Hence, the success of any diagnostic test as a colorectal cancer screening tool lies in its ability to accurately diagnose polyps measuring 1 cm and more as well as detect early colorectal cancers.
Optical colonoscopy has been the gold standard test for the evaluation of the colon with an excellent sensitivity and specificity for the diagnosis of colorectal cancer and polyps. However, it is an invasive test and carries the risk of significant complications such as perforation. Apart from its invasive nature, other issues such as cost, availability, and patients’ experience led to the introduction and development of diagnostic imaging methods for evaluating the colon and rectum.
Traditionally, double-contrast barium enema has been the workhorse for imaging the colon and rectum in patients with suspected colorectal cancer. As colorectal cancers were more common in the rectum and sigmoid colon, it was common practice to perform flexible sigmoidoscopy (which is quicker, safer, and technically easier compared to colonoscopy), and the rest of the colon was evaluated by barium enema. However, the sensitivity of barium enema was shown to be low, even for polyps greater than 1 cm. In a prospective study, Rockey et al. reported a sensitivity of only 48 % for polyps greater than 1 cm, and this fell even lower to 41 % for polyps between 6 and 9 mm [10]. Hence, it became essential to replace barium enema with a more sensitive test.
Computed tomographic colonography (CTC) was first introduced by Vining et al. in 1994 as an alternative imaging method for the evaluation of the colon. In this technique, a helical CT data is used to produce three-dimensional images and hence simulate a virtual endoluminal view (hence also called virtual colonoscopy). High sensitivity rates for colorectal cancer can be obtained by this method. In a recent meta-analysis, Pickhardt et al. have reported that the pooled sensitivity of CT colonography for colorectal cancer was about 96 % compared to 95 % seen in optical colonoscopy [11]. It has to be noted that this excellent sensitivity for colorectal cancer was achieved in spite of including studies from five different continents with considerable variability in CT colonography techniques and technologies (using both single- and multidetector CT scanners). This underlines the excellent potential of CT Colonography as a screening tool for colorectal cancer on a universal basis.
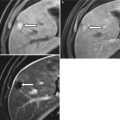
With regard to colonic polyps, the sensitivity of CTC is dependent on the size of the polyps (Fig. 11.1). The sensitivity and specificity of CTC in diagnosing polyps larger than 1 cm is 93 and 97 %, respectively. However, both the sensitivity and specificity reduces to 86 % for polyps measuring between 6 and 9 mm [12]. Though its sensitivity is reduced in small-size polyps, the risk of malignancy in a polyp less than 1 cm in diameter is less than 1 % [13].
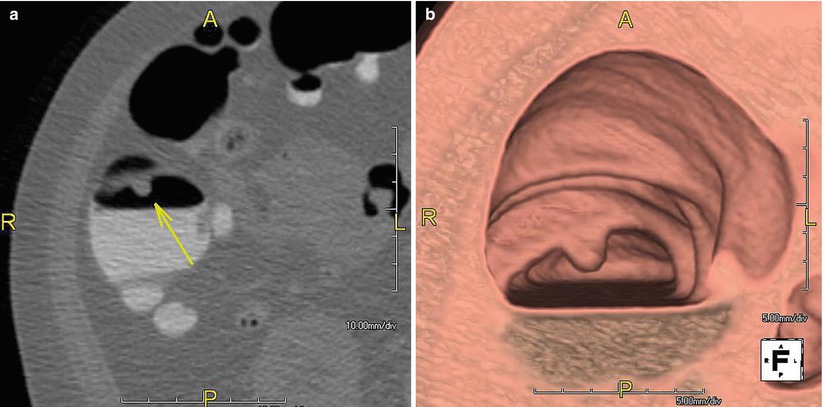

Fig. 11.1
CTC in a 54-year-old man shows an 8 mm polyp (arrow) in the ascending colon in the 2D image (a) and 3D image (b). Pathology confirmed adenoma
Besides being an excellent screening tool for colorectal cancer, it is minimally invasive, less time consuming, and does not require sedation. Further, it offers staging information, which is not possible with either optical colonoscopy or barium enema. Complications of colonic tumors such as obstruction, perforation, and fistula can be readily visualized with CTC. Finally, it also highlights the presence of any significant extracolonic pathology (Fig. 11.2), which may significantly alter the management.
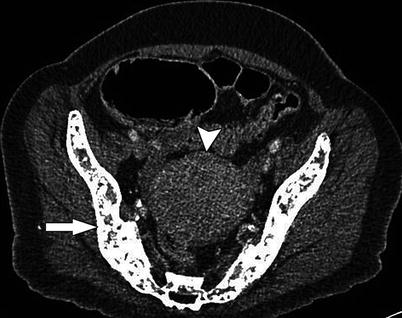

Fig. 11.2
CTC in a 69-year-old female with abdominal pain and constipation shows a large heterogenous 8 cm ovarian tumor (arrowhead) and bone metastases (arrow)
Indications and Contraindications for CTC
CTC is an established test for evaluating symptomatic patients in whom colorectal cancer is strongly suspected [14]. It is also a valuable test for colorectal cancer screening. The American College of Radiology has approved CTC for colorectal cancer screening in patients more than 50 years old, those with a positive fecal occult blood test, or individuals at moderate risk with a personal history of adenoma or colorectal cancer or with a family history of adenoma or cancer in a first-degree relative. High-risk individuals with hereditary nonpolyposis colorectal cancer are better evaluated with OC as there is a high pretest probability of identifying polyps/tumors in this group, and hence biopsy or polypectomy may be performed at the same sitting.
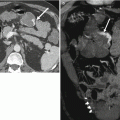
Patients with failed or incomplete colonoscopy or those who are reluctant to undergo OC or those who may be at an increased risk with OC can be evaluated by CTC [15, 16] (Fig. 11.3). CTC has been also used in the evaluation of patients presenting with obstructing colonic neoplasm as well as in inflammatory bowel disease [17–21] (Fig. 11.4).
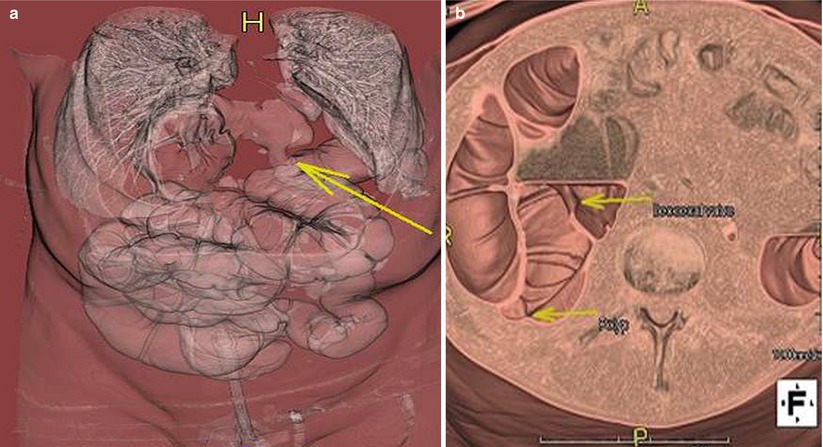
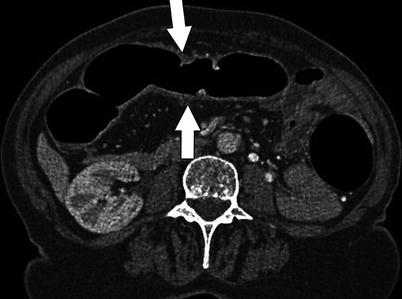

Fig. 11.3
3D reconstruction of CTC in a 60-year-old woman who had a failed colonoscopy shows the bowel herniating (long arrow in a) to the left hemithorax via a diaphragmatic hernia (which was the reason for the failed colonoscopy). Note the two polyps in the ascending colon (arrows in b)

Fig. 11.4
Axial 2D analysis of CTC shows subtle thickening in the ascending colon (arrows) in a 56-year-old man. Colonoscopy and biopsy confirmed Crohn’s disease
CTC Technique
CT colonography is based on a helical, thin-section CT of the cleansed and distended colon. Bowel preparation, bowel distension, CT protocol, and data interpretation are the essential components which ultimately decide a successful CTC study (Table 11.1) [12–57].
1. Bowel preparation (cathartic or noncathartic bowel cleansing, fecal tagging using oral iodinated contrast) |
2. Bowel distension (automatic insufflator) |
3. Proper CT technique (multidetector helical CT with or without IV contrast, obtain images in supine and prone positions, ensure adequate distension of all portions of bowel) |
4. 2D and 3D analysis (both are required as they complement each other) |
Step 1: Obtaining a Clean and Well-Distended Bowel
Following the introduction of CTC in 1994, various different cathartic agents including magnesium citrate, sodium phosphate, picolax, and polyethylene glycol (PEG) have been advocated and used worldwide for cleaning the bowel [25–27]. The choice of bowel preparation depends on patients’ comorbidities and the local departmental preferences. It has been shown that sodium phosphate gives significantly less residual liquid than polyethylene glycol solution [26]. However, sodium phosphate is contraindicated in patients with renal insufficiency and heart failure, and in such cases, it is best to use other alternatives such as magnesium citrate or PEG [28–30].
Oral contrast agents such as barium or iodinated contrast are commonly used in many centers to tag residual stool or fluid within the colon [31–33]. As the fecal material gets tagged with the high-attenuation contrast, they can easily be differentiated from the non-tagged soft tissue lesions such as polyps and tumors. The contrast also tags residual fluid thereby increasing the conspicuity of polyps which may be submerged in residual fluid based on attenuation differences.
Non-laxative-based bowel preparations have also been used in CTC by some authors, which spare the patients the burden of having to undergo rigorous and often troublesome catharsis [34–37]. A low-fiber diet with multiple oral doses of iodinated contrast over a 48-h period before CTC is used without any bowel catharsis. Using an advanced software (digital subtraction bowel cleansing process), the tagged fecal material can be subtracted from the source images, thereby allowing more accurate interpretation [38, 39]. Further research would be required before this can become a routine practice.
Bowel Distension
Besides having a clean bowel, it is paramount to have adequate distension of all segments of the colon for a complete CTC study [40, 41]. Gaseous bowel distension is achieved using an automated CO2 delivery via soft, rectal catheter. This is preferred as CO2 causes less discomfort compared to room air, and the automated system allows greater control in terms of flow rate, total volume administered (usually 2–4 l but highly variable), and maintaining adequate intracolonic pressure (maximum of 25 mmHg) [42–44]. Some centers advocate the use of intravenous antispasmodics (such as glucagon or hyoscine-N-butylbromide, Buscopan) prior to bowel distension as it is felt that they improve colon distension and reduce spasms. However, hyoscine-N-butylbromide is not currently licensed in the United States.
Step 2: Using Appropriate CT Protocol and Achieving Expertise in Data Interpretation
In general, a multidetector CT (MDCT) scanner is superior to single-detector row CT for performing CTC as it allows acquisition of multiple thin sections, superior multiplanar reconstructions, and faster imaging time [45, 46]. Judicious use of CT parameters including appropriate reduction in milliampere-seconds and using automated dose control software can help keep the radiation dose to a minimum [47–49].
Scanning is performed in supine, prone, and, in some cases, decubitus positions [50]. Obtaining CT datasets in different positions helps in many ways. Apart from helping to differentiate fixed lesions such as polyps/tumor from mobile feces, it also helps to ensure that the entire colon is distended in at least one or more sequences [41, 51, 52]. However, feces may be adherent and polyps can be very mobile, especially those with long stalks, but usually this can be distinguished by a careful interpretation of both 2D and 3D datasets. The use of intravenous contrast media is not absolutely necessary in CTC but may be helpful, especially in staging the cancer (when present) and in the detection of significant extracolonic pathology [45, 53–55].
Data analysis in CTC requires interpreting both 2D and 3D imaging as they complement each other [40, 41] (Fig. 11.5). There are certain instances where 3D imaging is better than 2D, and the opposite is also true. For example, 3D interpretation is reported to be more sensitive for polyp detection compared to 2D imaging, particularly for those on the folds [46, 56–59] (Fig. 11.6). On the other hand, 2D interpretation can help to differentiate between true lesion and fecal residue. Also, measuring polyp size, which is crucial for management, can sometimes be challenging, and in most cases, it would be useful to measure it both on 2D and 3D views to get a true estimate [60]. Some authors advocate polyp volume measurement to overcome this problem [61].
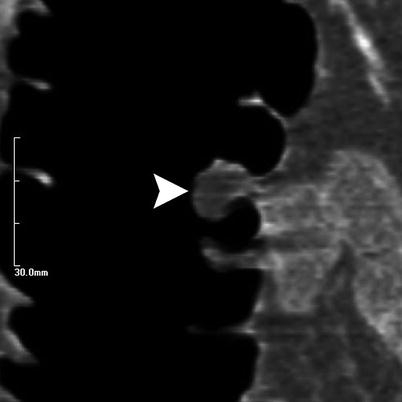
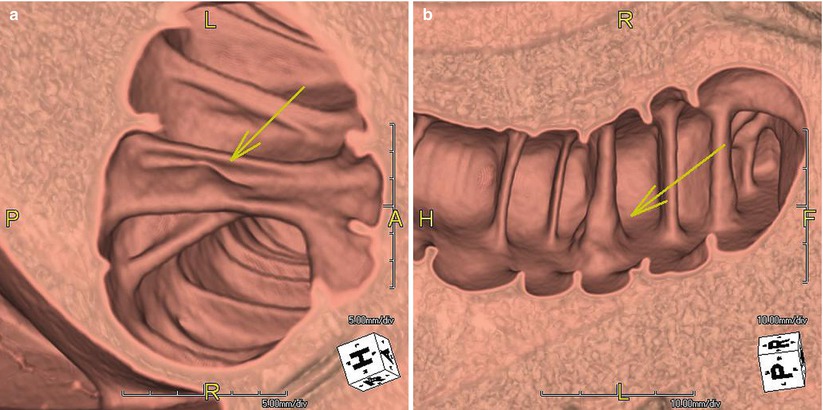

Fig. 11.5
3D analysis of CTC (not shown) in a 60-year-old woman showed a 9 mm polyp in the ascending colon. Correlation with 2D images confirmed that this was a benign lipoma (arrowhead) and hence did not require a colonoscopy

Fig. 11.6
3D images (a,b) of CTC in a 48-year-old woman show a polyp in the fold (arrow). These are usually easier to spot on 3D images
When using 3D views, it is important to realize that both prone and supine datasets will have to read in both directions, i.e., bidirectional “fly through.” Thus, when reading the supine images, rectum to cecum fly through is read first followed by a fly through in the opposite direction, and this is repeated for the prone images.
Various pitfalls encountered during CTC interpretation have been reported before [46]. This includes missing polyps on fold, especially those on the backside; misinterpreting complex folds as polyps; and mistaking a pedunculated polyp for feces due to its mobility [46]. Also, polyps, especially villous adenomas, may get coated with contrast which may lead to them being mistaken for tagged feces, but careful attention usually shows that it is only the interstices of the villous adenoma which has been coated. Flat and carpet lesions may often be missed when 2D images alone are evaluated. Diverticular disease may limit distension as well as interpretation (Figs. 11.7 and 11.8). Rectal catheters may sometimes hide lesions. Also, when using oral fecal tagging, it is important to realize that sometimes the contrast may be too dense such that polyps submerged in tagged residual fluid may still not be visible unless the window width and window level are adjusted accordingly.
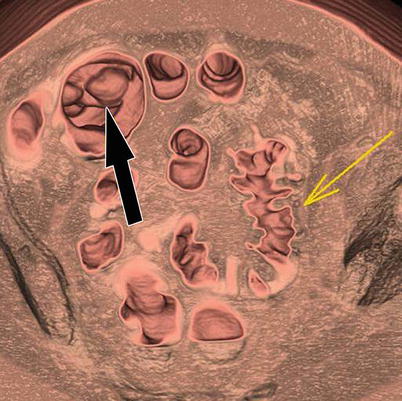
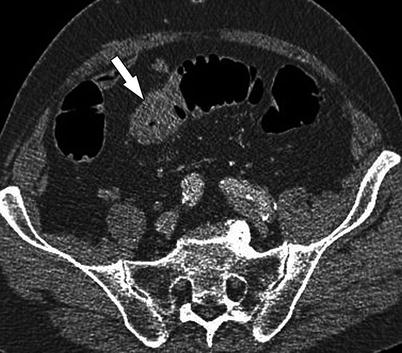

Fig. 11.7
3D images of CTC in a 56-year-old man who had a failed colonoscopy show severe diverticular disease in the sigmoid colon (yellow arrow), which is under distended, limiting interpretation. Note the 1 cm polyp in the cecum (black arrow), which proved to be malignant. Patient underwent right hemicolectomy. Failed optical colonoscopy is one of the most common indications for virtual colonoscopy

Fig. 11.8
CTC in a 76-year-old man with change in bowel habits shows abnormal thickening and stricture in the sigmoid colon (arrow). Although there are no pericolic nodes, this is a rather short segment stricture and neoplasm cannot be ruled out. As the patient was symptomatic, surgery was performed and pathology showed only diverticular stricture with no evidence of malignancy
Annular masses, extrinsic lesions, and impacted diverticula, are potential pitfalls seen at 3D evaluation [46]. Also, not performing a bidirectional fly through may lead to missing polyps on the backsides of folds. Most of these pitfalls can be overcome by complementing 2D with 3D interpretation.
Recent advances in 3D visualization have resulted in new 3D tools such as virtual dissection, panoramic views, unfolded, cube projections, and translucency rendering [62–65]. These have been designed with an idea to reduce time (eliminating need for bidirectional study as in virtual dissection) and increase specificity by assigning different colors for tissues of different attenuations such as feces and polyps (translucency rendering). It is also possible to track the visualized 3D endoluminal surface so that any areas which may have been missed initially can be re-interrogated, thereby reducing potential misses [66]. Further, computer-aided detection (CAD) systems are currently available in most CTC workstations, which can be helpful to increase the sensitivity of the radiologists, especially when they are relatively new to this technique [67–69].
Reporting CTC Studies and Training Issues
Following a standardized, structured reporting for CTC helps ensure consistency while reporting CTC as well as comparing CTC studies performed at different institutions which is an increasingly common phenomenon. In 2005, a standardized reporting scheme, “C-RADS—CT Colonography Reporting and Data System” was put forward by the working group on virtual colonoscopy [70]. They proposed that the report should include lesion size, number, morphology, location, attenuation, and recommendations for lesion surveillance.
It would also be very useful to create and save a 3D map and multiplanar reconstructed digital images highlighting the polyp locations in each segment such that the endoscopist can use it as a road map while performing optical colonoscopy.
Every diagnostic imaging tool is only as good as the radiologist who interprets it. Clearly, the sensitivity and specificity of CTC in the hands of experts are significantly higher than those who are less trained in this procedure. Hence, it is important to ensure high standards in the interpretation of CTC, and to meet this goal, several training and accreditation courses are advocated by various expert panels including the American College of Radiology [46, 71, 72].
Similarly, it would be very useful to educate the CT technicians to look for issues such as collapsed segments of bowel at the time of scan so that additional images in decubitus views may be obtained in the same sitting, which can help to obviate the need for a future repeat study or optical colonoscopy [73]. Likewise, it may be useful to teach the technicians to identify obvious colorectal tumors so that a staging scan of the chest can also be performed at the same time. In a well-organized population screening program, it should be possible to use CTC as a primary screening tool, and this can help to triage patients who may benefit from optical colonoscopy, rather than subjecting all patients to the more invasive colonoscopy.
Conclusion
CTC is an excellent diagnostic tool for a comprehensive evaluation of the entire colon. Its role in symptomatic population is well established. Recent studies have confirmed that when CTC is properly performed and evaluated, a high diagnostic accuracy can be achieved for clinically significant polyps and colorectal cancer. This has resulted in CTC being approved for colorectal cancer screening and surveillance by the American Cancer Society, the American College of Radiology, and the US Multisociety Task Force on Colorectal Cancer [74–78]. Further studies on the cost-effectiveness of CTC can help overcome the current reimbursement issues and strengthen the argument for the widespread use of CTC as a colorectal cancer screening tool in the United States.