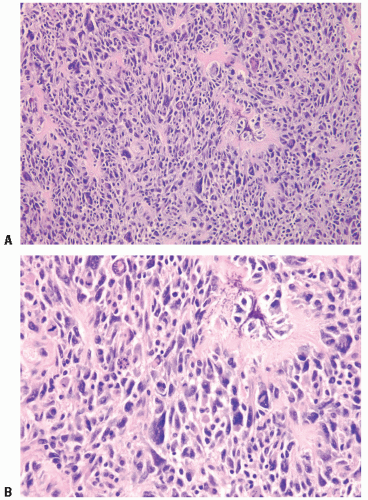
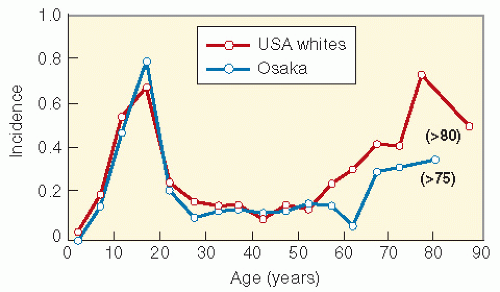
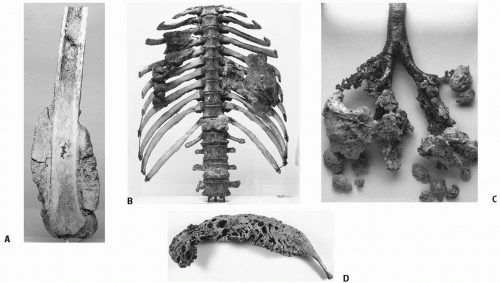
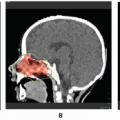
Study Name and Number of Eligible Patients |
Primary Objective |
Treatment Arms |
Results |
Comments (Reference) |
Pediatric Oncology Group (POG) |
POG-8651 (n = 100) |
To determine whether chemotherapy administered before and after definitive surgery is superior to surgery followed by adjuvant chemotherapy |
Arm A: HDMTX + AP, then surgery, then HDMTX + BCD + ADR + AP
Arm B: surgery, then HDMTX + AP + BCD |
The 5-year survival is 76% for patients assigned to neoadjuvant chemotherapy and 79% for patients treated with the more traditional approach (p = 0.6) |
There is no evidence of an advantage in event-free survival or survival for either treatment arm (41,78) |
European Osteosarcoma Intergroup (EOI, a combination of the European Organization for Research and Treatment of Cancer and the Medical Research Council) |
EOI-80831 (n = 307 registered, 207 evaluable, 163 completed allotted chemotherapy) |
This study began as a randomized phase II toxicity and response trial of two short, intensive chemotherapy programs |
Surgery, randomization of chemotherapy of AP vs. AP + MTX |
6-year survival: AP 65% + MTX 50% (p = 0.10)
6-year disease-free survival: AP 58% vs. AP + MTX 40% (p = 0.02) |
The dosage intensity of AP was greater in the two-drug arm than the three-drug arm. This may have produced the superior outcome. Disease-free survival was better for patients planned for conservation surgery than amputation (76) |
EOI-80861 (n = 407, 391 eligible) |
To compare short, intensive chemotherapy with complex, longer-duration program based on the Rosen T10 program |
Arm A: AP three cycles, then surgery, then three more cycles Arm B: ADR + V + HDMTX, then surgery, then BCD + ADR + V + HDMTX + AP |
The median follow-up is 5.6 years. Survival in both groups is almost identical. 5-year progression-free survival is 44% |
AP appears to be as effective as the more complicated program at a lower cost (75,77) |
Scandinavian Sarcoma Group (SSG) |
SSG-VIII |
To increase the number of good responders to neoadjuvant therapy with intensified |
HDMTX + AP, then surgery; good responders get HDMTX + AP postoperatively, poor |
The median follow-up is 6.9 years. The year event-free survival is 61% |
Female sex, small tumor volume, and high serum MTX levels predict better |
Children’s Cancer Group (CCG) |
CCG-741 (n = 166) |
To compare HDMTX with moderate-dose MTX in the context of a multiagent chemotherapy program |
Surgery, ADR, then randomize to arm: Arm A, HDMTX + AV; Arm B, moderate-dose MTX + AV |
38% disease-free survival at 48 mo. No difference between the two arms (p > 0.5) |
Lower disease-free survival was associated with the presence of spontaneous tumor necrosis at presentation (78) |
CCG-782 (n = 232) |
To use histologic response of the primary tumor to neoadjuvant chemotherapy to determine postoperative chemotherapy |
Biopsy, then HDMTX + AV + BCD, then surgery. All patients without local progression received BCD + AV + HDMTX. Then, if< 95% necrosis, receive BCD + HDMTX + AV |
5-year event-free survival was 53%. Patients with local disease progression in the induction phase had 48% 3-year event-free survival |
A poor prognosis is associated with an evaluated alkaline phosphatase at diagnosis or a primary tumor in the proximal humerus or proximal femur (40,79,76) |
Cooperative Osteosarcoma Study (COSS) Group of the German Society of Pediatric Oncology |
COSS-77 (n = 68) |
Improved survival from adjuvant chemotherapy |
Surgery, then HDMTX + AV |
46% 14-year metastasis-free survival |
Event-free survival was 48% for good responders and 48% for poor responders (80,81) |
COSS-80 (n = 101) |
Improved survival with neoadjuvant chemotherapy |
Arm A: ADR + HDMTX + P, then surgery, then ADR + HDMTX + P
Arm B: ADR + HDMTX + BCD ± β-IFN |
5-year event-free survival: Arm A = 66%, Arm B = 64% |
There was no difference in outcome between the two chemotherapy arms. There was no difference associated with the use of β-IFN (40,79, 80, 81) |
COSS-86 (n = 153) |
Stratified by risk group. High risk was large tumor, chondroid matrix, or poor bone scan response at week 5 |
Low risk: ADR + HDMTX + P, then surgery, then ADR + HDMTX + P
High-risk Arm A: ADR + HDMTX + intra-arterial IP, then surgery, then ADR + HDMTX + IP
High-risk Arm B: Same as Arm A except IP is intravenous |
77% 5-year metastasis-free survival, 66% 10-year metastasis-free survival, 72% 10-year overall survival |
Response rate is > 76%, better than in previous COSS studies (82) |
Children’s Oncology Group (COG) |
CCG-7921, POG-9351 (n = 662) |
To compare three-drug chemo with four-drug chemo. To determine if addition of MTP improves EFS and OS |
Reg A: AP + MTX ± MTP
Reg B: AP + MTX + I ± MTP |
Similar EFS and OS between Reg A and B without MTP
MTP-improved 6-year OS from 70% to 78% (p = 0.03) |
Addition of ifosfamide was of no benefit. Addition of MTP improved survival |
ADR, doxorubicin; AP, doxorubicin and cis-platinum; AV, doxorubicin and vincristine; BCD, bleomycin, cyclophosphamide, and actinomycin D; β-IFN, beta-interferon; EI, etoposide and ifosfamide; HDMTX, high-dose methotrexate; IP, ifosfamide and cis-platinum; MTX, methotrexate; P, cis-platinum; V, vincristine: MTP, muramyl tripeptide. |