Overview of Experience with Heavier Charged Particle Radiotherapy
Hirohiko Tsujii
Tadashi Kamada
Juergen Debus
In cancer therapy, the question is often raised whether improved local control could be associated with improved survival despite the fact that many patients eventually succumb to distant metastases. There has been ample evidence that in a number of malignancies, local relapse is correlated with distant metastasis and the impact of improved local control on survival is mediated through a reduction in deaths caused by local progression and reduction in distant metastasis.1 In this context, charged particle beams such as protons and heavier ions, compared with photon beams, provide an improved dose distribution and, in the case of heavier ions, a larger relative biological effectiveness (RBE), leading to a higher probability of tumor control while irradiating a smaller volume of surrounding normal tissues, thereby reducing the frequency and severity of radiation morbidity.
Since 1954 when charged particle radiotherapy (RT) was initiated using proton beams at Lawrence Berkeley Laboratory (LBL), the efficacy of heavier charged nuclei such as stripped helium, carbon, nitrogen, neon, silicon, and argon has been also assessed. Currently, only protons and carbon ions are employed worldwide and there are approximately 30 operating proton therapy facilities, whereas carbon ion RT is performed at three facilities worldwide. More than 3,000 patients have been treated with carbon ions since 1994 on the heavy ion medical accelerator (HIMAC) at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, and >300 patients have undergone carbon ion therapy at the Gesellschaft für Schwerionenforschung (GSI) in Germany since 1997. The third carbon ion therapy facility, in which protons are also available, started operations in 2004 in Hyogo, Japan. Three more facilities are under construction for carbon ion therapy at Heidelberg in Germany, Pavia in Italy, and Gumma in Japan and many more are planned.
PHYSICAL AND RADIOBIOLOGIC ASPECTS OF HEAVY IONS
Physical Properties
Conventional photon beams and neutron beams are characterized by an exponential absorption of dose with depth, whereas heavy ions such as carbon ions share with protons the property of forming a high-dose region known as a Bragg peak in the medium. The peak is typically narrow, a few millimeters at the 80% level, and the dose near the peak is several times greater than the dose in the plateau. In the region beyond the Bragg peak, almost no dose is deposited by protons but heavier ions do deliver a small dose as a result of secondary fragments. The original peak is too narrow and sharp to be used directly for the treatment of lesions with different shapes and sizes. Modification of the narrow peak is therefore necessary to conform to the size and shape of the lesion. This is performed with a passive scattering system using a range modulator, collimators, and compensators at NIRS and Hyogo,2,3 while at GSI an
active beam delivery system or beam scanning method is used.4,5
active beam delivery system or beam scanning method is used.4,5
In the passive scattering system, the peak is continuously moved and overlaid by a ridge filter to expand or spread out the peak width. The ridge filter is an energy absorbent that varies the amount of absorber, thereby sweeping the narrow peak over an extended region to create the spread-out Bragg peak (SOBP). In the scanning method, the peak position is dynamically moved within the target by changing the beam energy (see Chapter 5 for an in-depth discussion of beam delivery systems). At present, for both proton and carbon ion therapy, beam scanning is used only in a small number of facilities but it has the advantage of low maintenance costs and the potential to realize intensity-modulated particle radiotherapy (IMPT) with its promise of higher dose conformality.
Biological Properties
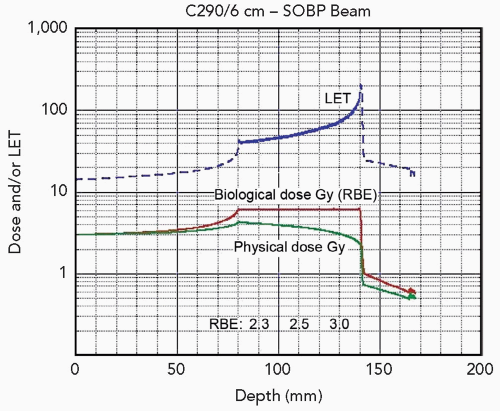
For an evaluation of biological end points, an estimate of the beam quality is required. For this purpose, the density of ionization events in tissue, that is, linear energy transfer (LET), is used to estimate the biological injury in radiation therapy. It is known that in the clinically relevant range, as the LET increases, the RBE increases, although the LET alone does not entirely explain the biological differences observed.6,7 Heavy ion beams such as carbon ion beams, in comparison to proton and photon beams, exhibit a larger energy loss and are described as high-LET radiation. In contrast to neutron beams where LET remains uniformly high at any depth, the LET of carbon ion beams increases steadily from the point of entrance in the body with increasing depth to reach a maximum in the peak region (see Fig. 10.1). This property is extremely advantageous from the therapeutic point of view, as the RBE of carbon ion beams increases as they advance deeper toward the tumor. In the passive beam scattering system, the shape of the ridge filter is designed to apply a gradient to the physical dose in order to achieve a uniform cell killing or isoeffect over the SOBP, whereas in the scanning technique, biological models are used to generate treatment plans with homogeneous biological effects in arbitrarily shaped target volumes.
POTENTIAL ADVANTAGES OF CARBON ION RADIOTHERAPY
Dose Distribution
The quality of dose distribution is dependent upon the energy spread and range straggling of the particles, whose magnitude is smaller for carbon ions than protons. It is also affected by the penumbra (the sharpness of the lateral edge) of the ion beam, which is dependent upon Coulomb scattering and becomes smaller as the mass of particle increases. In addition, carbon ions undergo nuclear interactions with some fragmentation of the primary beam into lower atomic number particles, producing a fragmentation tail after the distal end of the peak.2 The biological effect of this fragmentation tail is small because the tail contains only fragments with low atomic number. When comparing dose distributions between carbon ion beams and proton beams, the lateral falloff around the target volume is more rapid with carbon ion beams than proton beams (see Fig. 10.2). Both proton and carbon ions have another advantage over photons, which is related to generation of positron-emitting nuclei, which can be visualized by camera systems in situ and also offline.8,9 The detection and reconstruction of this nuclei has the potential to be used a method for quality control.
Improved Therapeutic Gain
Although the RBE of carbon ion beams is greater than that of proton and photon beams, the clinical interest lies in the existence of differential effects between the tumor and normal tissue that increases the therapeutic gain. In this respect, carbon ion beams are considered to have the best balance in terms of both physical and biological dose distributions. The biological advantage expected from the use of carbon ions are many fold: RBE is increased, oxygen enhancement ratio (OER) is reduced, repair of radiation damage is suppressed, and the cell cycle dependency of radiosensitivity is reduced. These characteristics, combined with improved dose localization, may play a major role in improving the
therapeutic ratio of carbon ion beams as compared to proton and photon beams. Accordingly, carbon ion RT could be effective against locally advanced, photon-resistant tumors and those located near critical structures.
therapeutic ratio of carbon ion beams as compared to proton and photon beams. Accordingly, carbon ion RT could be effective against locally advanced, photon-resistant tumors and those located near critical structures.
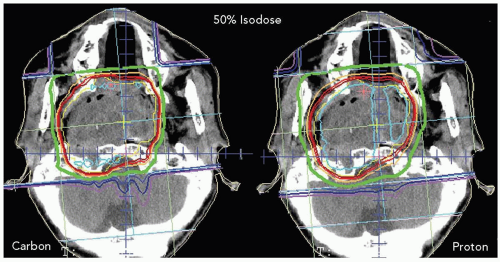 Figure 10.2 Comparison of therapeutic dose distributions between carbon ion beams and proton beams. The lateral falloff in carbon ion irradiation is sharper than in proton irradiation. |
Tepper10 and Goldstein11 investigated RBE values for single and fractionated doses for jejunal crypt cell survival after irradiation with various ions at different positions of the SOBP. They observed an increasing effectiveness of ions with increasing ion charge (and mass). As the ion mass increased, the increase of RBE was first observed in the peak region and then extended to the plateau region. When different ions were compared, carbon ions were characterized by the highest peak-to-plateau RBE ratio, which was also confirmed in early skin reactions of the mouse after irradiation with other ions.12 This opens up a promising potential for highly effective use of carbon ions in such tumors that are deeply located and resistant to photon beams.
Batterman13 investigated the relation between the RBE values and volume doubling time in lung metastases after fast neutron irradiation. It was found that the RBE of neutron beams increased as the volume doubling time increased; indeed, neutron beams had a higher RBE for slow-growing tumors such as salivary gland tumors, prostate cancer, and bone/soft tissue sarcomas. In the case of salivary gland tumors, the RBE for fractionated RT was found to be approximately 8.0 compared to values in the range of 3.0 to 3.5 expected for late damage in most normal tissues. Laramore14 summarized those tumor types and clinical situations in which fast neutron therapy offered an advantage. These advantages could also be realized with carbon ion therapy, which has an RBE similar to neutrons.
Hypofractionated Radiotherapy
Because of its unique physical and biological properties, it is theoretically possible in carbon ion RT to perform hypofractionated RT using a significantly smaller number of fractions than has been used in conventional RT. Experiments with neutron beams have demonstrated that increasing their fraction dose tended to lower their radiobiologic effect for both tumor and normal tissues.15 In these experiments, however, the RBE for the tumor did not decrease as rapidly as the RBE for normal tissues size is increased. This substantiates the fact that the therapeutic ratio increases rather than decreases with an increase in fraction size. Similar results have been obtained in experiments conducted with carbon ions,16 providing the biological rationale for the validity of short-course, hypofractionated regimens in carbon ion RT.
Progress in dose escalation has already been made at NIRS on a scale that permits RT for stage I lung cancer and hepatocellular cancer to be completed in one and two fractions, respectively. Even for other tumors such as prostate cancer and bone/soft tissue tumors that generally require a relatively prolonged irradiation time, it is possible to accomplish the treatment with carbon ion beams in approximately 16 to 20 fractions.17 In carbon ion RT at NIRS, the average number of fractions per patient have been reduced from 18 fractions in 6 weeks to 12 fractions in 3 weeks.
Potential Suppression of Metastases
Ogata et al.18 reported that carbon ion irradiation induced DNA damage which possibly suppressed the metastatic capabilities of tumor cells, leading to suppression of pulmonary metastases in vivo. They postulated that the suppression of metastases might have been caused by carbon ion irradiation producing a higher proportion of double-strand DNA breaks than photon irradiation. This may be another advantage of carbon ion RT but further studies are warranted to confirm these findings.
THE CARBON ION TREATMENT FACILITY AT NIRS
The HIMAC in Chiba is the world’s first heavy ion accelerator complex dedicated to medical use in a hospital
environment. The facility is approximately 100 m × 50 m × 30 m in size. It has one story above ground and three stories underground. It consists of two types of ion sources, an radio-frequency-quadrupole linac (RFQ) and an Alvarez drift tube linac (DTL) chain, dual synchrotron rings (42 m in diameter), high-energy beam transport lines, and irradiation facilities for patient treatment and experiments.19 The ions are generated and accelerated by 800 keV with an RFQ linac, and by 6 MeV with a Alvarez linac. The accelerated beams are injected into the dual synchrotron and are accelerated with a maximum of 800 MeV/nucleon. Beams are extracted every 2 minutes. The accelerated beams are transported to three patient treatment rooms and biology and physics experimental rooms. Of the three clinical treatment rooms, room A has a vertical port, room B has both vertical and horizontal beam ports, and Room C has a horizontal beam port. The HIMAC facility is located adjacent to the hospital and medical cyclotron area. The available accelerated carbon ion beam energies are 290, 350, and 400 MeV. The range of the beams is 15 to 25 cm in water. An appropriately sized ridge filter corresponding to the tumor size is selected to form the SOBP. The compensation bolus is fabricated for each patient to make the distal configuration of the SOBP similar to the shape of the target volume. A multileaf collimator defines the margins of the target volume.
environment. The facility is approximately 100 m × 50 m × 30 m in size. It has one story above ground and three stories underground. It consists of two types of ion sources, an radio-frequency-quadrupole linac (RFQ) and an Alvarez drift tube linac (DTL) chain, dual synchrotron rings (42 m in diameter), high-energy beam transport lines, and irradiation facilities for patient treatment and experiments.19 The ions are generated and accelerated by 800 keV with an RFQ linac, and by 6 MeV with a Alvarez linac. The accelerated beams are injected into the dual synchrotron and are accelerated with a maximum of 800 MeV/nucleon. Beams are extracted every 2 minutes. The accelerated beams are transported to three patient treatment rooms and biology and physics experimental rooms. Of the three clinical treatment rooms, room A has a vertical port, room B has both vertical and horizontal beam ports, and Room C has a horizontal beam port. The HIMAC facility is located adjacent to the hospital and medical cyclotron area. The available accelerated carbon ion beam energies are 290, 350, and 400 MeV. The range of the beams is 15 to 25 cm in water. An appropriately sized ridge filter corresponding to the tumor size is selected to form the SOBP. The compensation bolus is fabricated for each patient to make the distal configuration of the SOBP similar to the shape of the target volume. A multileaf collimator defines the margins of the target volume.
TREATMENT PLANNING FOR HEAVY ION RADIOTHERAPY
As in modern photon therapy, the initial procedure to ensure the proper administration of carbon ion therapy is the fabrication of an immobilization device for each patient. Computed tomography (CT) scans for treatment planning are taken with the patient immobilized in the device. If the patient requires respirationsynchronized irradiation, the respiratory gated irradiation system is also used at the time of these CT scans.20 The CT image data obtained in this manner are then transferred to the treatment planning system. At this stage, the irradiation parameters in terms of the number of irradiation portals and beam directions are determined in conjunction with the delineation of the target volume.
Treatment planning for carbon ion RT is performed using HIPLAN at NIRS,21 whereas at GSI it is based on physical dose optimization by VOXELPLAN from DKFZ Heidelberg with biologic plan optimization using the TRiP treatment planning software based on the Local Effect Model (LEM) proposed by Scholz et al.22 Plans consist of two to three isocentric fields using an intensity-modulated beam delivery system based on the raster scanning technique developed at GSI, enabling precise delivery of carbon beams to the target.5,23
CLINICAL RESULTS OF HEAVY ION RADIOTHERAPY
In 1954, ion beam RT was initiated using proton beams at LBL, followed by the use of helium ions in 1957, and neon ions in 1975.24 The treatment outcomes in the 299 patients treated with neon ion doses of 10 Gy or above demonstrated favorable results for salivary gland cancer, paranasal sinus cancer, advanced bone and soft tissue tumors, and locally advanced prostate cancer.24,25 Clinical research at LBL was discontinued in 1992 because of budget constraints and aging of the machine.
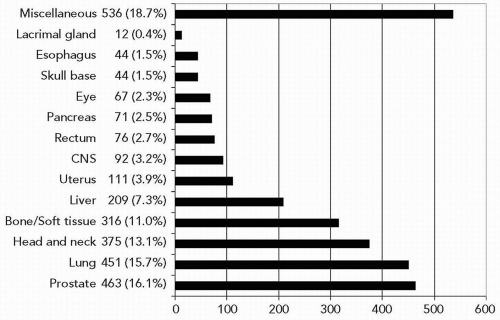
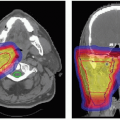
Since 1994, carbon ion RT has been performed at three institutions including NIRS, GSI, and Hyogo. In the initial phase, clinical studies were focused on an attempt to identify tumor sites suitable for this therapy and to determine the optimal dose-fractionation and irradiation methods.17,23,26 Currently, we can say that carbon ion RT can be used for treatment of certain types of tumors, including head and neck cancer, skull base tumors, lung cancer, liver cancer, prostate cancer, bone/soft tissue sarcoma, pelvic malignancies, and selected others (see Fig. 10.3). Of note, there have been no randomized controlled clinical trials to date comparing carbon ion with photon or proton RT. A brief overview of the results with heavy charged particle RT is provided in the following text. Additional information is also provided in greater detail in the chapters on the different anatomic areas.
Head and Neck Tumors
In proton therapy, locally advanced head and neck tumors involving the oropharynx and paranasal sinus have been treated with combined proton/photon irradiation or hyperfractionated, accelerated proton/photon RT.27,28 Improved local control has been obtained but distant metastasis was the predominant pattern of failure for locally advanced paranasal sinus cancer. The role of proton RT as compared to modern photon intensity-modulated radiation therapy (IMRT) in the treatment of locally advanced head and neck tumors remains undefined.
At NIRS, carbon ion RT was first applied to patients with advanced or recurrent head and neck tumors in 1994.17,29 In prospective dose escalation trials, carbon ion RT has been found to offer radiobiologic advantages in non-squamous cell tumors such as adenocarcinomas, adenoid cystic carcinomas, and malignant melanomas. With regard to the long-term survival of malignant melanoma, however, prevention of distant metastasis is needed, and a combined regimen of carbon ion RT and chemotherapy is being tried with some initial encouraging results. Preservation of visual acuity is of paramount importance and a clear relation has been found to exist between visual acuity and radiation dose.30
At GSI, patients with paranasal sinus cancers are treated with combined stereotactic photon RT to the clinical target volume (CTV) and a carbon ion boost to the gross
tumor volume (GTV). Locoregional control rates for the combined photon and carbon ion RT were better than those observed in historical series of patients treated with photon IMRT alone. The difference was, however, not statistically significant at the time of analysis.31
tumor volume (GTV). Locoregional control rates for the combined photon and carbon ion RT were better than those observed in historical series of patients treated with photon IMRT alone. The difference was, however, not statistically significant at the time of analysis.31
Skull Base Tumors
Various histologic tumor types can involve the skull base including chordomas, chondrosarcomas, meningiomas, and neurofibromas. In most cases, complete resection is difficult because of the proximity of critical normal structures and relative resistance to conventional radiation. While improvements have been achieved with proton therapy, the variance of local control rates among the different proton centers is significant, possibly due to patient selection and differences in the treatment techniques used.32, 33, 34 It has also been noted that, in the case of chordoma, there are late recurrences even 5 years after proton therapy.35 Carbon ion RT holds a promising potential for improving these poor long-term results because of increased biological effect and sharp lateral falloff.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree