, Ahmad Ameri1 and Mona Malekzadeh2
(1)
Department of Clinical Oncology, Imam Hossein Educational Hospital, Shahid Beheshti University of Medical Sciences (SBMU), Shahid Madani Street, Tehran, Iran
(2)
Department of Radiotherapy and Oncology, Shohadaye Tajrish Educational Hospital Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran
Almost no patients that are undergoing radiation therapy of head and neck cancers can run away from radiation-induced oral mucositis, especially if the oral cavity is included in the treatment target. Trotti et al. have confirmed the high incidence of induced oral mucositis in a systematic review with rates of 97% during conventional radiation therapy, 100% during altered fractionation radiation therapy, and 89% during chemoradiation therapy [1]. In a retrospective study of 204 head and neck cancer patients that received radiation therapy with or without chemotherapy, oral mucositis occurred in 91% of patients; the rates of mucositis grades 1, 2, 3, and 4 were 4%, 21%, 60%, and 6%, respectively [2].
Another study of 450 head and neck cancer patients that received radiation therapy with or without chemotherapy found that the majority of patients (83%) developed oral mucositis (mild in 19%, moderate in 35%, and severe in 28% of patients).
Oral mucositis has significant pain, dysgeusia, and odynophagia that can result in dehydration, malnutrition, and reduced quality of life scores.
Oral mucositis also can reduce radiation therapy tolerance and consequently affect treatment results. Unplanned breaks/delays in radiation therapy were reported in 2.4%, 15.8%, and 46.8% of patients with mild, moderate, and severe oral mucositis, respectively [3].
6.1 Mechanism
The oral mucosa is covered by stratified squamous epithelium. The basal layer of mucosal epithelium has columnar cells with rapid division properties to maintain a constant epithelial population as cells are shed from the surface [4]. The lamina propria underlies the epithelium, which consists of fibroblasts and connective tissue, capillaries, inflammatory cells (macrophages), and extracellular matrix [4, 5]. Radiation-induced oral mucositis is not a simple epithelial process and results from complex pathways embracing all different cellular and tissue compartments of the mucosa. It has been proposed that endothelial and connective tissue damage precedes epithelial changes in irradiated oral mucosa [5].
Radiation directly damages cellular DNA of rapidly dividing cells of the oral basal epithelium and cells in the underlying tissue [6]. Radiation also generates oxidative stress and reactive oxygen species (ROS) that lead to further tissue damage by activating a number of transcription factors such as nuclear factor-κB (NF-κB) in the epithelium, endothelium, macrophages, and mesenchymal cells. Subsequently, the upregulation of genes and the production of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 occur, leading to an injury to the connective tissue, endothelium, and apoptosis of cells within the basal epithelium. Pro-inflammatory cytokines also activate molecular pathways that amplify production of TNF-α, IL-1, and IL-6 and resulted in further tissue damage (positive feedback loop).
Until this stage, the clinical appearance of mucosa seems to be normal, and only the thinning of the mucosa and edema related to acute vascular response may develop and cause early signs and symptoms of mucositis (mild erythema and burning sensation). But as radiation therapy continues, all events from the prior phase result in further injuries and loss of the basal epithelial stem cells with continuing cell loss from the mucosal surface, reducing the cellular population of the mucous membrane. Atrophic changes and breakdown of mucosa occur and patients experience obvious symptoms of mucositis [6–12].
The ulcerative phase of mucositis is accompanied by significant inflammatory cell infiltration. Beside the intrinsic factors, this phase may be affected by extrinsic factors that have reciprocal relation together. Damaged epithelium of the ulcerative mucositis is susceptible to bacterial colonization. Following the colonization with a mixed microbial flora, including mostly gram-negative bacteria, bacterial cell wall products penetrate the injured mucosa and increasingly stimulate pro-inflammatory cytokine release, amplifying the severity of mucositis and tissue injury [6]. When radiation therapy is completed, tissue repair begins with renewal of epithelial proliferation and differentiation and reestablishment of the local microbial flora [13].
6.2 Timing
In conventional fractioned radiotherapy (2 Gy per fraction daily), mucosal erythema with mild discomfort occurs within 1–2 weeks of treatment at doses of approximately 10–20 Gy [13]. Patchy pseudomembranous formation can develop after 2 weeks (20 Gy), and ulcerative radiation-induced mucositis often arises at doses of more than 30 Gy and peaks during the fourth to fifth week of therapy. This timing of symptom presentation is varied based on treatment schedule. In accelerated radiation therapy, mucositis reaches its peak within 3 weeks [11, 14]. The symptoms usually become more severe with treatment and remain for about 2–3 weeks after the end of therapy at its peak [2, 15]. Healing then begins and may take weeks to months (generally 3–6 weeks) to resolve [16]. However, chronic open wounds recognized as soft-tissue necrosis may occur in a few cases due to excessive depletion of mucosal stem cells depending on their recovery [17].
6.3 Risk Factors
Several factors have been identified for the development of more severe oral mucositis during radiation therapy. These factors could be related to treatment, tumor, and/or patient characteristics.
6.3.1 Tumor Site
Primary tumors of the oral cavity, oropharynx, or nasopharynx [16] increase the risk of oral mucositis due to including higher volumes of the oral mucosa and salivary gland in irradiation fields.
In the treatment planning of oral cavity, oropharyngeal, and nasopharyngeal cancer, a significant surface of oral mucosa includes in radiation field that increase the rate of oral mucositis. In the oral cavity, some areas, including the lateral borders and ventral surface of the tongue as well as the soft palate and floor of the mouth, have increased susceptibility to developing oral mucositis due to higher cell turnover rates. Instead, buccal mucosa has less sensitivity to radiation-induced mucositis [13].
6.3.2 Concomitant Systemic Therapy
When chemotherapy is combined with radiation therapy, both normal tissue and tumor response alter due to their additive effects. Both radiation therapy and antineoplastic agents disrupt normal cell division of the oral mucosa and result in increased oral mucositis incidence [13, 19, 20]. Dose, type, and schedule of systemic therapy all affect severity and frequency of mucositis in the setting of concurrent chemoradiotherapy (weekly versus every 3 weeks; cisplatin or paclitaxel are associated with higher rates of oral mucositis) [21, 22].
There is a paucity of data about oral mucositis rates when cetuximab is used concurrently with radiation therapy. Some proposed no exacerbation of the common mucositis associated with radiation therapy [23, 24], and others found an increase of oral mucositis compared to radiation alone or conventional cisplatin with radiation therapy [25–27]. Future clinical trials are needed to more precisely evaluate the incidence and severity of mucositis, the time of occurrence, and the impact on quality of life and treatment interruption of patients treated with cetuximab and radiation therapy.
6.3.3 Radiation Dose
Higher total radiation dose to the oral cavity and higher dose per fraction are significantly correlated to the grade of acute mucositis. It has been reported that patients with cancer of the larynx, hypopharynx, oral cavity, nasopharynx, or oropharynx that are treated with radiation therapy with cumulative radiation dosage more than 50 Gy are at an increased risk for oral mucositis [3, 16].
With the introduction of high conformal radiation therapy, the correlation between the dose to the oral cavity and the severity of acute mucositis has been evaluated more precisely.
In a study of patients undergoing intensity modulated radiation therapy (IMRT) for head and neck cancer, it was demonstrated that a cumulative point dose of 39 Gy resulted in mucositis for 3 weeks or longer; mild severity and short duration of mucositis were found at cumulative point doses less than 32 Gy [28]. Another study found that the percentage of oral cavity volume receiving doses higher than 15, 30, 40, 45, and 50 Gy significantly correlated with acute mucositis grade [29].
6.3.4 Radiation Fractionation Schedule
6.3.5 Patient-Related Factors
The incidence of mucositis is related to various patient variables including younger patients, smoking, alcohol consumption, metallic dental restorations, preexisting periodontal disease, low body mass index, poor functional status, low leukocyte count, advanced disease and stage, a prior history of severe mucositis, and various comorbid conditions [3, 35–37].
It has been suggested that with increasing number of patient-related factors, the risk, duration, and severity of mucositis increases (see below).
Some argue that younger cancer patients have a higher rate of mucosal turnover that increases their susceptibility to mucositis [38].
The use of dental guards is proposed in the areas of metallic restoration because it reduces back scatter exacerbated radiation doses to adjacent mucosa caused by metallic restoration materials [13].
Genetic polymorphisms may be a susceptibility factor for radiation-induced mucositis (XRCC1 polymorphisms, NBN polymorphisms) [39, 40]. It has been seen that cytokine phenotype may be correlated to the risk of radiation-induced injury. During radiation therapy, an elevation in serum levels of cytokines IL-6, TNF-α, and IL-1β and low levels of IL-8 seem to correlate with mucositis severity [35, 41–43].
A weak correlation between high pretreatment epidermal growth factor (EGF) levels and decreased severity of mucositis has been seen that is suggestive of the protective EGF effect for oral mucosa damage [44]. Further study is needed to clarify cytokines and growth factors role in predicting, preventing and treating oral mucositis.
Suresh et al. evaluated a collection of patient-related risk factors and proposed a comprehensive tool to predict the probable incidence and severity of mucositis in head and neck cancer patients receiving chemoradiotherapy. In this suggested system, patients are scored based on age > 40, erythrocyte sedimentation rate (ESR) >3-times the upper limit, albumin <3.0 g/dL, white blood count (WBC) less than 3000/μL, Eastern cooperative oncology group (ECOG) performance status (PS) of more than 2, stage III or higher disease, use of tobacco, and presence of any comorbid conditions. One point is given to each of these parameters. Scores of 3 or less and 6 or above predicted the differences in the incidence of mucositis [45].
6.4 Symptoms
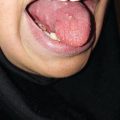
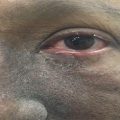
The early signs of radiation mucositis are red appearance of the oral mucosa due to dilation of capillaries in the endothelial layer and reduced epithelial thickness. A whitish appearance may be seen due to transient hyperkeratinization prior to erythema. Patients are mostly asymptomatic or complain of a mild burning sensation or intolerance to spices or hot food in this early phase. Erythema (Fig. 6.1) is followed by fibrinous pseudomembranous formation, followed by erosion and ulceration (Fig. 6.2). Under the pseudomembranes, the epithelial surfaces are denuded, and hemorrhage occurs easily. Pseudomembranous ulcerative lesions are very painful, and patients complain of severe pain and difficulty in chewing that interferes with their oral intake or speaking, eventually leading to weight loss.



Fig. 6.1
Grade 1 of oral mucositis at second week (14 Gy) of radiation therapy

Fig. 6.2
Grade 2–3 of oral mucositis at fifth week (48 Gy) of radiation therapy
Oral pain follows a similar pattern of objective clinical findings of oral mucositis but it may begin sooner and reach its peak earlier (between weeks 2 and 4) [2]. Correlation between patient-reported and clinical manifestations of mucositis is low in early parts of treatment [46].
Bacterial infection (usually gram-negative) or viral infections (such as herpes simplex virus (HSV)) and fungal infections (usually candidiasis) can sometimes be superimposed on oral mucositis [13, 37]. Infectious mucosal lesions often extend beyond the field of radiation. An infected oral mucosa usually manifests with a deep ulceration with a yellow-white necrotic center and raised borders. Fungal mucositis presents with white removable fungal plaques [13]; however, erythematous forms may occur and complicate the exact diagnosis [47].
6.5 Scoring
Patients undergoing radiation therapy to the oral cavity should be seen at least once a week. At each visit, patient symptoms and their oral intake should be assessed; their oral mucosa should also be examined.
A variety of assessment scales exist for measurement of oral mucositis. Three of the most commonly utilized scales (Table 6.1) for radiation-induced mucositis are toxicity criteria of the Radiation Therapy Oncology Group (RTOG), the European Organization for Research and Treatment of Cancer (EORTC) [48], the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [49], and the World Health Organization (WHO) oral toxicity scale [50].
Table 6.1
RTOG, WHO, and CTCAE mucositis grading
RTOG | WHO | CTCAE | |
|---|---|---|---|
Grade 1 | Irritation/may experience mild pain not requiring analgesic | Mild Soreness ± erythema | Asymptomatic or mild symptoms; intervention not indicated |
Grade 2 | Patchy mucositis that may produce an inflammatory serosanguinous discharge/may experience moderate pain requiring analgesia | Moderate Erythema, ulcers; patient can swallow solid food | Moderate pain; not interfering with oral intake; modified diet indicated |
Grade 3 | Confluent fibrinous mucositis/may include severe pain requiring narcotic | Severe Ulcers with extensive erythema; patient cannot swallow food | Severe pain; interfering with oral intake |
Grade 4 | Ulceration, hemorrhage, or necrosis | Life-threatening consequences; urgent intervention indicate | |
Grade 5 | Death |
6.6 Prevention
Maintenance of good oral hygiene is critical to preventing oral mucositis. All patients should be informed of proper oral hygiene before starting treatment:
Cleaning teeth with toothpaste and a soft toothbrush after each meal and at bedtime [51], using a mild-tasting toothpaste or a solution of 1 teaspoon of salt added to 4 cups (1 quart) of water (if not tolerating any toothpaste) [19].
Dental flossing once daily.
Dental screening at least several weeks before the beginning of therapy extractions and major surgeries should be planned 10–21 days and 4–6 weeks before beginning radiation therapy, respectively [52].
Rinsing the mouth two to four times a day (1 tablespoon (15 mL) of oral rinse, swish in oral cavity for 30 s, then spit out).
Recommended oral rinses [51]:
Water
Sodium chloride 0.9% for irrigation
Salt solution by adding a little salt (1/4 to 1/2 a teaspoon) to a cup of warm water
Be aware that alcohol-based mouth rinses should be avoided.
Moisten lips with a moisturizing cream.
Adequate oral fluid intake (daily fluid intake of 8–12 cups, 2–3 L).
Avoiding alcohol, tobacco, caffeine, fluid or foods with high sugar, highly acidic fluids and foods (e.g., lemon glycerin swabs, vitamin C lozenges), hot and spicy foods, and crunchy foods.
6.6.1 Prophylactic Interventions
Benzydamine is a nonsteroidal anti-inflammatory drug with local analgesic, anesthetic, and antimicrobial properties that inhibits pro-inflammatory cytokines including IL1 and TNF-α. It has been shown to reduce the severity and frequency of mucositis in patients with head and neck cancer undergoing radiation therapy. Benzydamine oral rinse appears to be effective in the prevention of radiation-induced oral mucositis in head and neck cancer patients [53–58]. Benzydamine (15 mL of oral rinse 0.15%) is used as a mouth rinse. It should be held or rinse within the mouth for at least 30 s, followed by expulsion from the mouth and try not to swallow, 3–4 times per day. It is recommended to begin the day prior to radiation therapy and continue during treatment.
Benzydamine is well tolerated. If patients complain of numbness or irritation or a burning sensation as result of benzydamine usage, diluting the liquid with an equal amount of warm water helps alleviate this issue. Other side effects are nausea and vomiting, xerostomia, headache, cough, and pharyngeal irritation. It can be absorbed through the oral mucosa and should be used with caution in patients with renal impairment.
6.6.2 Prophylactic Intervention Under Evaluation
Intensity-modulated radiation therapy (IMRT) allows sparing a greater area of the oral mucosa from higher doses of radiation. However, an obvious superiority of IMRT to conventional three-dimensional conformal radiotherapy (3D–CRT) and conventional two-dimensional radiotherapy (2DRT) in the prevention of the acute oral mucositis is not established [59, 60].
Low-level laser therapy seems to be effective in the prophylaxis of radiation-induced oral mucositis [61–66]. As a noninvasive measurement, it can decrease the incidence and severity of oral mucositis [67]. Laser therapy modulates three main biological effects: an analgesic effect with increase in endorphin release and pain signal inhibition, an anti-inflammatory and wound healing properties with energy production at the mitochondrial level, and an increase in vascularity and regeneration of injured tissues [2, 63, 68]. It refers to local application of a high-density monochromatic narrow-band light source with the output power range from 5 to 500 milliwatts and a wave-length between 600 and 1000 nanometers [69]. It is a simple procedure that is administered immediately after radiation therapy and takes only about 6–8 min to administer. All is done extraorally unless intraoral lesions develop [47].
Glutamine is a nonessential amino acid in the body. Glutathione, a byproduct of glutamine metabolism, protects normal tissues against oxidative injury [70, 71]. Glutamine depletion may develop in cancer patients due to cachexia and normal tissue damage from radiation or chemotherapy [72].
It has been found that oral glutamine administered daily during radiation therapy can reduce the severity and frequency of oral mucositis in head and neck cancer patients. These results warrant further investigation in future trials [72–77].
Zinc renders multiple essential functions in the body such as cell proliferation, wound healing, free radical protection, immunity, and anti-inflammatory effects. It has been shown that oral zinc sulfate can prevent and cause delay in development of oral mucositis [78].
Payayor (Clinacanthus nutans) is a traditional herbal medicine originating from Thailand with anti-inflammatory and analgesic properties. There are some studies that support payayor as a prophylactic intervention for radiation-induced oral mucositis [79, 80]. However, no guideline for use exists currently due to insufficient evidence.
Calendula officinalis, commonly known as Marigold, possesses some anti-inflammatory and antioxidant properties. It has been suggested that Calendula officinalis can be effective in decreasing oral mucositis severity [81, 82].
Natural honey has been recently shown to be effective in reducing radiation-induced mucositis. Honey has immunomodulatory properties and antibacterial activity and can accelerate wound healing. Honey is an easily available agent that warrants further trials to validate its effect [83–85].
Royal jelly is a secretion of hypopharyngeal and mandibular glands of worker bees with antioxidative, antibiotic, and anti-inflammatory action. Royal jelly could improve the signs and symptoms of oral mucositis and shorten healing time [86].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been reported to regulate proliferation and maturation of leukocytes, macrophages, and dendritic cells. GM-CSF can improve wound healing by enhancing fibroblasts and keratinocyte growth [87].
Subcutaneous GM-CSF has been shown to decrease pain and severity of oral mucositis [88, 89]. However, topical administration of GM-CSF to treat radiation-induced oral mucositis has mixed results [87, 90, 91]. Saarilahti et al. studied topical use of GM-CSF and reported promising effects in prophylaxis of radiation-induced oral mucositis [92]. There are also conflicting data about effectiveness of subcutaneous GM-CSF in radiation-induced mucositis prophylaxis [93, 94].
Keratinocyte growth factor (KGF) is an epithelial cell growth factor expressed by fibroblasts and endothelial cells and has an important role in wound healing by increasing proliferation and maintaining integrity of epithelial cells and enhancing neovascularization and collagen deposition [95].
Primary results show that recombinant human keratinocyte growth factor Palifermin resulted in reduction of radiation mucositis with no stimulation of the proliferation of tumor cell lines [96–98], which needs further evaluation.
Oral recombinant human epidermal growth factor (rhEGF) has been shown to improve wound repair by stimulating epithelialization. rhEGF appears to be effective for the treatment of radiation-induced mucositis [99, 100]. Another growth factor with promising results in preclinical study is velafermin (recombinant human fibroblast growth factor-20, rhFGF-20) [101]. Further studies are needed to determine these agents’ efficacy and safety for prevention and treatment of radiation-induced mucositis.
Amifostine is a thiol compound that selectively protects normal tissue from radiation effects [102]. Amifostine decreases the frequency and severity of xerostomia. The salivary preservation by amifostine may offer a protective effect against oral mucositis [17]. However, the amifostine studies for the prevention of oral mucositis offer insufficient evidence to support its use for this purpose. Additional investigation is needed to clarify the role of amifostine as an intervention for oral mucositis prevention [103].
Fluconazole (100 mg/daily) in head and neck cancer patients receiving radiation therapy can lead to decreased candida carriage and incidence of severe mucositis [5, 104–107]. Patients that are receiving head and neck radiation therapy are at increased risk of developing oral candida infection (17–29%) and colonization (93%) [108].
PTA is a multi-agent lozenge containing a mixture of polymyxin E, tobramycin, and amphotericin B and has a broad spectrum of antibacterial and antifungal effects. It has been used to prevent radiation-induced mucositis with inconclusive results. Results of the topical antibiotic approach in prevention of oral mucositis are insufficient and need to be further studied [109–114].
Chlorhexidine antimicrobial oral rinse is not recommended for the prevention of radiation-induced oral mucositis. It has no clinical benefit for the reduction or prevention of radiation-induced oral mucositis [18]. Chlorhexidine oral rinse is not well tolerated and may cause a degree of discomfort (e.g., taste alteration, burning sensation and teeth staining) without any clinical benefit [115].
Sucralfate is an oral ulcer coating complex of sucrose-sulfate-aluminum salt. Clinical trials found no significant advantage for sucralfate in the prevention or treatment of radiation-induced mucositis [116–120].
Prostaglandins are known to be cytoprotective. Misoprostol is a synthetic prostaglandin E1 analogue. Misoprostol mouthwash has been studied to prevent oral mucositis in head and neck cancer patients, and the data does not demonstrate a prevention benefit [121, 122]. There are limited studies for prostaglandin E2 application in the radiation-induced mucositis prevention and treatment that do not draw any conclusions.
Steroids have several effects on different systems of the human body and have been studied for radiation-induced oral mucositis prevention. Currently, there is insufficient evidence to support using systemic steroids for reducing the frequency or severity of oral mucositis [47].
Early morning radiation delivery has been shown to marginally reduce the severity of mucositis because of circadian variations in cell cycle proteins. The most radiosensitive phase of the cell cycle (G2-M) occurs in the late afternoon and evening in human oral mucosa [123, 124]. Currently, there is no recommendation for this observation in clinical practice [125].
Pilocarpine has not shown any preventive effect on oral mucositis during radiation therapy in head and neck cancer patients [125].
Supplemental antioxidants during radiation therapy have been proposed to protect the normal tissue and reduce side effects caused by radiation therapy. However, these antioxidants may act unselectively and protect cancer cells against the damaging effects of reactive oxygen species induced by radiation. Antioxidant supplement safety during radiation therapy is particularly controversial. The American Cancer Society and most other national nutrition guidelines recommend that patients with active cancer treatments limit the usage of supplements to obvious deficiency of essential agents [126].
RK-0202 (RxKinetix), an oral rinse, comprises the potent antioxidant N-acetylcysteine in a polymer matrix. RK-0202 has demonstrated primarily positive results in reducing the incidence of severe mucositis in patients treated with radiation therapy for head and neck cancer [127].
Vasoconstrictor agents such as phenylephrine with transient vasoconstriction, tissue hypoxia, and suppression of mucosal cell death during irradiation would prevent radiation-induced oral mucositis based on preclinical studies [128]. Further clinical trials should be conducted to prove the preventive effect of these agents on radiation-induced mucositis.
Caphosol (Cytogen Corp) is an electrolyte solution with high ionic content (Ca2+ and PO43− ions) that help tissue repair by diffusing ions into the intercellular spaces in the epithelium, thus permeating the mucosal lesion and modulate the inflammatory process. Recent studies did not find significant reduction in the incidence or duration of severe oral mucositis in patients receiving head and neck radiation therapy [129, 130].
Oxygen nebulization that uses high-flow oxygen could improve local mucosal oxygen content leading to angiogenesis, anti-inflammatory effect, and improved wound healing. It has been studied in patients with nasopharyngeal cancer to prevent radiation-induced mucositis with promising results. Future studies are required to better determine effectiveness [131].
Several herbal mouth rinses like Korean red ginseng [132], manuka (Leptospermum scoparium) and kanuka (Kunzea ericoides) [133], or chamomile [134] have been studied with a positive effect on the development of radiation-induced mucositis. Future investigation is needed to confirm the efficacy and safety of these products.
Persian traditional medicines have different compounds with various local and systemic effects on the mucosal surface and can theoretically be used to reduce mucositis [135]. One of the combinations (Malva sylvestris L and Alcea digitata (Boiss) Alef), which is effective in reducing xerostomia [136], has also been evaluated in a small randomized trial in our patients and primary results are promising (not published yet).
The mammalian target of rapamycin (mTOR) inhibition plays a role in the protection of normal oral epithelial cells from radiation-induced epithelial stem cell depletion. mTOR inhibition with rapamycin may have a potential effect on the prevention of radiation-induced mucositis [137]. Clinical studies need to evaluate rapamycin efficacy in this setting.
Mothers against decapentaplegic homolog 7 (SMAD7) is a protein encoded by the SMAD7 gene and has an antagonistic effect on transforming growth factor beta (TGF-β) and NF-κB signaling. Its prophylactic and therapeutic effects on radiation-induced oral mucositis as a well as its safety need to be determined in future studies [138].
Wobe-Mugos is a combination of proteolytic enzymes comprised of papain, trypsin, and chymotrypsin that has an anti-inflammatory effect. It seems not to be efficient in preventing radiation-induced oral mucositis [139].
6.7 Management
Principles of management consist of patient assessment, oral care, management of oral pain, treatment of secondary infection, and consideration of nutritional support.
6.7.1 Patient Assessment
All patients treated with radiation therapy should be seen as least once weekly, and the oral mucosa should be examined at each visit. In the patients with oral mucositis, baseline grading of oral mucositis and the patient’s general status should be determined. After initial assessment, decision-making about treatment protocol can be provided. Most cases can be managed in an outpatient setting except in the setting of grade 4 mucositis, fever (more than 38.3 °C), or severe neutropenia. Patients with inadequate fluid intake may require oral supplementation or IV hydration. Complete blood count is proposed in patients that have severe mucositis, fever, or at risk of developing neutropenia.
6.7.2 Mouth Care
Mouth care includes all measurements noted for maintaining good oral hygiene (see above), with intensity and frequency modification based on mucositis grading [140].
6.7.3 Management of Oral Pain
Pain management is the most important aspect of symptom control. Systemic analgesia may be prescribed in addition to topical agents in moderate to severe pain. Pain control can encourage patients to eat and drink more and wash the mouth more efficiently, resulting in improved medication effects.
6.7.3.1 Benzydamine Mouth Wash
Benzydamine can reduce the severity of oral mucositis and associated pain in radiation-induced oral mucositis [51, 141].
Benzydamine (15 mL of oral rinse 0. 15%) is used as a mouth rinse. It should be held in the mouth for at least 30 s, followed by expulsion (should not be swallowed), up to every 1–2 h.
Benzydamine is well tolerated. If patients complain of numbness or irritation or a burning sensation, dilution with an equal amount of warm water may reduce these symptoms. Other side effects are nausea and vomiting, xerostomia, headache, cough, and pharyngeal irritation. It can be absorbed through the oral mucosa and should be used with caution in patients with renal impairment.
6.7.3.2 Doxepin
Doxepin is a tricyclic antidepressant. Topical application is prescribed for pruritus and neuropathic pain [142]. Topical doxepin rinse has been shown to be an adequate analgesia for oral mucositis pain up to 4 h after application. Patients usually have good compliance; however, mild burning or stinging, unpleasant taste, and drowsiness could develop as common adverse effects [143].
Usage: oral rinse at a dosage of 25 mg (10 mg/mL × 2.5) diluted in 5 mL of water for 1 min 3–6 times per day.
6.7.3.3 Magic Mouthwash
Magic mouthwash usually contains at least three of these basic ingredients [144]:
An antibiotic
An antihistamine or local anesthetic
An antifungal
A corticosteroid
An antacid
Magic mouthwash is used every 4–6 h, maintained in the mouth for 1–2 min before being either spit out or swallowed (in pharyngeal or esophageal involvement). It’s recommended not to eat or drink for at least 30 min after using magic mouthwash [144].
There are various formulations for magic mouthwash with no standard mixture. Some of the more common formulations are defined here [145]:
80 mL viscous lidocaine 2%, 80 mL Mylanta, 80 mL diphenhydramine (12.5 mg per 5 mL elixir), 80 mL nystatin 100,000 U suspension, 80 mL prednisolone (15 mg per 5 mL solution), and 80 mL distilled water
1 part viscous lidocaine 2%, 1 part Maalox (do not substitute Kaopectate), and 1 part diphenhydramine (12.5 mg per 5 mL elixir)
30 mL viscous lidocaine 2%, 60 mL Maalox (do not substitute Kaopectate), 30 mL diphenhydramine (12.5 mg per 5 mL elixir), and 40 mL Carafate (1 gm per 10 mL)
100 mL dexamethasone (0.5 mg per 5 mL elixir), 60 mL nystatin 100,000 U suspension, 100 mL diphenhydramine (12.5 mg per 5 mL elixir), and 3 capsules tetracycline 500 mg
Different contributors of magic mouthwash are added for different purposes:
Diphenhydramine provides local analgesia but can exacerbate xerostomia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree