, Ahmad Ameri1 and Mona Malekzadeh2
(1)
Department of Clinical Oncology, Imam Hossein Educational Hospital, Shahid Beheshti University of Medical Sciences (SBMU), Shahid Madani Street, Tehran, Iran
(2)
Department of Radiotherapy and Oncology, Shohadaye Tajrish Educational Hospital Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran
Dry mouth (xerostomia) is one of the most common complications of radiation therapy in head and neck cancers due to the high radiosensitivity of the salivary glands.
Xerostomia is a common debilitating side effect of radiation therapy for head and neck cancers that affects chewing, eating, tasting, swallowing, tooth decay, and speaking. Xerostomia has a negative impact on quality of life in cancer patients.
It has been estimated that the incidence of acute xerostomia induced by radiation therapy of head and neck cancers is 60–90% depending on tumor site, irradiation technique, and time of evaluation; it is observed in 30% of patients with advanced cancer in need of starting a palliative care program [1, 2].
In regard to timing of radiation therapy, the incidence of xerostomia is 6%, 93%, and 83% before, during, and 1–3 months after treatment, respectively [3]. In regard to type of radiation therapy, xerostomia occurs in 81%, and 71% of patients underwent conventional head and neck radiation therapy during and 1–3 months after treatment, respectively. New radiation techniques such as intensity-modulated radiation therapy (IMRT) may spare salivary glands more than conventional therapies and decrease xerostomia rate and severity [3].
Moderate to severe xerostomia occurs in 60.2% of patients with nasopharyngeal carcinoma and in 32.9% of patients with other sites of head and neck cancer 3 months after treatment with intensity-modulated radiation therapy [4].
7.1 Mechanism
In order to understand the mechanism of xerostomia induction in radiation therapy, salivary gland physiology needs to be understood.
The terms of major and minor salivary glands refer to their anatomic size. There is a difference in the quality of content and quantity of production during different time points through the day, which may affect the clinical symptoms related to each salivary gland dysfunction.
Normal salivary flow is highly variable and is usually 0.25 mL/min (1–1.5 L per day). A salivary flow of less than 0.12–0.16 mL/min is considered to be abnormal [5].
The parotid glands have only serous-secreting acinar cells and exclusively produce serous watery secretion. Up to 50% of stimulated saliva and approximately 20% of unstimulated saliva secretion volume comes from parotid glands secretion.
The submandibular glands have both serous and mucous acinar cell types (10% mucous cells and 90% serous cells) and produce a mixed serous and mucous saliva. They contribute 65% of unstimulated saliva secretion and 35% of stimulated saliva secretion.
The sublingual glands mainly have mucous acinar cells and produce predominantly thick, viscous saliva. They contribute less than 10% of unstimulated saliva volume.
The minor glands, which are distributed throughout the upper aerodigestive mucosa (e.g., labial, buccal, lingual, and palatinal mucosa), are mixed glands largely comprised of mucous acinar cells. The minor glands produce less than 10% of the total volume of saliva [6].
During the day, major saliva production in stimulated and unstimulated conditions is related to parotid and submandibular glands, respectively [7].These major salivary gland dysfunctions are major causes of xerostomia induced by radiation therapy.
Salivary glands are highly radiosensitive. Serous acinar cells of the salivary glands are well differentiated and have a slow mitotic rate and turnover, but they behave like acutely responding tissues to radiation and undergo interphase cell death by apoptosis [8, 9]. Mucous acinar cells of salivary glands have a lower radiosensitivity than serous acinar cells, and they have a trend to retain their function for some time later [10]. The parotid glands seem to lose more function than do the other salivary glands, resulting in a decrease in watery saliva and accumulation of sticky mucus. However, the difference in radiosensitivity between the parotid and submandibular/sublingual salivary glands is still controversial [11–13].
It has been found that saliva production reduces during the first days of radiation therapy. Membrane damage of saliva-producing cells confounding with receptor-mediated signaling pathways of water excretion and functional loss is responsible for the early hyposalivation. Cell death does not occur in the acute phase but is a late event [14].
