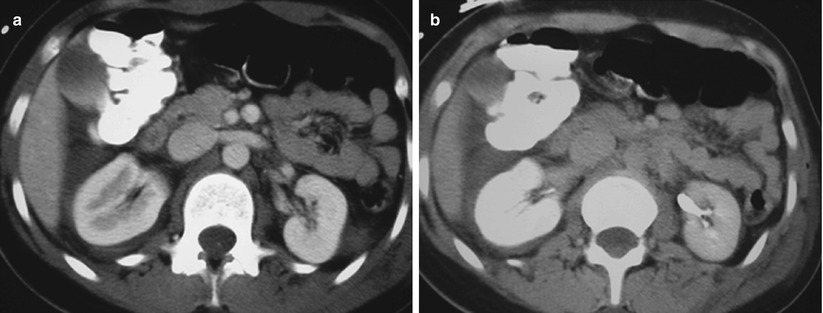

Fig. 7
Renal contusion. (a, b) CT images acquired post blunt trauma in arterial and portal venous phases show enlarged right kidney with delayed nephrogram and pyelogram compared to the left kidney. Finding is most consistent with diffuse renal contusion. Free fluid is seen in hepatorenal recess
6.2 Minor: Focal Renal Infarct
Segmental renal infarcts are relatively common from blunt renal trauma and usually involve the poles. These injuries result from stretching of renal branch vessels, an accessory renal artery, or capsular arteries and subsequent thrombosis (Lewis et al. 1996) (Fig. 8). These infarcts appear as sharply marginated wedge-shaped regions. Often there are no concurrent renal injuries. Renal angiography is usually not indicated as these injuries, in the author’s experience, do not lead to subsequent hemorrhage and do not require embolization as isolated injuries.
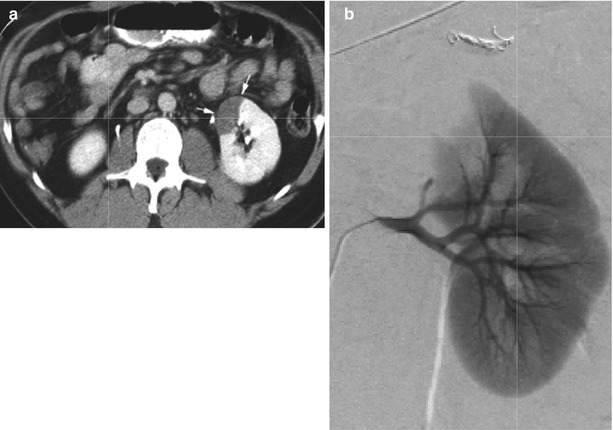

Fig. 8
Segmental renal infarct after trauma. (a) CT image acquired in the portal venous phase shows wedge-shaped area of nonenhancement in the anterior upper segment of left kidney (arrows). (b) Selective renal arteriogram confirms occlusion of segmental vessels supplying superior pole of the kidney. No specific treatment was undertaken
6.3 Minor: Subcapsular Hematoma
Subcapsular hematomas are relatively rare, particularly in older adults as it is difficult to strip the capsule from the cortex to allow bleeding into the potential space. Usually, the capsule tears resulting in a perinephric hematoma. These hematomas are limited in expansion by the renal capsule creating a convex bulge into the renal parenchyma (Fig. 9). Large subcapsular hematomas may produce enough pressure against the parenchyma to delay renal perfusion. In most cases, these injuries will resolve without intervention and will be self-limited by increasing subcapsular pressure.
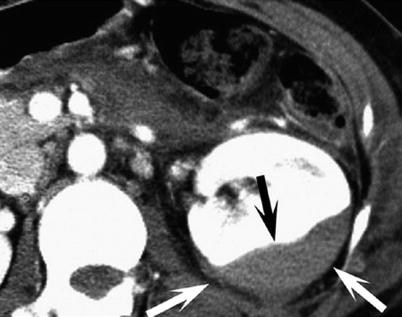

Fig. 9
Subcapsular renal hematoma. Arterial phase CT image shows compression of the enhanced renal parenchyma (black arrow) from overlying hematoma confined by renal capsule (white arrows)
7 CT Finding of Major Renal Injury
Grade III injuries are more significant and may require intervention. Among them are renal fractures extending to the collecting system with contained urine leak, large perinephric hematomas, without ongoing bleeding, and renal parenchymal splits (Fig. 10




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree