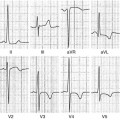
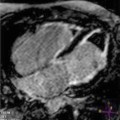
Fig. 7.1
Late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) images in patients with ischemic (a, b) and nonischemic (c, d) dilated cardiomyopathy (DCM). Note the absence of LGE in patients with nonischemic DCM and the presence of subendocardial LGE distributed along coronary segments in ischemic DCM images (arrows)
7.3 Macroscopic vs Diffuse Fibrosis
LGE sequences are not able to identify diffuse interstitial fibrosis [4] (Fig. 7.2). T1 mapping sequences are able to evaluate interstitial fibrosis and are shown to correlate well with histologically detected fibrosis [5–8], although they require some postprocessing and are time consuming.
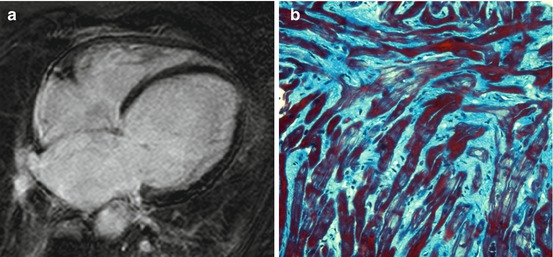

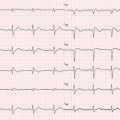
Fig. 7.2
Absence of late gadolinium enhancement (LGE) at cardiac magnetic resonance (CMR) in a patient with dilated cardiomyopathy (DCM) (a). Histologic specimen (Masson trichrome stain) from endomyocardial biopsy of the same patient showing diffuse interstitial fibrosis (b)
7.4 Left Ventricular Dyssynchrony
CMR is able to assess LV dyssynchrony [9] by assessing segmental radial wall motion in short-axis steady-state free precession (SSFP) views. Patients with heart failure (HF) display increased dyssynchrony despite normal QRS duration. Increasing degrees of dyssynchrony are predictive of poor outcomes in patients who undergo CRT and patients who do not [10, 11]. LV dyssynchrony may also be evaluated with velocity-encoded sequences, with results similar to tissue Doppler imaging [12]. This may be useful in patients with poor acoustic window.
7.5 Assessment of Left Ventricular Thrombosis
Contrast-enhanced CMR is more sensitive and specific in detecting intraventricular thrombus compared with standard 2D echocardiography [13], particularly through SSFP sequences and especially through inversion recovery (IR) sequences with a very long inversion time early after gadolinium administration (Fig. 7.3).
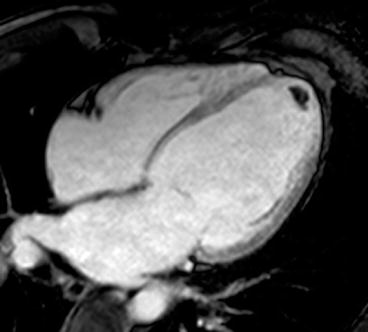

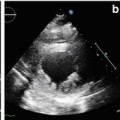
Fig. 7.3
Advanced dilated cardiomyopathy (DCM). Cardiac magnetic resonance (CMR) image acquired early after contrast administration, with long inversion time, showing a small intraventricular thrombus as hypointense (dark) area at left ventricular (LV) apex
7.6 Single Photon Emission Computed Tomography
Single-photon-emission computed tomography (SPECT) was evaluated to assess ventricular volumes and function [14], myocardial stress, rest perfusion, and sympathetic nervous activity. The presence and extent of perfusion abnormalities, possibly reflecting fibrosis, correlate with poor prognosis [15–17]. The pattern of occurrence may be helpful to differentiate ischemic from nonischemic forms of DCM [18–20]. SPECT imaging may be an alternative to CMR—although it is less reliable—in identifying patients with areas of LV scarring responsible for poor response to CRT [21, 22]. Iodine 123-metaiodobenzylguanidine (MIBG), a norepinephrine analog, is used as a tracer to investigate cardiac sympathetic nervous function [23]. In some studies, altered cardiac sympathetic nervous activity, assessed by MIBG imaging, is predictive of ventricular arrhythmias, appropriate implantable cardioverter defibrillator (ICD) therapy [24], and adverse prognosis in DCM [25–27].
7.7 Positron Emission Tomography
Myocardial metabolic activity may be assessed with [18F]-fluorodeoxyglucose positron emission tomography (PET). In patients with DCM, finding heterogeneous regional myocardial metabolic activity is predictive of a poor outcome, whereas a homogeneous pattern is predictive of functional improvement [28]. Absolute myocardial blood flow measured by dynamic PET and [13N]-ammonia or 15O-labeled water (15O-H2O) [29] is impaired both at rest and in response to vasodilating stimuli in DCM patients [30, 31]. The degree of impairment is independently associated with disease progression and adverse outcome [31, 32]. Conversely, patients on beta-blockers who experience improved LV ejection fraction (EF) also show improved myocardial blood flow assessed by PET [33, 34].
7.8 Computed Tomography
Computed tomography (CT) is a noninvasive cardiac imaging technique that is mainly used to test for the presence of coronary artery disease. Multidetector CT is thus accurate in identifying patients with idiopathic versus ischemic forms of DCM and may represent a valid alternative to coronary angiography [35, 36]. Data acquisition in CT is continuous throughout the cardiac cycle. Therefore, end-diastolic and end-systolic images may be used to analyze ventricular volumes and function, with good correlation with CMR and contrast-enhanced echocardiography [37, 38].
7.9 Dystrophinopathy-Associated Cardiomyopathy
CMR studies in patients with dystrophinopathy-associated CMP show abnormalities of myocardial strain through SSFP and tagging sequences [39–41], midwall/subepicardial fibrosis by LGE imaging [42–44], diffuse fibrosis identified by T1 mapping techniques [45], fatty replacement [46, 47], and inflammation through T2 and early gadolinium enhancement sequences [48]. The latter sign is a harbinger of rapid LV function deterioration [48]. Subclinical CMP is also reported at CMR (SSFP and LGE) in mother-carriers of Duchenne and Becker muscular dystrophy [49]. Myocardial perfusion with 201Tl imaging shows distinct perfusion defects in the LV free wall in patients with Duchenne muscular dystrophy [50–52]. Large perfusion defects have adverse prognostic significance [50, 53]. PET identifies areas of myocardial perfusion/metabolism mismatch (increased [18F]-FDG activity and decreased [13N]-ammonia activity) in the LV free wall in patients with Duchenne and Becker dystrophy [54, 55].
References
1.
2.
3.
4.
5.
Sibley CT, Noureldin RA, Gai N et al (2012) T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology 265:724–732PubMedCrossRefPubMedCentral
6.
7.
8.
Piechnik SK, Ferreira VM, Dall’Armellina E et al (2010) Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 12:69PubMedCrossRefPubMedCentral
9.