Saliva is a complex fluid, mostly composed of water (99%) and a minority of various nonorganic and organic substances such as enzymes, hormones, antibodies, antimicrobial constituents, and growth factors. Saliva quality changes during radiation therapy, including increased viscosity, decreased transparency, yellow/brown discoloration, declined production of glycoproteins (e.g., immunoglobulin A), decreased salivary pH (from 6.8 and 7.0 to 5.5), altered salivary electrolyte levels (increases in the concentrations of sodium, chloride, calcium, and magnesium with slight potassium level change), and shift in certain intraoral microbial populations (increase in Streptococcus mutans and species of Lactobacillus, Candida (primarily Candida albicans), and Staphylococcus, with parallel decreases in Streptococcus sanguinis and species of Neisseria and Fusobacterium) [13, 15–18].
7.2 Timing
Radiation-induced xerostomia usually initiates early during radiation therapy for head and neck cancers containing salivary glands within the radiation fields. When the radiation dose reaches 10–20 Gy with 2 Gy per day, which corresponds to the first to second week of treatment, a 50–60% decrease in salivary flow occurs, and the patient may begin to experience mild to moderate dryness of the mouth, which may progressively worsen over the course of treatment. After 7 weeks of radiation therapy, salivary flow decreases to approximately 20% and continues to further drop for more than 6 months after completion of treatment [11, 19, 20]. Some recovery is possible until 12–18 months after radiation therapy depending on the dose received by the salivary glands and the volume of the gland tissue included in the irradiation volume; there is also a compensatory hypertrophy of the non-irradiated salivary gland tissue. Xerostomia may rarely recover a few years after radiation therapy [19, 20].
Radiation-induced salivary gland damage may be reversible or permanent based on the radiation dose. With a dose less than 60 Gy, changes in the salivary glands, including edema and inflammation, are reversible. Although, for acceptable function, the radiation doses to salivary glands should be more lower than 60 Gy. When the dose exceeds 60 Gy, changes may be permanent, with fibrosis and glandular degeneration [13].
7.3 Risk Factors
The occurrence and severity of radiation therapy-induced xerostomia are dependent on radiation-related factors, mostly salivary gland radiation dose and volume within the radiation field [21]. Altered fractionation schedules’ impact on the incidence of xerostomia is not clearly defined. It seems that accelerated and hyperfractionated radiation therapy both increase acute side effects of treatment including xerostomia [22].
Concurrent chemotherapy is associated with a significant risk of developing acute and late xerostomia [21]. However it has been shown in some studies that concomitant chemotherapy and intensity-modulated radiation therapy do not increase the incidence of acute or late xerostomia relative to intensity-modulated radiation therapy alone [23]. Concomitant cetuximab (a monoclonal antibody against EGFR) doesn’t increase the xerostomia rate when it’s prescribed concurrently with radiation therapy [24].
Patient age is also a contributing factor. With increasing age, the vulnerability of salivary glands to radiation injury and development of xerostomia increases [7]. It should be noted that increasing age does not cause hyposalivation by itself [25]. The higher incidence of xerostomia in elderly patients is mainly due to their comorbidities and use of medications with the potential to develop xerostomia.
No significant association between the risk of developing xerostomia and ethnicity, marital, or socioeconomic status has been observed [21].
It has been proposed that the progression of xerostomia can be improved by mixing clinical and dose-volume factors. The mean dose given to the contralateral and ipsilateral parotid glands is the most significant predictors in multivariable normal tissue complication probability models for xerostomia. Age, financial status, T stage, and educational level are proposed clinical predictive factors for radiation-induced xerostomia. However some of these clinical datasets such as financial status and education may affect the patient-reported xerostomia, many of which need to be investigated before they are incorporated into the models [4].
The volumes of parotid and submandibular glands are decreased due to radiation therapy. The parotid gland volume reduces about 30% during radiotherapy. The lateral regions of the irradiated parotid glands move inward. The irradiated submandibular glands also shrink and move upward. Parotid shrinkage during treatment is accompanied by a decrease in tissue density consistent with a relative increase in fat over glandular tissue [26, 27].
Parotid gland density and volume variations during radiation therapy may possibly predict acute xerostomia. It has been found that a higher score of acute xerostomia is predicted by higher density and volume variations in the first 2 weeks of treatment. Further studies are necessary to definitively assess the potential of early density/volume changes in identifying more sensitive patients at higher risk of developing xerostomia [28].
7.4 Symptoms
Dryness is one of the most unpleasant oral symptoms that adversely affects all oral functions and compromises oral health. Patients may experience dry oral mucosa and thick, sticky saliva requiring them to adjust their diet and keep the mouth moist with water [1, 29].
Both acute and chronic xerostomia induce functional alterations such as chewing, swallowing, speaking, burning, and pain, with a propensity to bacterial and fungal infection, demineralization of teeth, and increase in caries, dysgeusia, gagging sensations, a fear of choking, and odynophagia. The patient may have bad breath secondary to food stagnation in the oral mucosa, gingiva, teeth, or tongue [30, 31].
Cheilitis, a fissuring or ulceration in the angles of the mouth and erythematous tongue due to damage to the dorsal epithelium, can also be seen in patients with xerostomia [29, 30].
Quality of life significantly worsens along with the severity of xerostomia. With each milliliter decrease in saliva secretion, the quality of life score decreases by 2.25% [32].
7.5 Diagnosis
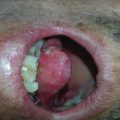
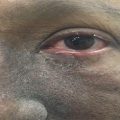
Xerostomia is a clinical diagnosis based on patient signs and symptoms (Fig. 7.1). The objective tests of salivary flow are not usually used for diagnosis because there is little correlation between patient symptoms and these tests. Therefore, clinical management should be based on patient symptoms [33].


Fig. 7.1
Grade 2 xerostomia in tongue cancer 1 month after radiotherapy
There are some differential diagnoses including oral infections, bad oral conditions, and mucositis, which may be ruled out with a detailed history and clinical exam. However, they can all occur in association with each other.
7.6 Scoring
For measuring the severity of xerostomia, there are assessment tools based on patient- and observer-reported data.
Observer-based toxicity scoring is generally based on the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) grading scale (Table 7.1). Because of the low correlation between the measured salivary output and observer-reported xerostomia [34] and underestimation of the severity of xerostomia with these scoring systems [35], several xerostomia questionnaires have been developed to permit patient self-reporting.
Table 7.1
RTOG/EORTC xerostomia scoring
Definition | |
|---|---|
Score 0 | No change over baseline |
Score 1 | Mild mouth dryness/slightly thickened saliva/ may have slightly altered taste such as metallic taste/these changes not reflected in alteration in baseline feeding behavior, such as increased use of liquids with meal |
Score 2 | Moderate to complete dryness/thick, sticky saliva/markedly altered taste |
Score 3 | – |
Score 4 | Acute salivary gland necrosis |
One of the most validated patient self-reported questionnaires is xerostomia questionnaire (XQ), which was developed by the University of Michigan (Table 7.2). It consists of eight questions; patients rate each item on a scale from 0 to 10. Higher scores are related to more severe xerostomia [35].
Table 7.2
Michigan University xerostomia questionnaire
0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
Rate your difficulty in talking due to dryness | |||||||||||
Rate your difficulty in chewing due to dryness | |||||||||||
Rate your difficulty in swallowing solid food due to dryness | |||||||||||
Rate the frequency of your sleeping problems due to dryness | |||||||||||
Rate your mouth or throat dryness when eating food | |||||||||||
Rate your mouth or throat dryness while not eating | |||||||||||
Rate the frequency of sipping liquids to aid swallowing food | |||||||||||
Rate the frequency of sipping liquids for oral comfort when not eating |
7.7 Prevention
There are some preventive approaches for radiation-induced xerostomia, including more conformal radiation delivery technology, radioprotective agents, and even preirradiation surgical techniques [36, 37].
7.7.1 Amifostin
Available data show that amifostine significantly reduces the incidence of acute and long-term xerostomia. Amifostine, an organic thiophosphate agent, is a prodrug that is dephosphorylated by alkaline phosphatase in tissues after administration, converting it into its active form. This active form enters the cells and acts as a scavenger against free radicals. Amifostine concentrations in tumor cells are lower than normal tissue due to lower alkaline phosphatase levels and the pH of tumors, and therefore normal tissue protection is provided [20]. However, amifostine’s effect on tumor cell protection is still a concern that precludes it from extensive administration as a radioprotective agent.
Brizel et al. in a phase III trial randomized 303 patients that received conventional radiation therapy for head and neck cancers (both postoperative and as primary treatment) to receive amifostine daily before each fraction (200 mg/m2 intravenously). They found that amifostine significantly reduced the incidence of grade 2 acute xerostomia from 78% to 51% and reduced the incidence of grade 2 chronic xerostomia from 57% to 34% without a difference in disease control or survival [38]. Amifostine was consequently approved by the US Food and Drug Administration (FDA).
Amifostine can be administered only with standard fractionated radiation therapy without chemotherapy and only when ≥75% of both parotid glands are exposed to radiation in the postoperative setting. Amifostine should not be administered in patients receiving definitive radiation therapy, except in the context of a clinical trial, because of insufficient data to exclude a tumor-protective effect in this setting [39]. Amifostine administration in the setting of concurrent chemotherapy with radiation therapy and in the setting of accelerated and hyperfractionated therapy has not been systematically studied [40–43].
7.7.1.1 Prescription
Each vial contains 500 mg of amifostine on the anhydrous basis, requiring reconstitution for intravenous infusion [44]. Prior to intravenous injection, amifostine is reconstituted with 9.7 mL of sterile 0.9% sodium chloride. The reconstituted solution (500 mg Amifostine/10 mL) is chemically stable for up to 5 h at room temperature (approximately 25 °C) or up to 24 h under refrigeration (2 °C–8°C). Amifostine is prepared in polyvinylchloride (PVC) bags and is available at concentrations ranging from 5 mg/mL to 40 mg/mL [45].
Standard amifostine intravenous administration: 200 mg/m2 over 3 min once daily 15–30 min prior to radiation therapy [46, 47].
Amifostine can also be administered subcutaneously (unlabeled route): 500 mg once daily prior to radiation therapy [46, 48, 49].
Note: Because of rapid clearance from the blood and tissue, the drug needs to be delivered shortly before radiation therapy.
7.7.1.2 Contraindications
Hypersensitivity to aminothiol compounds or any component of the formulation.
7.7.1.3 Advers effects
Several adverse effects have been reported for amifostine including hypotension, nausea and vomiting, hypocalcemia, and cutaneous reactions.
Blood pressure should be monitored every 5 min during the infusion and thereafter as clinically indicated. The infusion of amifostine should be interrupted if the systolic blood pressure decreases significantly from baseline [45, 48].
Amifostine is a moderately to highly emetogenic agent, and antiemetic medications (including dexamethasone 20 mg I.V. and a serotonin 5-HT3 receptor antagonist) should be administered prior to and in conjunction with amifostine [50].
Serum calcium levels at baseline should be checked and monitored in patients at risk for hypocalcemia, such as those with nephrotic syndrome or patients receiving multiple doses of amifostine for injection [45, 48].
7.7.2 IMRT
The volume of salivary tissue irradiated is related to the occurrence of xerostomia. With introduction of novel radiation therapy delivery techniques including intensity-modulated radiation therapy (IMRT), partial sparing of salivary glands may become possible, and thus acute and late xerostomia are significantly reduced [51, 52]. Mean dose to the parotid glands should be reduced as much as clinically possible. A mean dose of less than 20 Gy for at least one parotid gland or a mean dose of less than 25 Gy for both glands may prevent severe long-term xerostomia [53]. It has been suggested that the mean parotid dose for both sides together has a higher predictive value over considering each side separately [27].
The effect of amifostine combined with IMRT is not clear. It seems that the protective effect of IMRT in sparing saliva is much greater than the effect of amifostine [54, 55].
In patients that need bilateral radiation therapy of the head and neck including bilateral parotids within the radiation fields, the effect of IMRT in prevention of xerostomia is limited.
IMRT provides a way to spare all salivary glands and improves xerostomia-related quality of life during meals and at rest; however, the largest effect is still on xerostomia during meals [56]. As noted previously, the parotid glands are largely responsible for the stimulated saliva output, whereas the minor salivary glands and submandibular glands are mainly responsible for unstimulated saliva (lubrication in rest). The risk of xerostomia decreases or is even eliminated with sparing of at least one parotid gland and reduces with sparing of at least one submandibular gland [53]. It has been reported that a lower mean dose to the oral cavity (<40 Gy) and contralateral submandibular gland (<50 Gy) is each associated with lower patient-reported and observer-rated xerostomia [51].
7.7.3 Submandibular gland transfer
Another preventive approach is submandibular gland transfer. The submandibular gland is responsible for most of the unstimulated salivary volume, which is important in the subjective symptoms of xerostomia and oral homeostasis. Some argue that sparing the submandibular gland is preferable to sparing the parotid gland [29].
In this approach, a single submandibular gland is transferred into the submental space during a surgical procedure referred to as the Seikaly-Jha procedure (SJP). The borders of the transferred gland are marked with wire to help identify it during radiation therapy and can be shielded from the radiation [57, 58]. The surgical method of transfer is a quick, easy, and simple procedure that can be done during surgical treatment of the primary tumor [20, 59–61].
The submandibular gland may be damaged during the transfer, and complete prevention cannot be achieved, although it is still superior to some other preventive options including pilocarpine in regard to median salivary flow and saliva consistency. It has been estimated that the submandibular gland transfer could reduce 69% of the risk of acute xerostomia. However, after radiation therapy, the salivary gland repaired itself and the rate of late xerostomia can reach 19% [62]. Local/regional recurrence and survival outcome after submandibular gland transfer seem not to be compromised; however there is some controversy and need for more evaluation [41, 63].
Submandibular gland transfer should be conducted on the gland of the contralateral side of the primary cancer. The procedure should not be performed for patients with cancer of the oral cavity, bilateral neck lymph node involvement, submandibular or submental neck lymph node involvement, or advanced neck disease (N3). For accurate selection of patients that are candidates for submandibular gland transfer, suspicious nodes and all level I lymph nodes (submental and submandibular) are dissected and sent for frozen section evaluation before transfer. If any of the nodes are involved with cancer, the transfer procedure should be abandoned [62].
7.7.4 Pilocarpine
Pilocarpine has been approved for postradiation xerostomia. The administration of pilocarpine during radiation therapy has been studied to prevent the subsequent development of xerostomia.
Pilocarpine hydrochloride is a muscarinic-cholinergic agent that can stimulate salivary glands [64–66].
Several studies have shown positive results of pilocarpine in the prevention of radiation-induced xerostomia, especially when the doses of whole parotid glands are more than 45 Gy. However, others have concluded that oral pilocarpine could not be recommended to prevent xerostomia in patients receiving radiation therapy for head and neck cancers [67].
It has been found that pilocarpine administration during radiation therapy can result in a significant improvement in unstimulated salivary flow at the end of treatment but not in stimulated flow. This observation can be explained with the mechanism of pilocarpine’s protective effects [68]. Pilocarpine stimulates the residual function of salivary tissues outside the radiation fields, including non-irradiated parts of parotid and other major salivary glands/minor salivary glands, and it has no protective effect in the glands that are completely irradiated [69–72]. Prominently unstimulated salivary flow is preserved.
The other hypothesis proposed for the mechanism of pilocarpine’s protective effect is related to indirect inhibition of radiation-induced oxidative damage. Pilocarpine reduces heavy metals such as zinc, manganese, and iron, which are found in secretary granules, leading to reduction in intracellular leakage of proteolytic enzymes in secretary granules after radiation damage and reduction in subsequent serous cell autolysis [67, 70].
Data based on in vitro studies indicate that pretreatment pilocarpine administration does not protect tumor cells and has no effect on radiosensitivity of cell lines [73].
7.7.4.1 Prescription
Start pilocarpine 5 mg three times daily with irradiation, and continue until 3 months after the end of radiotherapy [74].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree