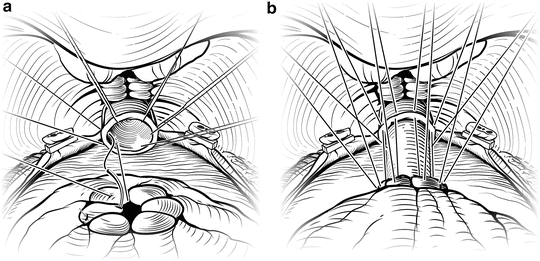
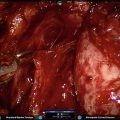
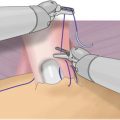
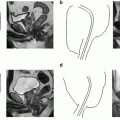
Fig. 7.1
Periprostatic anatomy. The nerves (NVB) run along the posterolateral aspect of the prostate, surrounded by the periprostatic (lateral pelvic) fascia. Until the nerves are fully released from the prostate, traction on the prostate should be kept to a minimum to avoid damaging these delicate structures. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Preoperative Assessment
A successful operation begins with proper patient selection and careful surgical planning, so that the operation best suited to the patient’s anatomy and the characteristics of his cancer can be performed. Before entering the operating theater, the surgeon must have a detailed understanding of the size, grade, and location of the patient’s cancer, as well as the tumor’s relationship to critical periprostatic structures. The patient’s serum prostate-specific antigen (PSA) levels; digital rectal examination (DRE) results; the grade, the location and extent of cancer in each biopsy core; and the presence of perineural and lymphovascular invasion and extraprostatic extension (EPE) can help to define the location and extent of the cancer. This information can also be incorporated into preoperative nomograms to estimate the probable pathologic stage of the cancer, the site of EPE, and the chances of long-term cancer control [5].
Magnetic resonance imaging (MRI) can be helpful in delineating the anatomic details to assist in tailoring the operation, particularly with regard to dissection of the neurovascular bundles [6], the apical dissection and vesicourethral anastomosis, and the extent of bladder neck excision [7]. Most tumors are grossly localized to the prostate, but areas of microscopic EPE may be present. Knowledge of the presence and location of EPE allows the surgeon to perform wider excision in the involved areas to decrease the risk of positive margins and cancer recurrence. Measurement of the length of the membranous urethra on MRI helps to predict the risk of postoperative urinary incontinence, and it allows the surgeon to avoid over-dissection of the urethra beyond the apex and to take small bites of the urethra with the anastomotic sutures. In a study of 211 consecutive patients treated with RP by a single surgeon from 1999 to 2001, 120 of the 134 patients (89 %) with preoperative urethral length greater than 12 mm were continent at 1 year, versus only 35 of the 46 patients (77 %) with a length of 12 mm or less [7]. After controlling for age and surgical technique, a multivariate analysis found membranous urethral length to be associated with a shorter time to stable postoperative continence (p = 0.02). Thus, in a patient with a long urethra, variations in surgical technique may not be significant, but in a patient with a short urethra, imprecise surgical technique may substantially reduce recovery of continence.
Patients with newly diagnosed clinically localized prostate cancer (T1–T2) have a low probability of occult metastases. “Extent-of-disease” imaging, or a metastatic work-up, is unnecessary unless the patient’s PSA levels or Gleason grade are high. Bone scans are rarely positive if the PSA is less than 20 ng/mL [8, 9]. However, patients with an aggressive cancer (PSA > 20 ng/mL or Gleason sum > 7), advanced local lesions (T3–T4), or symptoms suggestive of metastases should undergo axial skeletal imaging with bone scan or body MRI before a final treatment decision is made. An MRI of the prostate is adequate to assess the pelvic lymph nodes, but in high-risk patients an abdominal pelvic CT should be obtained if an MRI is not performed.
Pelvic Lymph Node Dissection
Pelvic lymph node dissection (PLND) has not been shown to improve survival in patients with localized prostate cancer, but long-term observational studies consistently show freedom from biochemical and clinical recurrence in 10–30 % of patients with nodal metastases, suggesting a therapeutic benefit of PLND [10–12]. In a recent article by Schumacher et al., 122 patients with limited lymph node metastases at RP had 60 % 10-year cancer-specific survival without the use of immediate adjuvant androgen deprivation therapy [13]. Because PLND may delay or prevent biochemical recurrence and the need for additional therapy, we recommend PLND if the risk of nodal metastases predicted by nomogram is ≥2 %. Few patients whose risk is <2 % are appropriate candidates for RP; most are managed expectantly by active surveillance [14–16].
No prospective studies have delineated the appropriate anatomic limits of PLND, and much controversy exists over appropriate templates. Some surgeons remove only the few nodes near the inguinal ligament (node of Cloquet); others take only the external iliac lymph nodes above the obturator nerve, while others routinely perform a more extensive dissection that includes the obturator and hypogastric areas. Extended lymph node dissections not only remove a greater number of lymph nodes, they also are more likely to detect positive lymph nodes. In a study by Heidenreich et al., lymph node metastases were diagnosed in 27 % of patients with extended PLND, compared with only 12 % of patients who underwent standard PLND [17]. We confirmed Studer’s finding that 60 % of all positive nodes lie beneath the obturator nerve. Thus, performing a full, extended PLND in intermediate- and high-risk patients is important, both for its increased staging accuracy and for its potential therapeutic benefit. (See Chap. 4 for further details on pelvic lymphadenectomy.)
Surgical Technique for RP
The patient is positioned flat on the operating room table in the supine position, with the pubis over the break in the table. As the operation proceeds, the table can be flexed to improve exposure (Fig. 7.2). Excessive flexion should be avoided and the duration of flexion minimized, particularly in patients with a history of lower back pain or sciatica, to decrease the risk of spinal flexion injuries. Prophylactic antibiotics should be given before the incision is made. The patient is prepped with antiseptic solution (chlorhexidine), and an 18 Fr Foley catheter is placed in the bladder. An 8–10-cm midline skin incision is carried down through subcutaneous tissues. The fascia is sharply incised along the linea alba in the midline, exposing both rectus muscles, which are freed from the posterior rectus sheath laterally, elevating the inferior epigastric vessels. The fascial dissection can be 4–6 cm longer than the skin incision, allowing sufficient exposure. A Turner Warwick retractor is placed superiorly to gain full exposure of the deep pelvic nodes. The vas is mobilized anteriorly but not transected, exposing the external iliac vessels to the hypogastric artery. The prevesical space is opened and the lymphadenectomy is performed at this time, if indicated. Care is taken to seal all large lymphatic vessels with clips or sutures to minimize lymphocele formation.
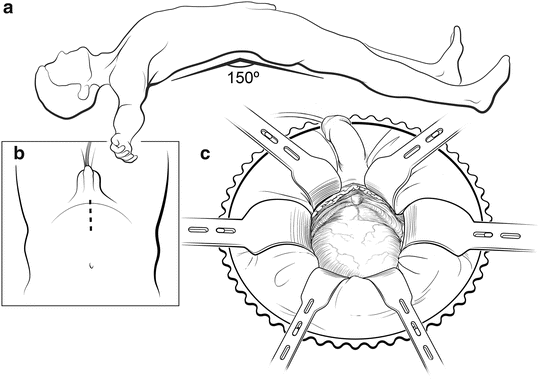

Fig. 7.2
Patient positioning. The patient is placed in the supine position with arms perpendicular to the body (a). The table is slightly flexed and placed in Trendelenburg to make the suprapubic area level. An 8- to 10-cm midline skin incision is made to expose the retropubic space (b). A Turner Warwick retractor is used to expose the operative field (c). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Dorsal Vascular Complex
The first step in mobilizing the prostate is to divide the endopelvic fascia and isolate and ligate the dorsal vascular complex (DVC). The fat anterior to and lateral to the prostate is teased away to expose the underlying anatomy, and the endopelvic fascia is incised laterally in the deep natural groove between the prostate and the levator ani muscles (Fig. 7.3). Only the fascia itself should be incised (a few millimeters thick); the remaining fibers of the levators attached to the prostate should be dissected bluntly to minimize bleeding. Extensive cauterization of bleeding in the levators could damage the pudendal nerve, leading to incontinence. The fascial incision is extended anteriorly and the puboprostatic ligaments divided only if necessary. The surgeon should be able to pass his index and middle fingers around the apex of the prostate (Fig. 7.4). It is critical that this step be done using tactile and not visual feedback. The apex of the prostate need not be completely visualized, as this often results in over-dissection and possible damage to the external rhabdosphincter. The puboprostatic ligaments need not be divided if the apex of the prostate is adequately mobilized, but if necessary, these ligaments should be sharply incised at their attachment to the pubic bone, with care taken to not damage the DVC.
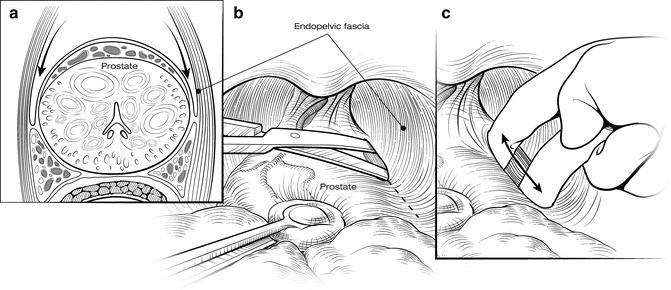
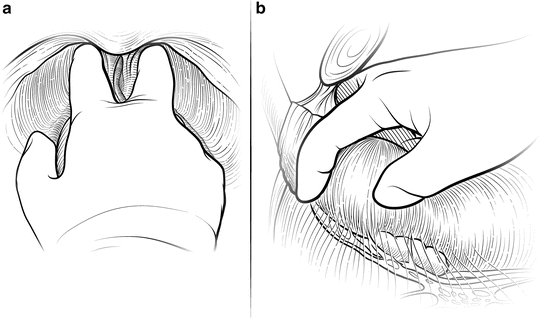

Fig. 7.3
Opening the endopelvic fascia. Adipose tissue overlying the apex of the prostate and endopelvic fascia is teased free and sent for pathology examination, because it may contain lymph nodes. The endopelvic fascia is incised within the natural groove between the prostate and levator ani muscles (a, b). Blunt digital dissection is used to accentuate the incision (c). Fibers of the levator ani muscle can be swept off the prostate bluntly. Care must be taken to avoid rupturing the venous plexus on the lateral aspect of the prostate. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission

Fig. 7.4
Mobilizing the apex of the prostate. Once the endopelvic fascia has been opened bilaterally, blunt dissection is used to gently mobilize the apex before proceeding with ligation of the dorsal vascular complex (DVC). The surgeon should be able to pass his index and middle fingers around the apex and feel the urethra to ensure adequate mobilization. This step should be done only with tactile feedback. If the apex can be completely visualized, it has probably been over-dissected, potentially risking damage to the external rhabdosphincter. Any suspicious nodules at the apex, particularly with an anterior tumor, should be noted. The puboprostatic ligaments do not typically need to be divided to gain adequate exposure. However, in patients with a wide DVC or large gland, releasing the puboprostatic ligaments may assist with adequate mobilization. Scissors can be used to incise the ligaments at their attachment to the pubic bone to avoid lacerating the superficial dorsal veins. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
A figure-of-eight suture ligature is then placed approximately 1 cm cephalad to the anterior bladder neck, to ligate the superficial DVC roughly at the equator of the Foley balloon when placed on tension. This suture is also helpful in identifying the proper location for dividing the bladder neck later in the operation and to minimize bleeding. The first deep figure-of-eight suture is placed around the superficial and deep DVC at the mid-prostate, encompassing both cut edges of the endopelvic fascia. A Babcock clamp can facilitate the placement of this suture by cinching the fascial edges (Fig. 7.5a), or, alternatively, a sponge stick can be used to retract the fascial edges (Fig. 7.5b) as the suture is placed.
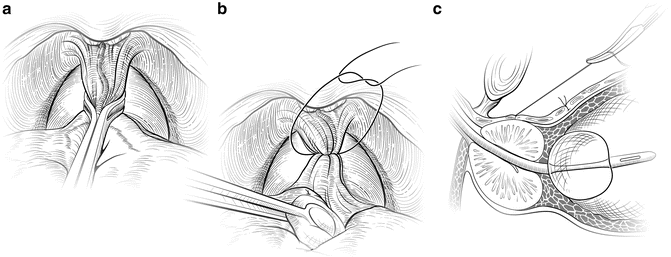

Fig. 7.5
Control of the deep dorsal vascular complex (DVC). The superficial branch of the dorsal vascular complex is ligated proximally (a and b) and distally (Fig. 7.7) before incising the DVC over the urethra. A second figure-of-eight suture is placed around the deep DVC at the mid-prostate. It may be helpful to gather up the DVC between the cut edges of the endopelvic fascia using a Babcock clamp (a) or a sponge stick (b). The suture should not be placed too laterally, to avoid tethering the neurovascular bundles. This stitch is kept long and tagged for later use in the apical dissection (c). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
The deep DVC is ligated distally after the DVC is isolated from the urethra just beyond the apex of the prostate. The fascial layer surrounding the DVC and urethra is bluntly ruptured (Fig. 7.6) with the index finger, allowing the surgeon to appreciate an indentation between the urethra, filled with the Foley catheter, and the DVC. Gentle traction on the Foley catheter can help identify the urethra. A long, blunt-tipped right-angle clamp is passed between the DVC and the urethra and is used to grasp a 26-gauge stainless steel wire (Fig. 7.7). The wire is pulled through, and the ends are grasped with a heavy clamp. The wire loop will be used as a guide to transect the DVC after the distal suture ligature is placed through the DVC a few millimeters dorsal and caudal to the wire. The suture is anchored through the posterior periosteum of the pubis and tied securely, controlling bleeding from the DVC when it is divided. Care is taken to not encompass the levator muscles or the urethra. This maneuver reduces blood loss by several hundred cubic centimeters (cc).
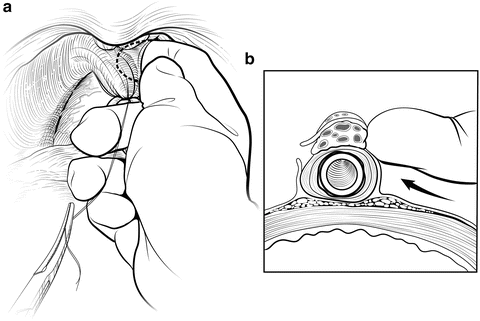
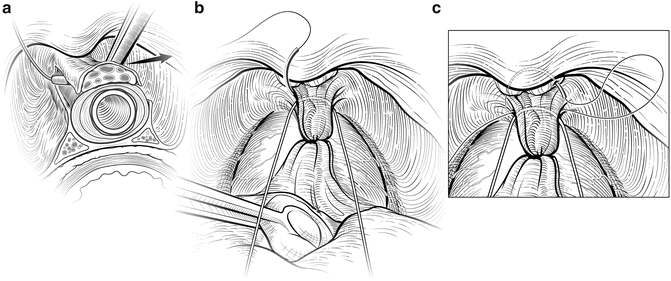

Fig. 7.6
Disruption of the lateral pelvic fascia over the dorsal vascular complex (DVC) and urethra. Tension is maintained on the tagged suture to provide countertraction. The surgeon uses his pointer finger to feel the subtle indentation between the urethra and DVC (a). Manipulation of the Foley catheter can assist in properly identifying the limits of the urethra. The fascia is digitally disrupted on both sides (b). With one finger in the space as a guide, the surgeon passes a long-tipped right angle clamp beneath the DVC. The clamp must be placed as close to the apex as possible to avoid damaging the rhabdosphincter. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission

Fig. 7.7
Ligation of the dorsal vascular complex (DVC). A blunt-tipped (Mixter) right-angle clamp is used beneath the DVC to grasp a 26-gauge stainless steel wire, which is pulled through and clamped. This wire serves as a guide to delineate the DVC. A suture ligature is placed a few mm caudal and dorsal to the wire (b) and is then anchored through the periosteum of the symphysis pubis (c). The stitch is slowly tightened, cinching the DVC against the public bone to secure hemostasis. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Holding the steel wire vertically as a template, the DVC is transected sharply with a fresh No. 15 surgical blade (Fig. 7.8). The surgeon can adjust the amount of upward tension on the wire and downward traction on the prostate with a sponge stick to divide the DVC sufficiently distal to the apex, minimizing the chance of a positive anterior surgical margin. Transection too distally, however, risks incontinence by damaging the anterior supporting structures of the distal sphincter mechanism. Any residual DVC bleeding can be controlled by sewing the incised edges of the lateral pelvic fascia over the DVC with a running vertical suture. Again, this suture is anchored through the periosteum of the symphysis pubis to compress the vessels and reestablish the suspension provided by the puboprostatic ligaments. If necessary, back-bleeding from the prostate can be controlled with a hemostatic suture, clips, or bipolar cautery. Care should be taken to not draw the neurovascular bundles (NVB) medially if a suture is used.
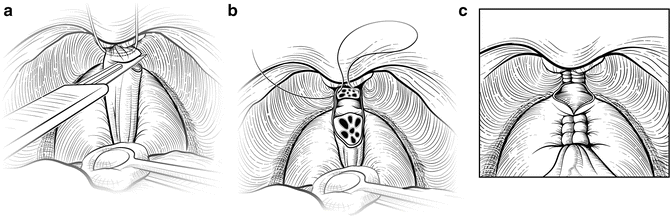

Fig. 7.8
Division of the dorsal vascular complex (DVC). The wire serves as a guide to allow a guillotine transection of the DVC. With the wire held vertically and a sponge stick on the prostate for countertraction, the surgeon uses a No. 15 blade scalpel to incise the DVC (a). Tension on the wire and sponge stick can be adjusted to ensure that the DVC is divided sufficiently distal to the apex to minimize the chance of a positive anterior surgical margin. The anterior urethra should be fully visible once the DVC is fully transected. If necessary, a scalpel or scissors can be used to cut any remaining DVC tissue to fully expose the urethra. Bleeding is controlled, if necessary, by sewing the incised edges of the pelvic fascia lateral to the DVC with a running vertical suture (b), which is anchored through the periosteum. A running suture can be placed on the prostate side to minimize back-bleeding (c). Care must be taken to avoid medial displacement of the neurovascular bundles (NVBs). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Nerve Sparing
Once adequate hemostasis is achieved, the apex of the prostate and the urethra should be readily visible. The surgeon must now mobilize the prostate away from the NVBs while minimizing traction or cautery on these delicate nerves. Gentle dissection is essential at this stage of the operation. The surgeon should feel as if (s)he were operating on the sacral nerve roots of the spinal cord. The patient’s recovery of erectile function is highly dependent on the care with which these fragile structures are handled. The following procedural steps are neither serial nor exclusive; they are often done concurrently, depending on the patient’s anatomy.
Early Lateral Release of the NVB
The NVBs may be released using a lateral approach (Fig. 7.9) [18]. Early release of the NVB minimizes traction on the nerves during further manipulation of the prostate. A sponge stick is used to retract the prostate medially, and the fascia is incised medial to the NVBs using scissors or a scalpel. A Kittner (“peanut”) dissector helps to gently widen the plane between the NVB and the prostate. The incision can be made more medial or lateral, based on the extent and location of the patient’s cancer. At this point the surgeon must decide whether to dissect between Denonvilliers’ fascia and the capsule of the prostate (“intrafascial dissection”), leaving the fascia covering the medial NVB, or to dissect more widely, outside the fascia, preserving the NVB but leaving Denonvilliers’ fascia on the prostate (“interfascial dissection”). Small venous branches of the NVB are encountered that can be isolated and ligated with small hemoclips placed parallel to the NVB. The fasciotomy is extended from the apex to the level of the seminal vesicles. Once a sufficient plane has been developed, it can be extended dorsally and medially around the prostate in the same plane. Once the NVB is released, Denonvilliers’ fascia must be sharply incised posteriorly, leaving Denonvilliers’ fascia covering the posterior prostate to minimize the risk of a positive margin. The yellow anterior fat of the rectal wall should be clearly visualized, indicating a proper depth of the dissection. Throughout this dissection, the surgeon must follow the contour of the prostate to assure complete resection of the cancer and avoid injury to the NVB.
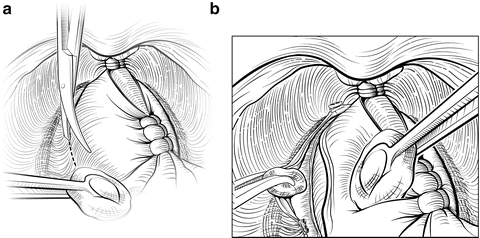

Fig. 7.9
Lateral release of the left neurovascular bundle (NVB). Early release of the NVB will minimize traction on the nerves while mobilizing the prostate. A sponge stick is used to rotate the prostate to the contralateral side. A small fasciotomy is made in the groove between the NVB and the prostate (a). Depending on the location of the tumor, the incision can be made more medial or lateral. The fasciotomy is then extended using a Kittner or sharp tipped right-angle clamp (b). Small vessels are controlled with small hemoclips or bipolar focused electrocautery. Once the NVB has been completely released anteriorly, Denonvilliers’ fascia will be encountered posteriorly once the NVB is mobilized. This layer should be sharply incised so that the anterior rectal fat is visualized, allowing for complete lateral release. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Retrograde Release of the NVB
In some patients, the lateral fascial planes may not develop easily, and release of the NVBs may be easier if the dissection starts at the apex of the prostate. The anterior two-thirds of the urethra is sharply divided at the apex of the prostate (Fig. 7.10), with care taken to maximize the length of the urethral stump. When the prostate is retracted cephalad, the plane between the urethra medially and the NVB laterally can be gently developed using a Kittner inserted just distal to the apex of the prostate. The Kittner is repeatedly inserted into the groove and rotated with gentle but persistent downward force toward the rectum, following the contour of the prostate. This manipulation will slowly push the NVB lateral, away from the urethra and expose the digital apex. Further dissection with a Kittner, scissors, or a right-angle clamp can then be used to extend this plane around the apex, releasing the NVBs laterally.
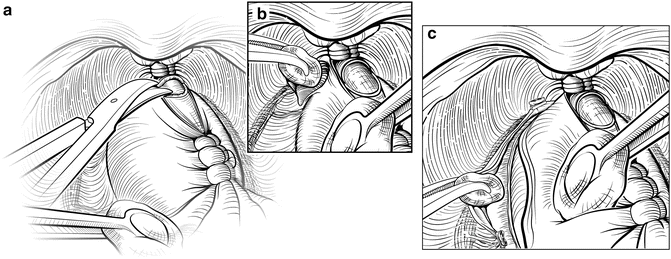

Fig. 7.10
Retrograde release of the neurovascular bundles (NVB). An alternate approach to the lateral release is to explore the plane between the NVBs and the urethra distal to the apex of the prostate. The anterior urethra is divided at the apex of the prostate (a). A Kittner dissector is gently inserted between the urethra medially and the NVBs laterally, developing a space between the urethral stump and apex of the prostate (b). This plane can then be extended laterally around the remainder of the prostate. Finally, Denonvilliers’ fascia is incised to fully release the NVBs (c). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Planes of Dissection of NVBs
Using a combination of these techniques, the NVBs can be spared routinely. Preservation of NVBs is generally not an all-or-nothing phenomenon. Often, all or most of each bundle can be spared, even in patients with a high risk of EPE. A more detailed description of periprostatic anatomy [19, 20] has further defined the alternative planes of dissection for releasing the NVBs (Fig. 7.11). The initial fasciotomy can be made directly adjacent to the prostatic capsule for an intrafascial plane of dissection (complete nerve sparing), which preserves the layer of Denonvilliers’ fascia over the NVBs, protecting the nerve for optimal recovery of function. The fasciotomy can be made more laterally, leaving Denonvilliers’ fascia on the prostate (interfascial plane; partial nerve sparing) to minimize the risk of a positive margin. A wide extrafascial dissection, with complete resection of a nerve, is rarely necessary except when the presence of gross extension of cancer into the NVB is readily apparent. The proper plane for dissection should be chosen based on careful preoperative and intraoperative evaluation of the location, extent, and grade of the cancer. We find MRI particularly useful in this setting to help evaluate the anatomy of the prostate and of the cancer. During the operation, palpable indication or fixation of the layers of fascia can help identify areas of possible extracapsular extension.
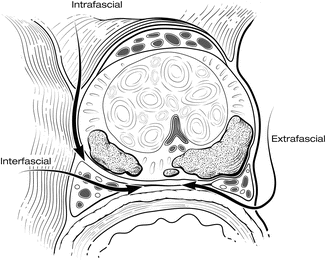

Fig. 7.11
Transverse section of the prostate depicting the planes for release of the neurovascular bundles (NVB). The type of nerve dissection performed must be tailored to the tumor location. The initial fasciotomy can be made directly adjacent to the prostatic capsule for an intrafascial plane dissection (complete nerve sparing), within the lateral prostatic fascia (interfascial plane; partial nerve sparing), or outside the lateral prostatic fascia (extrafascial plane; nerve resection). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
In some high-risk patients, if an extrafascial dissection may be required to achieve negative margins, a CaverMap device (Uromed, Boston, MA) can be used to confirm the functionality of the remaining NVBs [21, 22]. If one or both NVBs are resected, a sural nerve graft is then considered. We believe that this step is best accomplished in collaboration with a plastic surgeon who has microsurgical expertise.
Apical Dissection and Urethral Division (Anastomotic Sutures)
Once the NVBs have been released laterally, the apex of the prostate can be mobilized. If not already completed, the anterior two-thirds of the urethra is sharply divided using scissors or a scalpel. The anterior and lateral anastomotic sutures are placed at 9, 11, 1, and 3 o’clock positions in the urethral stump with the Foley catheter in place (Fig. 7.12). We use a 2-0 monofilament suture on a 5/8 circle needle (UR-6 Monocryl, Ethicon, Inc.). If the urethra is thin, 3-0 Monocryl can be substituted. With the urethra on mild traction and the prostate retracted cephalad, the urethral sutures can be placed, including only 3–4 mm of urethral tissue. Larger bites will shorten the effective urethral length and compromise recovery of urinary continence. We prefer to place these urethral sutures from inside to outside. The catheter is then removed, exposing the undivided posterior urethra. The posterior anastomotic sutures are placed from outside to inside at 5 and 7 o’clock positions so that the deep fibrous layers of Denonvilliers’ fascia are included for added strength. The remaining posterior urethra is then divided, and the dissection continued, lifting the prostate off the rectum beneath Denonvilliers’ fascia.
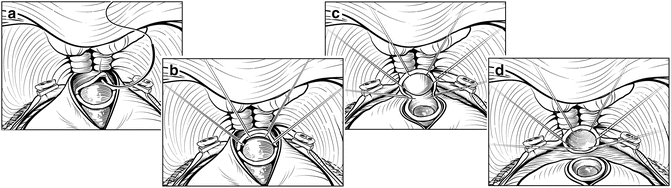

Fig. 7.12
Placement of urethral sutures. If not already done, the anterior urethra is incised over the Foley catheter. Care must be taken to maximize urethral length without creating a positive apical margin. The urethral sutures are then placed in an inside-to-outside fashion. Three-millimeter bites are taken with the urethra under slight traction with a sponge stick. Once the 9, 11, 1, and 3 o’clock sutures are placed, the Foley is withdrawn. The final 5 and 7 o’clock sutures are placed outside-to-inside and should include the fascia posterior to the urethra. The remainder of the urethra is then incised (d). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Posterior Dissection of Lateral Vascular Pedicle and Excision of Seminal Vesicles
As the prostate is mobilized off the rectum, upward retraction on the apex of the prostate allows sharp and blunt dissection all the way to the base of the prostate. If a large, high-grade cancer invades through this thick posterior fascia (rare), this plane can be further deepened to keep perirectal fat on the prostate. Once the prostate has been mobilized, a plastic Foley catheter can be inserted to assist with upward traction. While blunt digital dissection is potentially quicker, we discourage it because it creates traction on the NVBs and may leave some of Denonvilliers’ fascia on the rectum, risking positive posterior margins.
At the base of the prostate the NVBs give off a vascular pedicle, which must be carefully identified, clipped, and divided, allowing the NVBs to separate from the prostate fully. Once the NVBs are completely freed, the thick lateral vascular pedicles of the prostate can be felt on each side as tight bands (Fig. 7.13) as the prostate is retracted ventrally and cephalad. The pedicles contain the large prostatic artery and must be isolated, securely clipped or ligated, to expose the seminal vesicles and fully mobilize the prostate. The pedicles can be isolated with a sharp right-angle clamp, clamped with a long tonsil clamp, or cut and ligated with 2-0 ties. All of the lateral vascular pedicle must be isolated, ligated, and divided to fully expose the seminal vesicles.
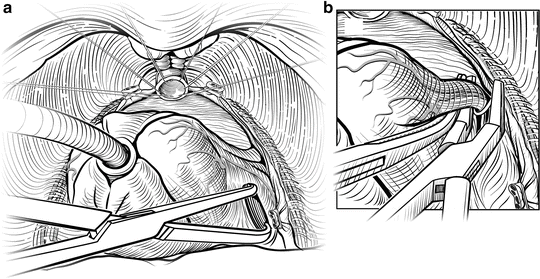

Fig. 7.13
Posterior dissection of the prostate. Once the urethra has been divided, the prostate is placed on upward traction and sharp dissection is used to develop the plane below Denonvilliers’ fascia. A Foley catheter is placed into the bladder to assist with traction. The lateral vascular pedicles require sequential ligation with clips or ties from superficial to deep. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Blunt finger dissection between the seminal vesicles and bladder at the upper third of the seminal vesicles will separate the seminal vesicles (Fig. 7.14), exposing a large arterial pedicle between the bladder and seminal vesicles about midway along the seminal vesicles. These vessels and the small arteries at the tip of the seminal vesicles must be carefully ligated or controlled with bipolar cautery. The vas deferens are also clipped and divided at the level of the tip of the seminal vesicles.
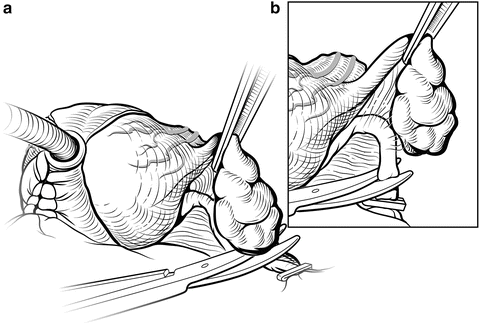

Fig. 7.14
Dissection of the seminal vesicles. A plane is created between the bladder and the seminal vesicles to expose the large vessels at the tips of the seminal vesicles (a). These are controlled with hemoclips and divided (b). Fibrovascular tissue surrounding the seminal vesicles is taken en bloc. The ampulla of the vas deferens is clipped to include the vasal arteries and divided. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Once the seminal vesicles and vasa deferentia are free, the tips can be bluntly dissected off the base of the bladder all the way to the bladder neck. The remaining lateral vascular pedicles contain the prostatic artery and the major vessels from the bladder to the prostate [23]. This “beard” of tissue in the hilum of the prostate should be widely dissected to provide a margin of resection, comparable to the tissue in the hilum of the kidney during a radical nephrectomy.
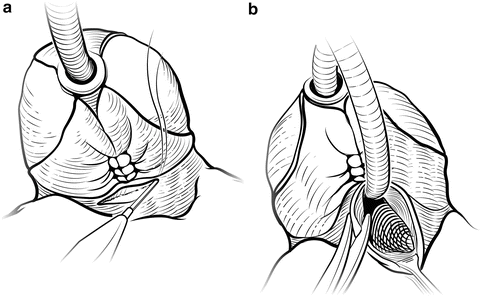

Fig. 7.15
Bladder neck incision. Cautery is used to incise the bladder neck anteriorly over the Foley catheter balloon (a). The balloon is deflated and the Foley is looped to provide traction. The bladder neck incision is carried circumferentially to free the specimen (b). Care is taken to avoid damage to the ureteral orifices. Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Bladder Neck Division and Reconstruction
The prostate is divided from the bladder at the bladder neck, beginning anteriorly where the superficial dorsal vein was marked with a stitch. Electrocautery helps control bleeding (Fig. 7.15). Attempting to spare the bladder neck by tapering it into the prostatic urethra should be avoided to minimize the risk of a positive surgical margin [24]. Sparing the bladder neck has not been shown to improve long-term continence. Traction on the prostate facilitates the dissection. Care is taken to identify the ureteral orifices, which can be confirmed by inserting Number 5 Fr pediatric feeding tubes or simply administering indigo carmine or methylene blue to ensure that the ureters are patent, steps rarely necessary.
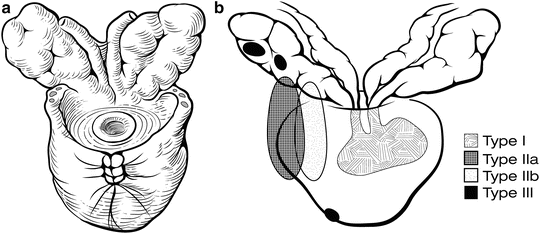

Fig. 7.16
Possible areas of seminal vesicle involvement [22]. The removed specimen should contain both entire seminal vesicles as well as the surrounding fibrovascular tissue at the seminal vesicle base (a). High-risk patients may have seminal vesicle involvement that is direct spread from the ejaculatory duct complex (Type 1); direct spread from the base of the prostate (Type IIa); retrograde spread from the tumor through periprostatic tissue into the seminal vesicles (Type IIb); or isolated skip metastases (Type III) (b). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
The Specimen
Once the specimen is freed, we carefully inspect the entire surface to ensure that there are no obvious positive surgical margins that might require more tissue to be removed (Fig. 7.16). Frozen sections can be used to confirm a positive margin but are of little value for routine detection of positive margins during the operation [23]. Next, the field is irrigated and meticulously inspected for bleeding. Postoperative bleeding after RP inevitably leads to a pelvic hematoma, which can disrupt the bladder anastomosis and lead to a bladder neck contracture or urinary incontinence [25]. In rare cases, patients with postoperative bleeding will need to be re-explored to evacuate the hematoma and control bleeding.
When adequate hemostasis has been achieved, the divided bladder neck is reconstructed. The bladder neck is closed posteriorly in a tennis-racquet fashion tight to a 32 Fr red rubber catheter (Fig. 7.17). The mucosa is everted with fine 4-0 polyglactin absorbable sutures. Sutures are placed at 10, 12, 2, and 4 o’clock, approximately 8 mm apart. At 6 o’clock a suture is placed deep through bladder muscle on both sides to coapt the mucosal edges around the 32 Fr red rubber catheter, which should slide with some resistance through the newly created bladder neck. The placement of the 6 o’clock suture can be adjusted, if necessary, to ensure proper diameter of the bladder neck before this suture is tied. The mucosa should be fully everted 360° around the reconstructed bladder neck. The remaining posterior bladder neck is closed with a running two-layer 3-0 polyglactin absorbable suture. Care must be taken to avoid the ureteral orifices near the trigone by incorporating large bites of detrusor muscle but very small bites of mucosa.
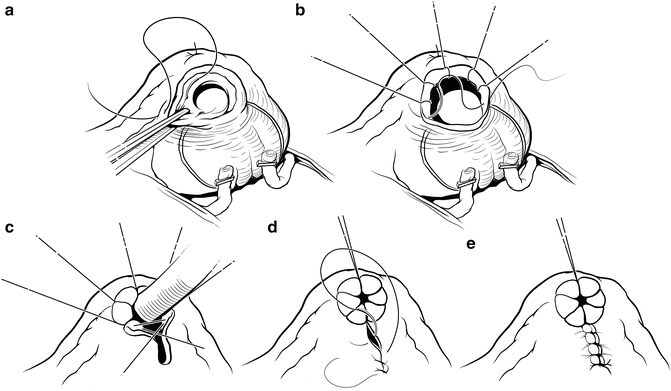

Fig. 7.17
Bladder neck reconstruction. The mucosa is everted anteriorly with interrupted sutures placed at 8, 10, 12, 2, and 4 o’clock approximately 5 mm apart (b). The 6 o’clock suture is placed through muscle on both sides to coapt the mucosal edges and sized to 32 Fr using a red rubber catheter (c). The remaining posterior bladder neck is closed with a running suture (d, e). Courtesy of Memorial Sloan-Kettering Cancer Center © 2013. Used with permission
Once the bladder neck is reconstructed, the retractor is removed and the table leveled, releasing any flexion. An 18 Fr 5 cc Foley catheter is inserted through the urethra. The previously placed urethral sutures are now placed inside out through the newly reconstructed bladder neck using a Ferguson needle (Fig. 7.18). Mucosa and muscle should be included in each of these stitches. The Foley catheter is inflated with 15 mL of sterile water. The catheter is placed on traction to bring the bladder neck down to the urethra, slack is taken out of the sutures, and the bladder is gently retracted with the anastomotic sutures tied in an anterior-to-posterior fashion. The bladder is irrigated to remove clots and to test that the anastomosis is watertight to 180 cc. A proper anastomosis will be watertight with no traction on the catheter. A small self-suction drain is then placed through the anterior abdominal wall through a separate stab incision, and the incision is closed with a running suture. The skin is closed with a running 4-0 Monocryl stitch, and the catheter is secured.