Chapter 1 Opening Round
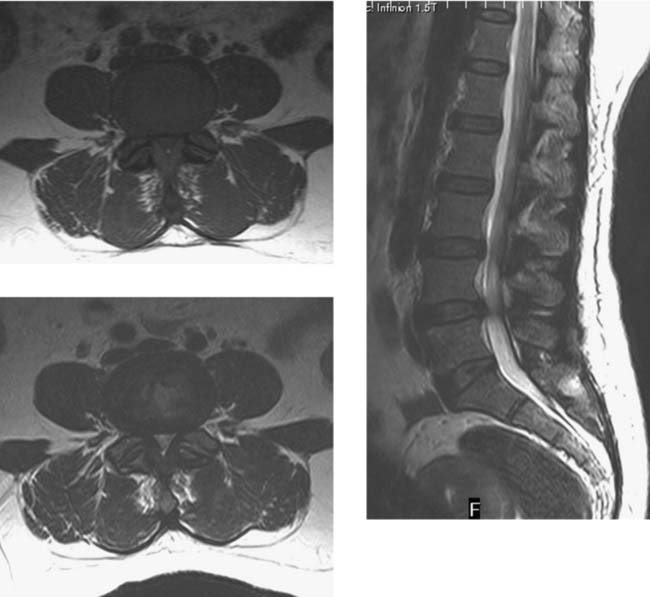
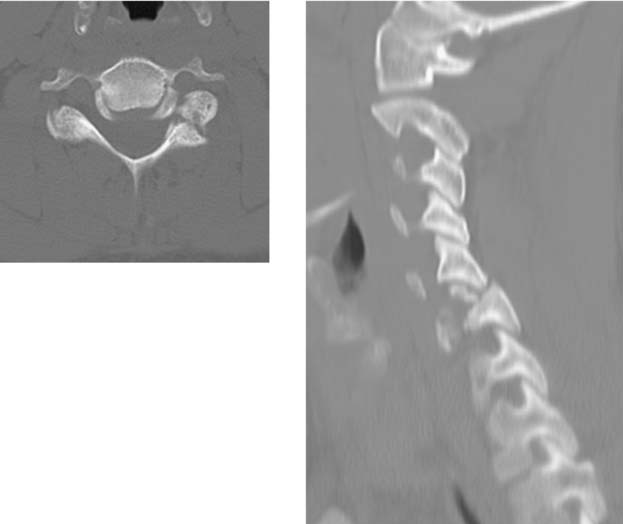
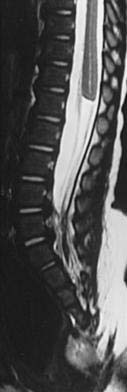
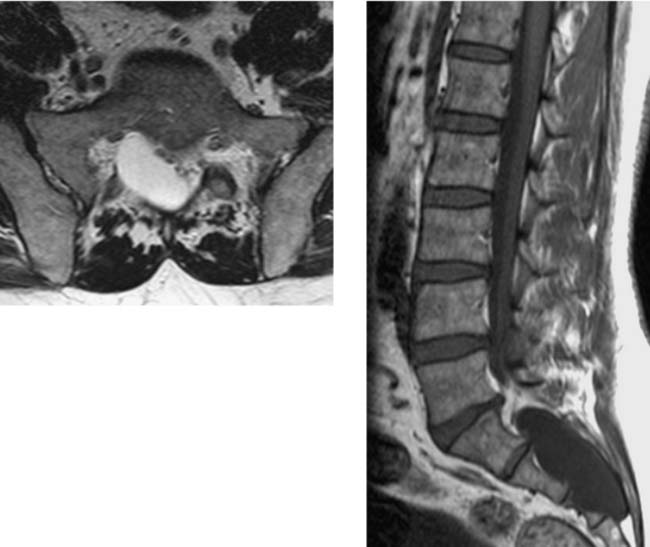
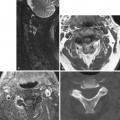
Spinal Stenosis, Lumbar
Goh KJ, Khalifa W, Anslow P, et al. The clinical syndrome associated with lumbar spinal stenosis. Eur Neurol. 2004;52:242-249.
Hiwatashi A, Danielson B, Moritani T, et al. Axial loading during MR imaging can influence treatment decision for symptomatic spinal stenosis. AJNR Am J Neuroradiol. 2004;25:170-174.
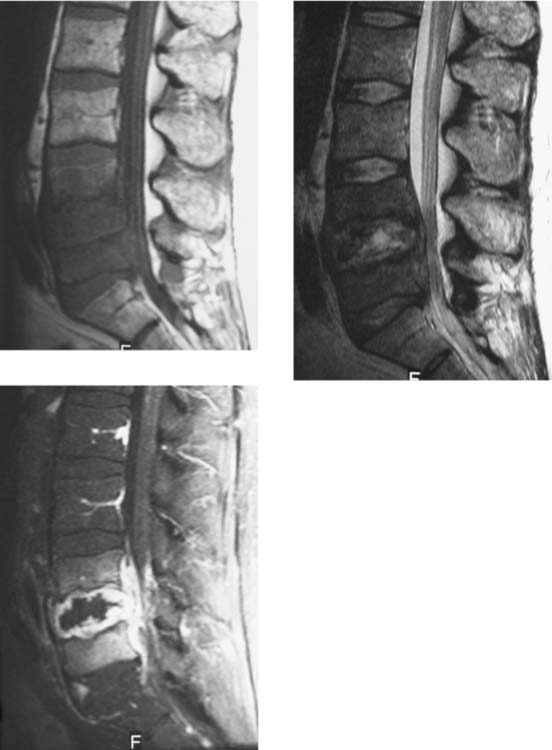
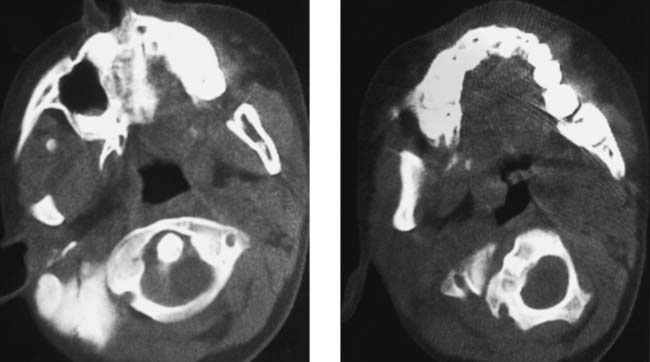
Diskitis/Osteomyelitis, Lumbar
CSF Flow Imaging
Haughton VM, Korosec FR, Medow JE, et al. Peak systolic and diastolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control participants. AJNR Am J Neuroradiol. 2003;24:169-176.
Levy LM. MR imaging of cerebrospinal fluid flow and spinal cord motion in neurologic disorders of the spine. Magn Reson Imaging Clin N Am. 1999;7:573-587.
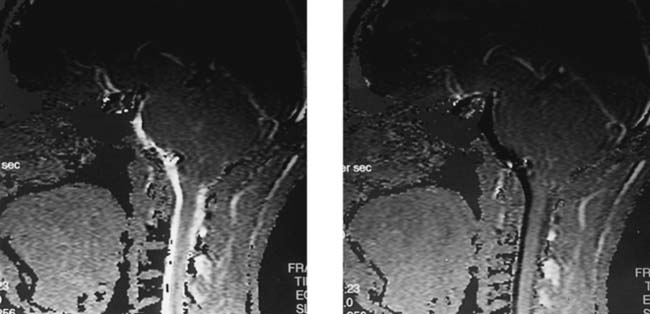
Postoperative, Recurrent Disk Herniation, Lumbar
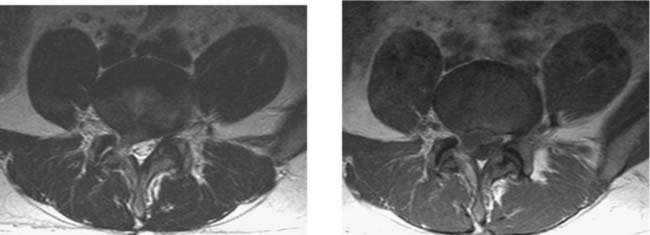
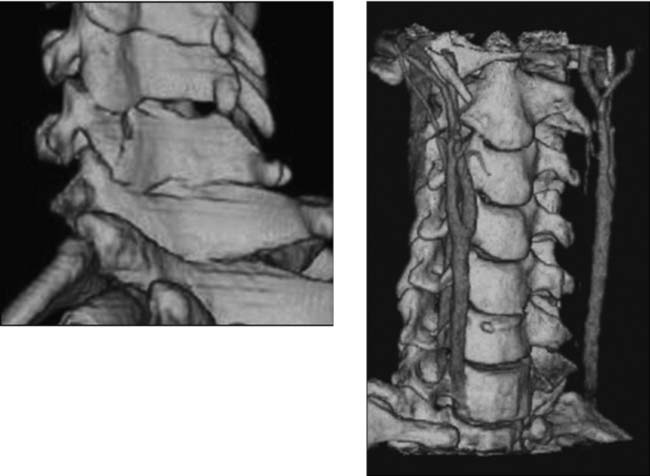
Unilateral Facet Dislocation, Cervical
Levine AM. Facet fractures and dislocations. In: Levine AM, Eismont FJ, Garfin SR, Zigler JE, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:331-366.
Shanmuganathan K, Mirvis SE, Levine AM. Rotational injury of cervical facets: CT analysis of fracture patterns with implications for management and neurologic outcome. AJR Am J Roentgenol. 1994;163:1165-1169.
Atlantoaxial Rotatory Deformity
Currier BL. Atlantoaxial rotatory deformities. In: Levine AM, Eismont FJ, Garfin SR, Zigler JE, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:249-267.
Kowalski HM, Cohen WA, Cooper P, et al. Pitfalls in the CT diagnosis of atlantoaxial rotary subluxation. AJR Am J Roentgenol. 1987;149:595-600.
Comment
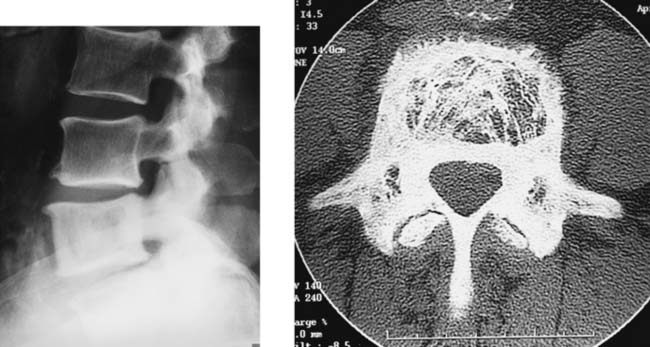
Paget Disease, Lumbar
Traumatic Vertebral Artery Occlusion
Friedman D, Flanders A, Thomas C, et al. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. AJR Am J Roentgenol. 1995;164:443-447.
Sanelli PC, Tong S, Gonzalez RG, et al. Normal variation of vertebral artery on CT angiography and its implications for diagnosis of acquired pathology. J Comput Assist Tomogr. 2002;26:462-470.
Veras LM, Pedraza-Gutierrez S, Castellanos J, et al. Vertebral artery occlusion after acute cervical spine trauma. Spine. 2000;25:1171-1177.
Caudal Regression Syndrome