Feature
Comments
Usual anatomic relationships
Anterior: obliterated umbilical artery
Posterior: ureter
Superior: external iliac vessels
Anatomic location
Location of the ovaries varies, depending on multiple factors:
Pregnancy may cause permanent displacement of the normal ovaries
Supporting ligaments may be lax or absent (e.g., after hysterectomy):
Suspensory ligament: connects the superior pole of the ovary to the pelvic sidewall
Ovarian ligament: attaches the lower pole of the ovary to the uterus
Mesovarium: attaches the ovary to the broad ligament. Contains the neurovascular bundle
Ovarian vessels
Dual arterial supply: ovarian arteries from aorta and ovarian branches of uterine artery (arises from the internal iliac artery)
Right ovarian vein drains directly into the inferior vena cava
Left ovarian vein drains into left renal vein
Ovarian nerves
Superior group: from the intermesenteric nerves and renal plexus
Middle group: from the superior hypogastric plexus or hypogastric nerve
Inferior group: from the inferior hypogastric plexus (pelvic plexus)
Table 1.2
Ovarian histology
Layer | Features |
|---|---|
Surface epithelium | Outermost layer, formed of simple cuboidal cells covering the ovarian surface |
Derived from the mesoderm (similar to the mesothelium of the peritoneum) | |
Site of origin of most ovarian tumors | |
Cortex | Comprises most of the ovary during reproductive age |
Contains ovarian follicles (including follicular cells, granulosa cells, and oocytes) | |
Medulla | Innermost layer |
Highly vascular; fed by branches of the ovarian vessels |
Ovarian cancer is the most lethal of all gynecologic tumors [1]. It is a genetically heterogeneous disease with a poor prognosis; and despite advances in technology and chemotherapeutic agents, the mortality from ovarian cancer has remained stable for the past 50 years [1]. Although it is sensitive to platinum-based chemotherapy, the 5-year survival for patients with advanced disease is only 30 % [1, 2]. The high mortality reflects advanced stage at presentation, including spread to serosal surfaces, abdominal or pelvic lymph nodes, and parenchymal metastases, as well as a high tendency for recurrence.
The standard of care for patients with newly diagnosed advanced ovarian cancer is comprehensive staging laparotomy and primary surgical cytoreduction followed by adjuvant chemotherapy [3]. The use of neoadjuvant chemotherapy followed by interval debulking surgery as a suitable alternative option has also been proposed [4]. The location and extent of location of peritoneal spread dictates the feasibility of cytoreductive surgery and predicts the likelihood of optimal primary cytoreduction.
Multiple imaging modalities have a role in the evaluation of the ovaries, including ultrasound, CT, MRI, and positron emission tomography (PET) (Table 1.3). In patients presenting with symptoms or findings on clinical examination suggestive of pelvic pathology, the main role of imaging is to distinguish ovarian origin from pathologies from adjacent organs (e.g., uterus or bowel) and to identify features that allow discrimination between benign and malignant ovarian tumors. In patients with ovarian cancer, imaging is used to identify the presence and extent of regional and distant spread of the disease, and serves as a “road map” to surgery. Following treatment, imaging is also used to identify potential sites of recurrence when this is clinically suspected. Ultimate diagnosis usually requires histologic confirmation, but interpretation of imaging findings in the context of other patient characteristics such as age, menopausal status, personal and family history, clinical examination, and tumor markers such cancer antigen 125 (CA 125) allows the formulation of an appropriate differential diagnosis and guides patient management.
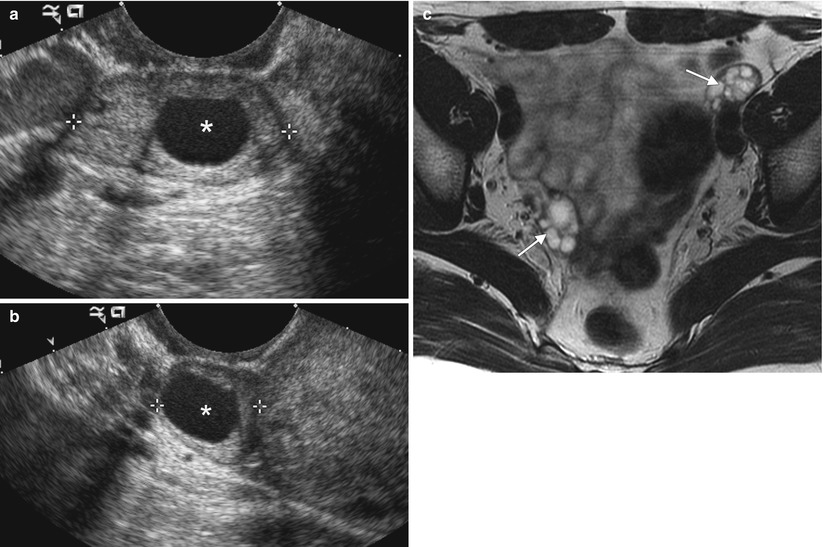
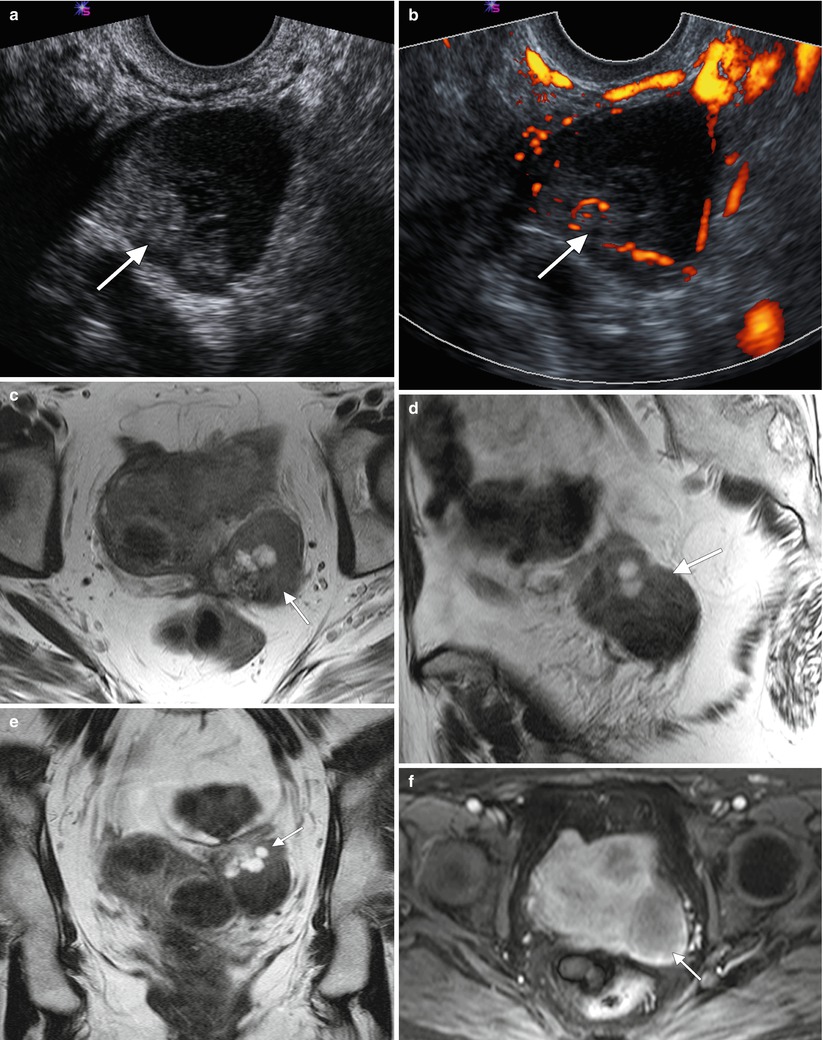
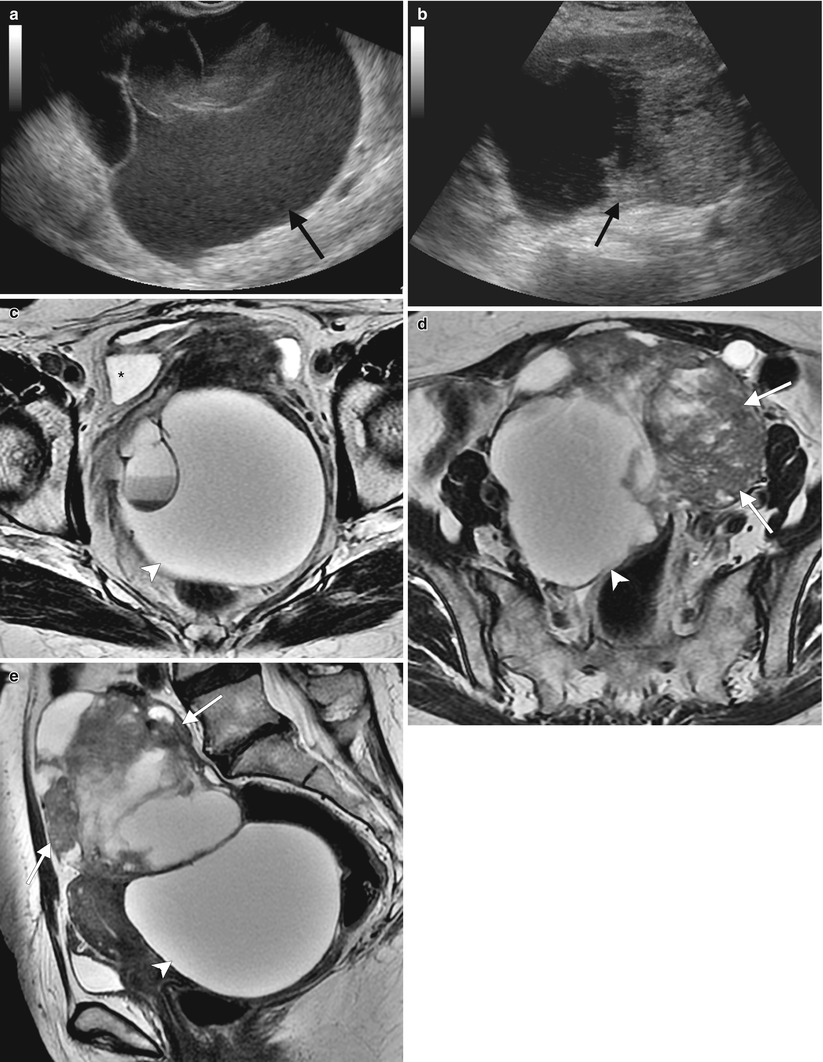
Table 1.3
Imaging techniques used for evaluation of the ovaries
Technique | Main use | Advantages | Disadvantages |
|---|---|---|---|
Ultrasound | First-line evaluation of ovarian pathology | Readily available | Operator-dependent |
Cost-effective | |||
Usually well tolerated | |||
CT | Staging of ovarian cancer | Fast | Involves ionizing radiation |
Evaluation of incidentally detected lesions | Reproducible | Soft tissue resolution is not as good as ultrasound or MRI | |
More widely available than MRI | |||
MRI | Characterization of lesions indeterminate on ultrasound or CT | Superb soft tissue resolution | More expensive and less readily available than ultrasound or CT |
No ionizing radiation | |||
PET/CT | Staging of ovarian cancer | Depicts function in addition to anatomy | Ionizing radiation from both the PET and CT components |
Evaluation of ovarian cancer recurrence |

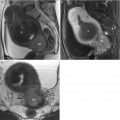
Fig. 1.1
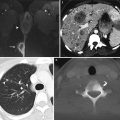
Normal ovaries. The size of the ovaries and the presence and size of follicles vary depending on the patient’s age and menopausal status. Immature follicles are usually smaller than 1 cm, but normal ovaries may contain follicles up to 3 cm in size. With the advent of high-resolution imaging, the ovaries are now frequently identified in postmenopausal women. The normal fallopian tubes are not routinely seen because of their small size and tortuous course. On ultrasound, the ovaries are typically isoechoic to muscle, and the normal follicles are hypoechoic. These images, from a 40-year-old patient, present longitudinal (a) and transverse (b) two-dimensional ultrasound views of the normal right ovary containing a physiologic follicle (asterisk). On T1-weighted MR images, the ovaries display homogeneous, low to intermediate signal intensity, whereas on T2-weighted MR images, the follicles become hyperintense compared with the surrounding stroma. This T2-weighted MR image (c) shows the normal appearance of the ovaries in a 34-year-old premenopausal woman (arrows). In postmenopausal women, the ovaries typically appear as predominantly solid structures with a relative increase in stromal tissue and may contain small T2-hyperintense follicles, although dysfunctional cysts up to several centimeters in size may also be encountered

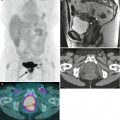
Fig. 1.2
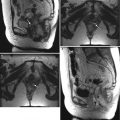
Ultrasound is usually the modality of choice for initial evaluation of suspected adnexal lesions. The other imaging modalities are reserved for the assessment of ultrasound-indeterminate lesions or the staging of suspected ovarian cancer. These are representative images from a 60-year-old woman with weight loss and mild abdominal discomfort. Two-dimensional (a) and color Doppler (b) ultrasound images demonstrate a complex solid/cystic mass with internal vascularity (arrows). Axial (c), sagittal (d), and coronal (e) T2-weighted images and a fat-suppressed T1-weighted image following intravenous gadolinium (f) showed that the mass arises from the left ovary (arrows) and has cystic and enhancing solid components. Benign uterine leiomyomas were also demonstrated. Pathology showed endometrioid adenocarcinoma of the left ovary

Fig. 1.3
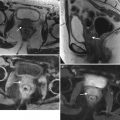
MRI can be used for further characterization of masses detected by ultrasound, as shown by these representative images from a 67-year-old woman who self-palpated a “lump” in her lower abdomen. Two-dimensional transabdominal ultrasound images (a, b) showed a large, complex pelvic mass, but its exact origin and relation to adjacent organs was difficult to evaluate because of its large size. Axial (c, d) and sagittal (e) T2-weighted images showed the large, central pelvic mass containing solid components (arrows) and cystic components (arrowheads). Some of the cystic components demonstrate fluid-fluid layering (asterisk), probably owing to hemorrhage. There is mass effect on the uterus and bladder, which are displaced anteriorly. Pathology showed high-grade serous ovarian carcinoma
1.2 Benign Ovarian Pathology
Follicular and corpus luteum cysts are common in women of reproductive age (Tables 1.4 and 1.5). Follicular cysts result from failure of the normal ovarian follicle to rupture or regress. The corpus luteum develops from an ovarian follicle following ovulation. It secretes progesterone, and is essential for establishing and maintaining pregnancy. If the ovum is not fertilized, the corpus luteum regresses spontaneously after one or two menstrual cycles. Hemorrhage into a corpus luteum cyst can produce a complex appearance that changes over time depending on the stage of clot evolution. Cysts larger than 3 cm in size should be followed up on ultrasound to ensure resolution. A summary of ultrasound features used to predict whether ovarian tumors are benign or malignant are presented in Table 1.6.
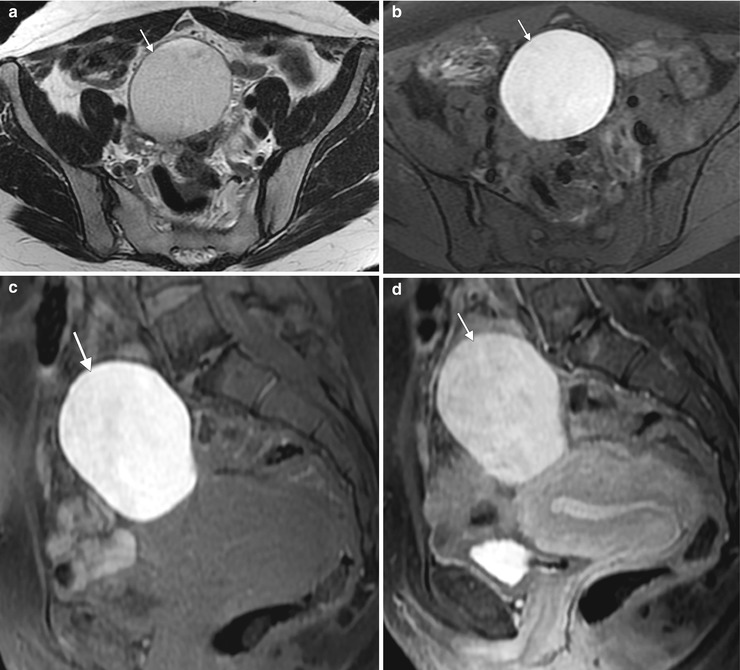
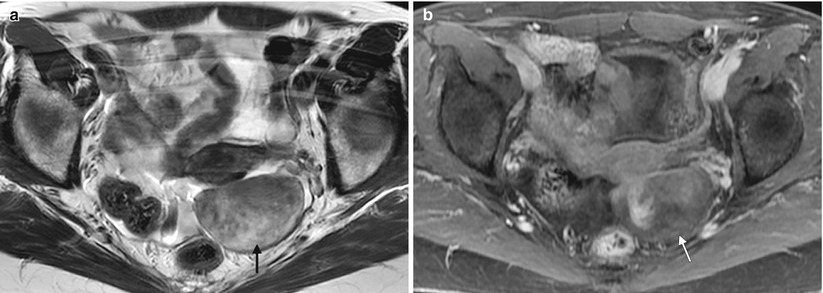
Table 1.4
Imaging features of benign ovarian tumors
Lesion | Ultrasound | T1WI | T2WI | T1+C | Other |
|---|---|---|---|---|---|
Follicular cyst | Anechoic, thin-walled, unilocular cyst | Low | High | None | Should be followed up to ensure resolution |
Corpus luteum cyst | Cystic lesion | Low | High | None | Lack of enhancement differentiates clot from solid nodule |
May contain clot | |||||
“Ring of fire” on Doppler | |||||
Mature teratoma | Fat-fluid level/“white balls”/“tip of iceberg” appearance | High | Variable | None | Low signal on fat-suppressed sequences |
Endometrioma | Simple/complex cyst, low-level echoes | High | Variable | None | No fat suppression |
Low-level echoes | Appearance influenced by iron content (from hemorrhage) | ||||
Fibrotic tumors | Solid lesions | Low | Low | Delayed enhancement | May be associated with pleural effusion (Meigs syndrome) |
Some secrete hormones | |||||
Tubo-ovarian abscess | May be solid, cystic, or complex | Low | Heterogeneous high with adjacent fat stranding or edema | Thick, enhancing walls | Usually present as a complication of pelvic inflammatory disease in young women |
Serous cystadenoma | Unilocular cyst | Low | High | None | Can be indistinguishable from follicular cysts on imaging |
Mucinous cystadenoma | Multilocular cyst | Low | High | None | May contain thin septations |
Table 1.5
Imaging criteria used to differentiate benign versus malignant ovarian massesa
Criteria | Benign | Malignant |
|---|---|---|
Primary criteria | ||
Cyst | Simple | Papillary projection |
Number of septae | <3 | ≥3 |
Wall/septal thickness (mm) | <3 | ≥3 |
Solid component | Homogeneous | Heterogeneous with necrosis or hemorrhage |
Size (cm) | <4 | ≥4 |
Ancillary criteria | ||
Ascites | Absent | Present |
Peritoneal/omental deposits | Absent | Present |
Lymph nodes | No enlarged lymph nodes | Enlarged lymph nodes |
Table 1.6
Positive predictive value of simple ultrasound features used to predict whether ovarian tumors are benign or malignanta
Ultrasound feature | Positive predictive value (%) |
|---|---|
For predicting benign histology | |
Unilocular | 99 |
Presence of solid components, of which largest solid component has largest diameter <7 mm | 100 |
Presence of acoustic shadows | 95 |
Smooth multilocular tumor with largest diameter <100 mm | 99 |
No blood flow | 98 |
At least one feature | 96 |
For predicting malignant histology | |
Irregular solid mass | 96 |
Presence of ascites | 97 |
≥ 4 papillary projections | 88 |
Irregular multilocular solid mass measuring ≥10 cm | 84 |
Strong Doppler blood flow | 88 |
At least one feature | 87 |

Fig. 1.4
Endometriosis and endometrioma. Endometriosis refers to the presence of endometrial glandular tissue outside of the uterus. Common locations include the ovary, uterine ligaments, fallopian tube, rectovaginal septum, pouch of Douglas, bladder wall, uterovesical fold, and umbilicus. Ovarian involvement results in the development of endometriomas, which are cystic lesions containing blood products. These images are from a 36-year-old woman with a family history of ovarian cancer and an ovarian lesion identified on screening ultrasound. An axial T2-weighted image (a) demonstrates a homogeneous, well-defined, hyperintense lesion arising from the right ovary. Fat-suppressed axial (b) and sagittal T1-weighted images before (c) and after (d) intravenous gadolinium show the lesion to be T1-hyperintense without fat suppression. This appearance is consistent with endometrioma

Fig. 1.5




Fibrotic tumors of the ovary. Fibromas, thecomas, and fibrothecomas are fibrotic tumors of sex-cord stromal origin that account for approximately 5 % of all ovarian tumors. They are usually asymptomatic and typically are detected in middle-aged women during routine gynecologic examination. Because of their solid appearance on imaging, they may mimic pedunculated uterine leiomyomas and even malignant ovarian tumors. They are associated with ascites in 15 % of cases and with pleural effusion (Meigs syndrome) in 1 %. Thecomas may be associated with endometrial thickening (due to estrogen secretion) or hirsutism and amenorrhea (due to androgen secretion). These images are from a 51-year-old woman with an adnexal mass. An axial T2-weighted MR image (a) and an axial fat-suppressed T1-weighted, contrast-enhanced MR image (b) demonstrate a well-defined, heterogeneous but predominantly T1- and T2-hypointense lesion in the left adnexal region. Pathology showed a fibrothecoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree