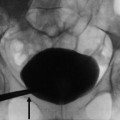
Fig. 1
(a, b) Contrast-enhanced CT. Normal anatomical changes after right radical nephrectomy. Metallic surgical clips (arrow) are evident on the right side
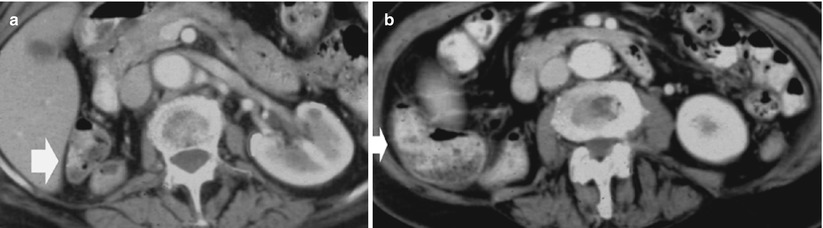
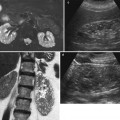
Fig. 2
(a, b) Contrast-enhanced CT. Postsurgical appearance in right nephrectomy bed in a 52-year-old man at follow-up. The right flexure of the colon (arrow) occupies the right side of the liver and the retroperitoneum
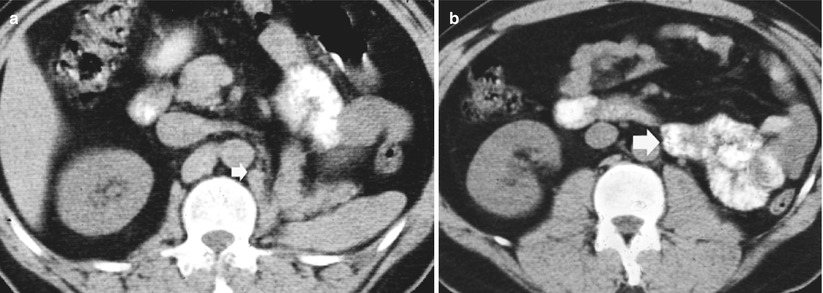
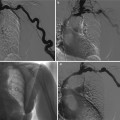
Fig. 3
(a, b) Contrast-enhanced CT. Normal anatomical changes after left radical nephrectomy. The jejunal loops (arrow) occupy the space previously occupied by the kidney
2.2 Partial Nephrectomy: Nephron-Sparing Surgery
Partial nephrectomy – nephron-sparing surgery – entails complete excision of the renal tumor with a margin of at least 0.5 cm of normal renal tissue and preservation of the largest amount of functioning renal parenchyma. Even though partial nephrectomy corresponds to nephron-sparing surgery, the last term includes different surgical techniques, namely, enucleation (nephron-sparing tumorectomy) reserved to benign tumors, polar resection or wedge resection with or without inclusion of the renal excretory tract (partial nephrectomy), or ex vivo resection. Some important changes in the manifestation of RCC have stimulated a growing trend toward nephron-sparing surgical techniques (Novic 1993). In particular, the widespread use of cross-sectional imaging techniques has led to a dramatic increase in the number of incidentally discovered small, asymptomatic renal masses. These asymptomatic tumors, which now constitute 25 % to almost 50 % of all surgically treated RCCs, are generally confined to the renal capsule, relatively small in size, and associated with an excellent prognosis after surgical removal (Konnak and Grossman 1985; Smith et al. 1989).
Partial nephrectomy is now considered the treatment of choice for small renal tumors of less than 4 cm in diameter in elective indication, especially if located in the renal poles or in the renal cortex far from the renal hilum and collecting system. The tumoral invasion of the collecting system represents an absolute contraindication to partial nephrectomy. Partial nephrectomy for hilar tumors (tumors in contact with a major renal vessel on preoperative cross-sectional imaging) is a cutting-edge procedure for which little data are available (Lattouf et al. 2008; Richstone et al. 2008). This surgical procedure avoids nephronic waste with an acceptable morbidity and similar oncologic outcomes compared to radical surgery. Radical nephrectomy presents a significantly higher risk for proteinuria due to hyperfiltration and chronic renal insufficiency in comparison to nephron-sparing surgery (Malcolm et al. 2009; Lau et al. 2000). Laparoscopic partial nephrectomy might allow a more aggressive approach in young healthy patients with an indeterminate mass.
Although radical nephrectomy was long considered the reference standard for the treatment of RCC, the results of numerous studies have demonstrated equivalent survival rates for patients who underwent radical nephrectomy and for those who underwent partial nephrectomy for small renal neoplasms (Lerner et al. 1996; Herr 1999; Lau et al. 2000; Ghavamian et al. 2002; Israel et al. 2006), with reported contralateral recurrence rates of <2 %, ipsilateral local recurrence and distant metastases of <5 %, and 5-year survival rates of 87–90 %, which are comparable to those from radical nephrectomy. Some authors are pushing the limit for partial nephrectomy above the threshold of 4 cm, and more surgeons are either considering anatomical location or technical expected difficulties rather than just the tumor size. Nephron-sparing surgery leads to higher risk of bleeding, especially in case of tumors larger than 4 cm, and it is absolutely necessary to investigate thoroughly the vascularization of the tumor to avoid such complications with exhaustive and accurate preoperative imaging (Peycelon et al. 2009).
The other most important present applications of partial nephrectomy are malignant renal tumors in a solitary functioning kidney in patients with congenitally absent kidney or who had previously undergone contralateral nephrectomy for RCC, malignant tumors in patients with compromised renal function, or multicentric bilateral malignant renal tumors (Provet et al. 1991; Light and Novick 1993). Multiple RCCs can be sporadic in 4–15 % cases, but are much more common in patients with von Hippel–Lindau disease and hereditary RCC (Sheth et al. 2001). In these patients, RCCs are often bilateral, multifocal, and manifest at a younger age. The challenge is to detect and delineate all lesions to ensure complete surgical excision, while preserving the maximal amount of functioning parenchyma. Nephron-sparing surgery has also been performed in young patients with bilateral synchronous Wilms’ tumor (Davidoff et al. 2008). Residual applications of partial nephrectomy correspond to large benign tumors, renal traumas, renal staghorn calculosis, complicated calyceal diverticula, renal duplications with segmental hydronephrosis, and complicated renal tuberculosis.
The appearance of the postoperative kidney depends to a great extent on the size and location of the resected tumor (Israel et al. 2006) and on the imaging time delay after resection. After partial nephrectomy, the kidney presents an abnormal contour (Fig. 4) and presents a posterior displacement adhering to the posterior abdominal wall. This finding, especially if accompanied by reactive or fibrotic changes in the perinephric fat, is indicative of previous renal surgery (Israel et al. 2006). Evidence of surgical metallic clips, visualized on CT or MR by using in-phase and out-of-phase sequences, represents a further usual imaging feature after partial nephrectomy. Several techniques are used for partial resection, which may also include packing the postsurgical defect with fat (Baumgarten and Baumgartner 2000) to help achieve hemostasis. If fat packing (Figs. 5 and 6) is used, the resulting appearance may mimic renal tumors by both CT (angiomyolipoma) and US (angiomyolipoma or hyperechoic RCC). Over time, the volume of fat used for surgical packing may decrease or remain unchanged, while the kidney is usually displaced posteriorly adjacent to the posterior abdominal wall (Fig. 6) (Israel et al. 2006). Extensive postoperative reactive changes in the retroperitoneum are also frequently visible (Figs. 7 and 8) and help in the differential diagnosis from fatty renal tumors.
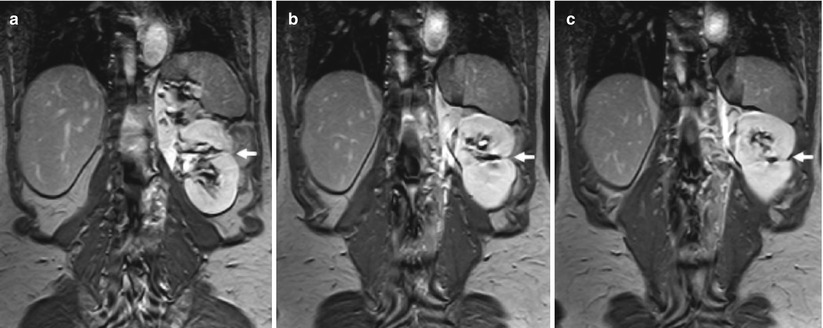
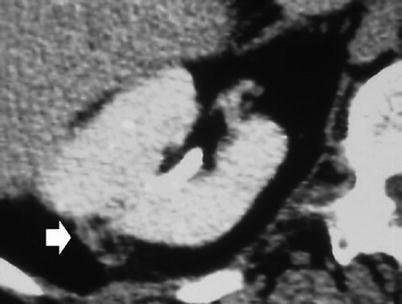
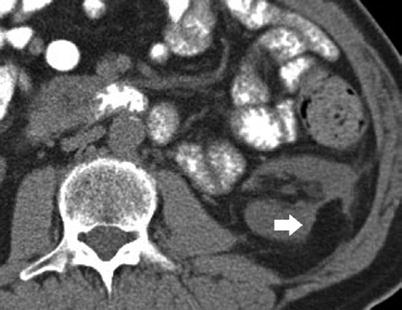
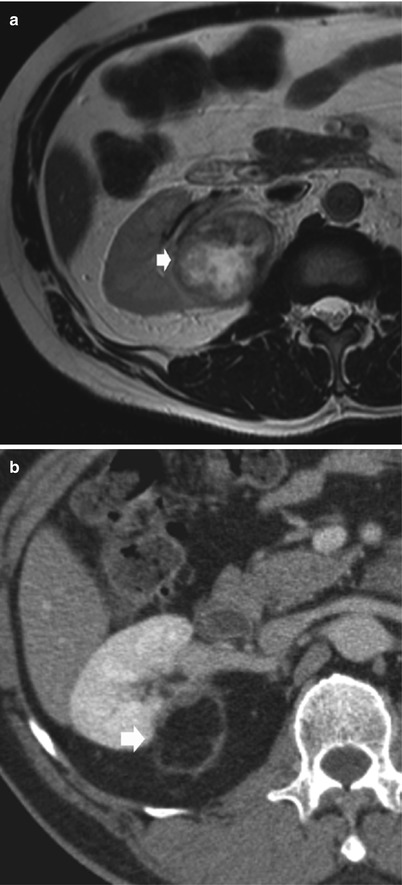
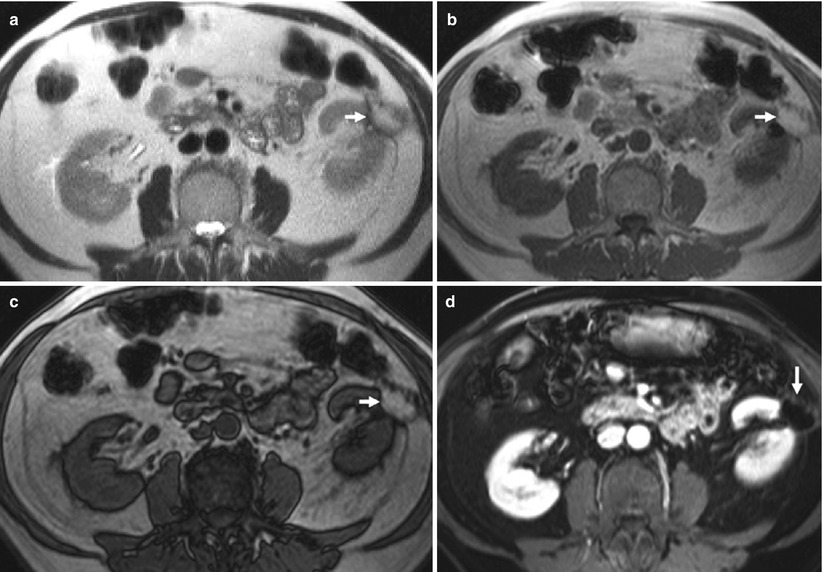

Fig. 4
(a–c) Postoperative findings in a 60-year-old man who underwent a left partial nephrectomy for renal cell carcinoma (RCC). Coronal 3D T1-weighted fast breath-hold spoiled gradient echo sequence for dynamic imaging after intravenous injection of gadolinium (15 mL). MR image obtained 1 year after surgery demonstrates a wedge-shaped postoperative defect (arrows) in the lateral aspect of the kidney. At the apex of the parenchymal defect, a small signal void corresponding to surgical clips

Fig. 5
Right partial nephrectomy. Contrast-enhanced CT, transverse image. Fat packing with retroperitoneal fat (arrow) used to cover the postoperative defect (Courtesy of Olivier Helenon, Paris)

Fig. 6
Postoperative findings after left partial nephrectomy for RCC. Axial unenhanced CT image demonstrates a wedge-shaped postoperative defect (arrow) in the lateral aspect of the left kidney. The postoperative kidney has a more posterior retroperitoneal location and adheres to the posterior abdominal wall. Extensive postoperative reactive changes in the retroperitoneum are visible as peripheral strands surrounding the region of partial nephrectomy (Courtesy of Mark Rosen, University of Pennsylvania)

Fig. 7
Postoperative findings after right partial nephrectomy for RCC. (a) Preoperative axial T2-weighted MR image demonstrates a solid and well-marginated right renal mass (arrow). (b) Axial contrast-enhanced CT image during nephrographic phase obtained 6 months after surgery demonstrates a wedge-shaped postoperative defect (arrow) in the lateral aspect of the right kidney. Reactive changes in the retroperitoneum are visible as a peripheral enhancing strand surrounding the region of partial nephrectomy (Courtesy of Alfredo Blandino, Messina, Italy)

Fig. 8
(a–d) Postoperative findings after right partial nephrectomy for RCC. (a) Axial T2-weighted, (b) in-phase and (c) out-of-phase T1-weighted, and fat-suppressed T1-weighted MR sequence (d) demonstrates a fat-containing mass (arrows) in the postoperative bed. At the apex of the defect, a small focal area of hypointense signal characteristic of postoperative scar tissue is visible (Courtesy of Mark Rosen, University of Pennsylvania)
Biologically absorbable hemostatic agents (Fig. 9) may also be used to help control intraoperative bleeding (Israel et al. 2006). Such materials may contain air pockets or bubbles (Fig. 10) that, on early postoperative images, may resemble a focal abscess (Young et al. 1993; Israel et al. 2006). To differentiate between a collection that consists of a hemostatic agent containing air bubbles and one that consists of infected fluid containing gas bubbles, it is necessary to consider the imaging findings in combination with the patient’s clinical history and symptoms (Israel et al. 2006). Anyway, some imaging findings may be important for the differential diagnosis. In most cases, the air in a hemostatic agent is rapidly resorbed during the first postsurgical week, even though air bubbles can be identified on images even 1 month after surgery (Fig. 10). In-phase and opposed-phase gradient echo MR sequences may be employed to detect the susceptibility artifact related to the surgical clips. The hemostatic agent typically reveals tightly packed gas bubbles with a geometric shape. The presence of an abscess should be suspected if a localized fluid collection that has an enhanced rim and contains gas bubbles or a gas–fluid level is seen. In addition, decreased density of the nephrogram because of edema in the surrounding renal parenchyma supports the diagnosis of an abscess.
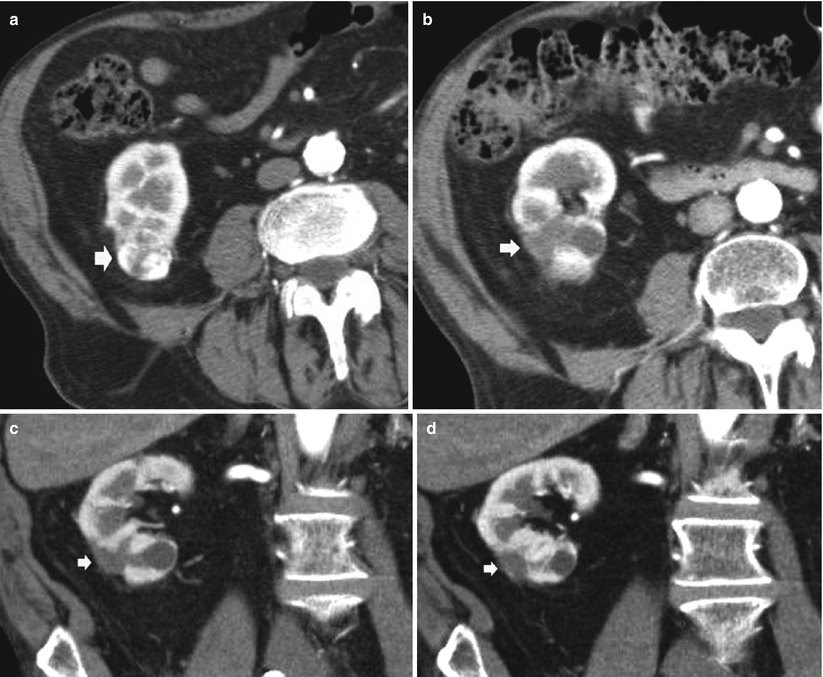
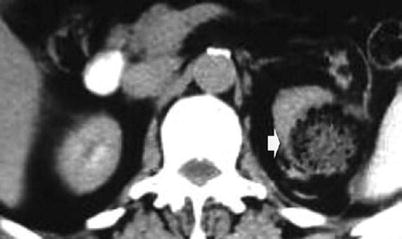

Fig. 9
Biologically absorbable hemostatic agent used to help control intraoperative bleeding at CT in a 65-year-old man after an open right partial nephrectomy for clear cell-type RCC. (a) Axial contrast-enhanced image obtained before surgery shows a hypervascular solid renal tumor (arrow) on the lower pole. Axial contrast-enhanced image (b) and coronal reformations (c, d) obtained after surgery show the biologically absorbable hemostatic agent filling the surgical bed (arrow)

Fig. 10
Tumorectomy for renal oncocytoma filled with biologically absorbable hemostatic agents. Contrast-enhanced CT, excretory phase performed 1 month after surgery. Evidence of air bubbles (arrow) within the hemostatic agent (Courtesy of Catherine Roy, Strasburg)
3 Complications After Renal Surgery
Even though radical nephrectomy is a more simple surgical procedure if compared to nephron-sparing surgery, both techniques may present postoperative complications and not related to tumoral recurrence which are classified as early (within 30 days from surgery) and late (30 days to 1 year from surgery) complications. The most common early complications are acute or progressive renal failure and proteinuria, even though these complications do not present typical imaging features. Other complications include pneumothorax (especially if a thoracoabdominal approach is used), trauma to adjacent structures (inferior vena cava, colon, duodenum, spleen, or pancreatic tail), lateral hernia when a flank incision is used, and postsurgical collections.
3.1 Complications After Radical Nephrectomy
After radical nephrectomy, different acute complications include formation of retroperitoneal fluid collections (hematoma – Fig. 11, lymphocele – Fig. 12, infectious collection – Fig. 13) and abscesses (Fig. 14). CT is the most reliable imaging technique to evaluate the principal complications after renal surgery. MR imaging can also be used to detect tumor recurrence, but the evaluation may be limited by artifact from metallic clips.
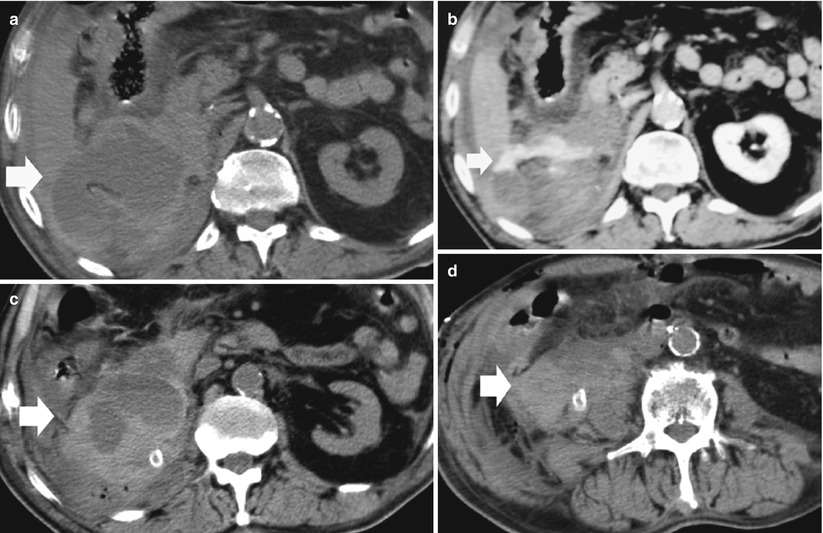
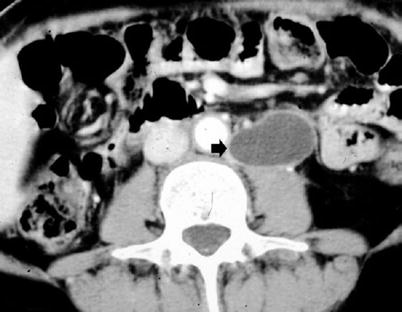
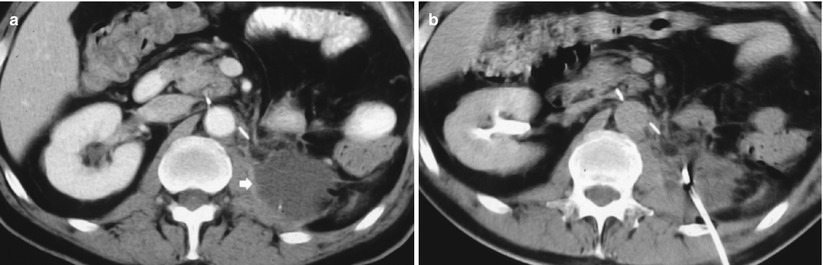
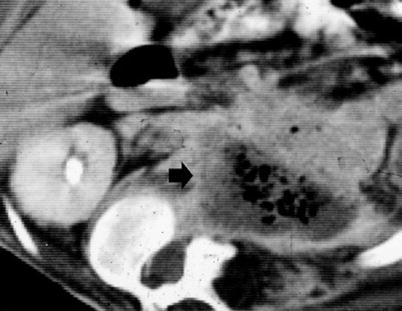

Fig. 11
(a–d) Retroperitoneal hemorrhage after right radical nephrectomy. (a) Unenhanced CT. Evidence of large heterogeneous collection (arrow) on the right retroperitoneum after right radical nephrectomy. (b) Contrast-enhanced CT. Arterial phase. Evidence of active blood extravasation (arrow). (c, d) Unenhanced CT performed 3 days later. CT-guided percutaneous drainage was positioned to drain the collection (arrow)

Fig. 12
Contrast-enhanced CT. Retroperitoneal lymphocele (arrow) after left radical nephrectomy

Fig. 13
(a, b) Contrast-enhanced CT. Retroperitoneal inflammatory collection (arrow) after left radical nephrectomy (a) and treated by CT-guided percutaneous drainage (b)

Fig. 14
Contrast-enhanced CT. Excretory phase. Retroperitoneal abscess (arrow) with air component observed after left radical nephrectomy
3.2 Complications After Partial Nephrectomy
After nephron-sparing surgery, different acute complications may occur including fat necrosis with evidence of fluid level, vascular complications, collecting system complications including urinomas, and infections. The procedure can be performed by using open or laparoscopic techniques. However, nephron-sparing surgery, especially when performed with laparoscopic techniques, is a more complex operation than traditional radical nephrectomy, and higher complication rates have been reported (Campbell et al. 1994; Israel et al. 2006). A worldwide literature review revealed a major complication rate of 10 % for patients who underwent laparoscopic partial nephrectomy. An overall complication rate of 23 % was reported for laparoscopic partial nephrectomy in a European multi-institutional series (Israel et al. 2006).
Vascular complications after partial nephrectomy may be determined by a damage of the main renal artery (hilar vessels) or the intrarenal vessels. During partial nephrectomy, the renal hilar vessels must be temporarily clamped to ensure a bloodless surgical field, and clamping may injure the arterial intima and lead to thrombosis (Israel et al. 2006). If that complication is not recognized at the time of surgery or in the immediate postoperative period, renal infarction (Fig. 15




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree