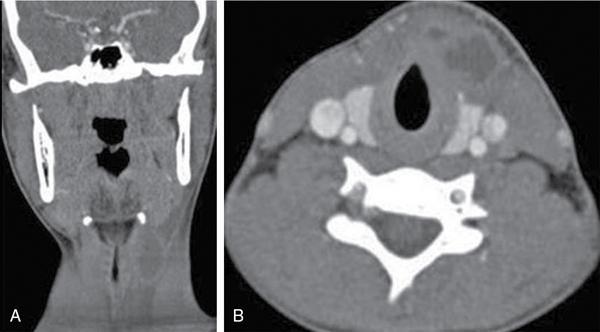
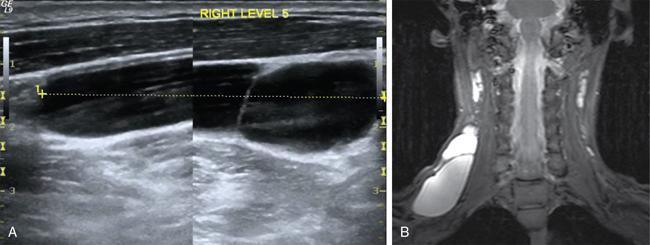
Silpa Kadiyala, UmamahaswaraReddy PAEDIATRIC CYSTIC NECK MASSES Characterization of paediatric neck masses is a frequent challenge faced by a radiologist. Neck masses may be congenital, acquired, inflammatory, infectious or neoplastic. Differential diagnosis to cystic neck masses in paediatric age group include the following lesions mentioned in Table 3.7.1.1. The origin can be from salivary glands, muscles, bones or thyroid. A meticulous approach includes clinical history, accurate physical examination, laboratory investigations and a multimodality imaging approach, which often leads to the accurate diagnosis. Lymphoepithelial cyst Chronic retention cyst Cervical lymph nodal abscess Submandibular gland cyst Cystic lymphangioma Necrotic malignant tumour Teratoma, vascular malformation Pharyngeal cleft cyst Thyroglossal duct cyst Dermoid cyst, ranula Thyroglossal duct cyst Brachial cyst, cystic lymph node Ranula, lymphatic malformation Thyroglossal cyst 2nd branchial cleft cyst Lymphatic and venous AVM Pyogenic granuloma Fibrosarcoma, haemangioma Langerhans cell histiocytosis Reactive lymph nodes Neurofibroma, lymphoma Ultrasound helps to differentiate solid neck masses from cystic lesions in most cases. CT is used to assess deep relations of the lesions, airway narrowing and vascularity of the lesion. In acute scenarios, CT is the preferred imaging modality. Despite its uses, CT is cautiously recommended, as it carries a disadvantage of radiation in the paediatric population. Good soft-tissue delineation provided by MRI is extremely useful for few conditions. However, because of longer times of image acquisition and the requirement for sedation, its use is limited. Rarely, sinogram (conventional or CT sinogram) may be essential to diagnose branchial fistulas. Scintigraphy or PET-CT may aid in diagnosis in certain circumstances. Image-guided biopsies and fine-needle aspiration play a pivotal role in establishing a final diagnosis. Based on location, paediatric neck masses are broadly classified into midline or lateral masses. Thyroglossal cyst, dermoid cyst and ranula are midline lesions. Lymph nodes, fibromatosis coli, branchial cleft cyst, lymphangioma, vascular malformations are lateral lesions (Fig. 3.7.1.1). Determining whether a lesion is solid or cystic is an important step in characterizing paediatric neck mass. Sonography easily differentiates clear cysts from solid masses. However, sometimes cysts contain echogenic debris and pose a diagnostic challenge. In such cases, eliciting mobile thick internal echoes or swirl sign can help differentiate them from solid lesions. Midline cysts such as thyroglossal cysts can be differentiated from solid lesions by eliciting movement with swallowing or with tongue protrusion. A multispatial, thin-walled cystic lesion, especially in the posterior triangle, is normally a lymphangioma. If the lesion is identified as solid, one must exclude whether it is a lymph nodal enlargement. Lymph nodal enlargement has many aetiologies, most common being reactive lymph nodes in which fatty hilum is maintained. Next most common is the tuberculous lymphadenitis in which necrosis, matting, liquefaction and surrounding inflammation are the ominous signs. Moderate homogeneously enhancing lymph nodes in CT/MRI are seen in lymphoma, and intensely enhancing lymph nodes are seen in Castleman’s disease. Nodal rim enhancement is seen in Kikuchi’s disease. If the solid lesion is confirmed to be nonlymph nodal, the next important step is to identify the origin of the lesion (whether from salivary glands, thyroid gland, sternocleidomastoid muscle or skin). Rarely, even after judicious approach, histopathological evaluation can only diagnose solid lesions. Branchial arches, clefts, pouches and membranes develop from the branchial apparatus. This process takes place in the third to seventh week of gestation. The buccopharyngeal membrane degenerates into mesodermal branchial bars, which undergo segmentation to form six pairs of branchial arches. Fifth branchial arch is rudimentary and disappears. The migration of neural crest cells stimulates growth and development of the branchial arches. Every arch has three parts: (1) outer epithelial lining of ectoderm separated by five clefts, (2) inner epithelial lining of endoderm with five corresponding pouches, (3) central cartilaginous core which is mesenchymal origin. The central cartilaginous core forms skeletal, muscular, ligamentous, vascular and neural components. The first and second arches undergo mesodermal proliferation, creating an epicardial ridge shortly after formation of branchial arches. Epicardial ridge forms the mesodermal precursor of the sternocleidomastoid, trapezius, infrahyoid and lingual muscles, along with the hypoglossal (CN XII) and spinal accessory nerves (CN XI). Proliferation of mesenchyme outgrowths of branchial arches II, III and IV and narrows branchial clefts II, III and IV, thus leading to the formation of an ectodermal pit. The cervical sinus of His obliterates with further development. Failure of obliteration results in the formation of branchial sinus, clefts or cysts of types II, III and IV (Tables 3.7.1.2 and 3.7.1.3). Trigeminal nerve (CN V) – maxillary and mandibular branches only Muscles of mastication, mylohyoid, anterior belly of digastric, tensor Tympani and velopalatine Malleus, incus Anterior ligament of malleus, sphenomandibular ligament Maxillary artery Facial nerve (CNVII) Muscles of facial expression, stapedius, stylohyoid, posterior belly of digastric Stapes, styloid process, lesser cornu and upper body of hyoid bone Stylohyoid ligament Stapedial artery (rarely) Glossopharyngeal nerve (CNIX) Stylopharyngeus, superior and middle pharyngeal constrictors Greater cornu and lower part of body of the hyoid Contributions to the carotid artery Superior laryngeal branch of vagus nerve (CNX) – fourth arch Recurrent laryngeal branch of vagus nerve – sixth arch Cricothyroid, levator veli palatini, inferior pharyngeal constrictors, intrinsic muscles of larynx, striated muscles of oesophagus Thyroid, cricoid, arytenoid, corniculate, cuneiform cartilages Fourth arch artery contributes to subclavian artery on right side and the aortic arch on left side. Sixth arch artery contributes to form ductus arteriosus and pulmonary artery Thyroglossal duct cyst (TGDC) is the most common congenital neck mass but usually present with infection during later life at around 5 years of age. TGDC is epithelial-lined cystic lesion formed from persistent thyroglossal duct remnant. Thyroglossal duct is a tubular structure which forms a connection between initial location of primitive thyroid at foramen cecum (tongue base) and terminal location at thyroid bed. TGDC is most commonly located at subhyoid region but can occur at any location of thyroglossal duct from foramen cecum to thyroid duct. TGDC is a midline cystic lesion which moves cranially with protrusion of tongue. On USG, TGDC appears as a well-defined round unilocular anechoic cystic lesion with posterior enhancement. Occasionally, cysts may have echogenic debris because of protein content secreted from epithelial lining, which may give pseudosolid appearance with posterior enhancement (Fig. 3.7.1.6). Presence of mural nodule within the cystic lesion should raise a suspicion of malignant papillary transformation of TGDC. Rarely, sublingual thyroid or ectopic thyroid at any location may be misdiagnosed as TGDC. So before performing surgery, it is essential to ensure the presence of a normal thyroid gland on ultrasound. CT shows fluid attenuation lesion in the expected location (Fig. 3.7.1.7A); extension into preepiglottic space should be mentioned in the report if present. Cysts can deviate to right or left side of midline and track under the strap muscles. Infection can occur and seen as thick-walled appearing cyst with surrounding oedema (Fig. 3.7.1.8). On MRI, it appears as a well-defined cystic lesion with thin-wall embedded in the strap muscles, which is hypointense on T1 and hyperintense on T2. Cyst when infected may show peripheral wall enhancement with internal septations. Treatment of noninfected cyst requires complete surgical excision (Sistrunk method) with removal of central portion of hyoid bone. These are localized congenital malformation of the lymphatic system involving the dilated lymphatic spaces. They commonly occur in the head, neck and axilla but can occur anywhere in the body. Based on the size of the cyst, they can be classified into microcystic, macrocystic and mixed lesions. They are subdivided into cystic hygroma, capillary lymphangioma, cavernous lymphangioma and lymphangiohaemangioma. Most lymphangiomas present at birth or within first 2 years of life. They most commonly occur in the posterior triangle and may have mediastinal extension. They are usually asymptomatic but when large can cause difficulty in respiration because of airway compression. The mortality rate and morbidity are low. This is the most common form of lymphatic malformation in cystic lymphangioma which is macrocystic. It may be associated with abnormalities such as Turner’s syndrome. They are formed because of the failure of juguloaxillary lymphatic sac to drain into the internal jugular vein. There will be progressive dilatation of the lymphatics secondary to accumulation of fluid, which results in cystic hygroma. This leads to the dilatation of lymphatic channels draining the extremities and chest, resulting in hydrops and peripheral lymphoedema. The cystic hygroma may reduce in size if there is reestablishment of lymphatic drainage and results in typical webbing of neck in Turner’s syndrome. This occurs in approximately 1 in 12,000 births and has a predilection towards left side of the neck, probably because of the subclavian vein on the left side. Endothelial cells producing lymphatic fluid line the cysts. Because of the gradual enlargement of the cysts, there would be compression and stretching of adjacent structures. They are usually painless, but when infected may show features such as tenderness or erythema. In the presence of infection, the cysts rapidly grow, and haemorrhage may occur into the cyst. Few lymphangiomas on the floor of the mouth and tongue have bleeding tendency and may enlarge eventually compressing the airways and causing respiratory difficulty. Skeletal distortion and overgrowth may be seen. Some lesions may appear solid or spongy on palpation and can manifest as diffuse infiltration and overgrowth of the region affected. Gorham–Stout syndrome in progressive osteolysis is due to diffuse soft-tissue and skeletal proliferation of lymphatic vessels. Associated Malformation: Foetal lymphangiomas are usually associated with foetal hydrops or demise. Approximately 73% are associated with Turner’s syndrome (45X0). Nuchal subcutaneous fluid accumulation can be visualized sonographically at around 10–14 weeks’ gestation and appears as increased hypoechoic nuchal thickness. Other syndromes associated with lymph nodes are Noonan’s syndrome, trisomy 21, trisomy 13, trisomy 18 and lethal multiple pterygium syndrome. There has been an association between periorbital lymphangioma and intracranial developmental venous anomalies and dural AVFs. Previously occult lymphatic malformation may present in childhood or adulthood, usually with a sudden increase in size because of haemorrhage or infection. Lymphangioma of head and neck presents as a mass or alteration of function due to mass effect on adjacent vital structures. The signs and symptoms vary with location of the lesion. When these lesions extend into the thoracic inlet, it may cause stridor, cyanosis or apnoea because of a mass effect on trachea and dysphagia when compressing the oesophagus. Extension into the mediastinum causes displacement and mass effect on airway which can be seen on a plain radiograph of the neck and chest. When complicated by haemorrhage, the fluid becomes echogenic and results in fluid levels. When the haemorrhagic fluid is spread uniformly, it may enhance postcontrast administration but the cystic fluid does not. Lymphangiomas are typically avascular masses causing destruction/distortion of adjacent vessels. Dilated or anomalous veins may be present. A pattern of early filling of multiple tiny veins and contrast puddling in venous channels has been seen in lymphatic venous malformation of tongue. The main DDs are teratoma and vascular malformations. Fluid–fluid levels, cystic fluid enhancement and absence of phlebolith help in differentiating lymphangiomas from vascular malformations. Unilocular lymphangioma has pharyngeal cleft cyst, TGDC, dermoid cyst and ranula as differential diagnosis. Sometimes a multilocular lymphangioma can mimic multiloculated abscess or granulomatous disorder such as nontuberculous mycobacterial infection. The treatment includes antibiotics for infection and surgical resection. Cervicofacial lymphangiomas need staged surgical excision. Aspiration of fluid only provides temporary relief. Intralesional sclerosant injection is usually more effective for macro cystic lymphangiomas. Ranula is most commonly acquired inflammatory mucous retention cyst with block or obstruction of ducts of sublingual salivary gland. Simple ranula is well-defined thin-walled unilocular lenticular-shaped hypoechoic lesion on ultrasound, which is hypodense on CT with no/subtle wall enhancement and is hypointense on T1 and hyperintense on T2 limited to sublingual space. If infected, it may have thick enhancing wall with altered T1 and T2 signals (Fig. 3.7.1.10). Dermoid and epidermoid are formed between 3 and 5 weeks of gestation age because of failure of segregation of surface ectoderm and neural tube and resulting in epithelium-lined epidermal inclusion cysts. The wall of dermoid cyst contains appendages of skin such as sweat and sebaceous glands, which produce oily substance into cyst cavity, giving it characteristic imaging appearance. Dermoids are midline in location and epidermoids are located at floor of mouth in SLS, SMS and at root of tongue. On imaging, dermoid is a well-defined cystic lesion with fluid, fat and mixed contents, whereas epidermoid is with fluid contents (Figs 3.7.1.11 and 3.7.1.12). Fibromatosis colli is a rare benign fibrous mass most commonly in lower third of sternomastoid muscle (SCM), resulting in congenital torticollis usually presented before 6 months of age. Some theories presume that the primary cause of this pseudotumor is because of presence of mature fibrous tissue in neonate suggesting that this condition is arising before birth, but some authorities claim that the main aetiology is birth trauma. Pathologically fibrous tissue is located in the middle of muscle fibres, leading to torticollis (face and chin turn to opposite direction, head tilted towards ipsilateral shoulder) because of fibrosis and shortening of SCM. On USG, normal SCM is hypoechoic with multiple thin linear echogenic lines dispersed in between (Fig. 3.7.1.13). In fibromatosis coli, there is increased echogenicity with fusiform enlargement of muscle. The USG picture varies with pathologic tissue within the lesion and is classified by Hsu et al. into four types (fibrotic mass, diffuse fibrosis intermixed with normal muscle, diffuse muscle involvement with no normal tissue, fibrotic cord in involved muscle). On CDFI, there is hyperaemia in acute phase and decreased flow in fibrotic phase. On CT, there is a fusiform enlargement of SCM muscle with no calcifications and lymphadenopathy. On MRI, fibrosis in SCM gives variable signal intensity, isointense to hypointense on T1, hypointensity on T2 and heterogenous enhancement on T1+C. Differentials include lymphadenopathy, cervical neuroblastoma, pseudomass from opposite SCM denervation. In most cases, torticollis responds to postural changes and physiotherapy. It is a rare cystic remnant of embryonic third pharyngeal pouch (tubular thymopharyngeal duct). They present as asymptomatic pain less mass in first and second decades, most commonly arising on the left side of neck with direct extension into anterior mediastinum in around 50%–60% cases. Embryologically, both thymus and thyroid gland develop from the outgrowth of the anterior aspect of third pharyngeal pouch. Failure of descent of thymus into mediastinum results in cervical thymic tissue. According to most of the theories, thymic cyst develops from the cystic degeneration of Hassall’s corpuscles. Thymic cysts are located along the thymopharyngeal duct from the angle of mandible to mediastinum adjacent to carotid sheath and parallel to sternocleidomastoid. Sometimes, thymic cyst results in vocal cord paralysis because of compression of recurrent laryngeal nerve. Sometimes, there are shortness of breath, hoarseness and pain because of extrinsic compression of surrounding viscera. Thymic cysts are usually unilocular thin-walled cystic lesions with clear fluid and can be multiloculated thick wall cyst with turbid contents. On USG, thymic cysts are well-defined anechoic uni- or multiloculated cystic lesion rarely with partial wall calcifications. On CT, thymic cysts are soft-tissue density lesions with 0–10 HU and can be more based on the contents of the cysts. On contrast-enhanced CT, they are nonenhancing low-attenuating cystic lesions in proximity to carotid sheath and may show direct connection with the mediastinum or with the fibrous cord along migratory tract of thymic tissue. On MRI, hypo on T1, hyper on T2 nonenhancing on T1+C. Cyst wall is thickened and shows enhancement if infected. Differentials include second branchial cleft cyst, dermoid and epidermoid cyst. Imaging is essential to know the mediastinal extension before surgical excision of the cyst. Vallecular cyst (VC) is a congenital cyst arising from the lingual surface of epiglottis due to predisposing obstruction of submucosal glands/cystic distention of laryngeal saccule. VC lies anterior to epiglottis, displacing it posteriorly. Diagnosis is by direct visualization of cyst by laryngoscopy in a child presenting with stridor and airway obstruction. On USG, VC is a well-defined, lobulated anechoic cystic lesion. CT reveals well-circumscribed soft-tissue density lesion with no enhancement on contrast. MRI shows cystic mass, which is hypo on T1 and hyper on T2. In imaging, it is very difficult to differentiate VC from TGDC, retention cyst of pharyngeal mucosal space and lymphatic malformation. Treatment of VC is by surgical excision, laser-assisted resection, marsupialization and radiofrequency ablation. Untreated VC may lead to life-threatening airway obstruction. Cervical lymphadenopathy is one of the common causes of paediatric neck mass. Normal lymph nodes are visible on ultrasound in children with short-axis diameter <10 mm but <15 mm for jugulodigastric nodes seen below angle of mandible. A normal lymph node is visualized as a well-defined reniform-shaped lymph node, which is having hypoechoic cortex and hyperechoic fatty hilum showing hilar vascularity with low PI and RI on ultrasound and CDFI. In case of viral, fungal and bacterial (streptococcus, staphylococcal being most common) infections in children of 1- to 5-year age group show lymph node enlargement with increased echogenicity of cortex and periadenitis which may further progress into central hypoechoic/anechoic necrotic area described as lymph nodal abscess formation with peripheral colour demonstration on CDFI. Another important cause for reactive cervical lymphadenopathy is mycobacterial disease, which accounts for 10% of extrapulmonary TB; it causes enlargement of lymph nodes with few of them involved in conglomeration, resulting in matting with no periadenitis. TB, like other bacterial infections, can form intranodal abscess in its subacute stage and calcifications in chronic stage of disease. Though malignancy is rare in children, few conditions such as neuroblastoma, lymphoma, leukaemia, rhabdomyosarcoma (RMS) are primary malignancies which can cause enlarged painless hard, nonmobile lymph nodes which on imaging are seen as round enlarged uniformly hypoechoic lymph node with loss of fatty hilum/eccentrically placed fatty hilum. On CDFI, there is displacement of hilar vasculature because of tumour infiltration and an increase in PI and RI because of compression of intranodal vasculature with infiltrated tumour. On CT, normal lymph nodes are iso- to hypodense compared with muscle. Intranodal abscess is seen as central hypodense area with peripheral rim enhancement and adjacent inflammatory change. On MRI, discrete or conglomerate nodes can be there with variable T1W and T2W appearances (Fig. 3.7.1.14). It has been published that using diffusion-weighted sequences helps to differentiate benign lymph nodes from malignant lymph nodes, but significant overlap still persists; guided FNAC/biopsy is always indicated for definite patient care. Haemangiomas are the most common soft-tissue vascular tumours in paediatric age with greater incidence in females and low-birth-weight premature infants. They are usually present at birth showing brisk growth and proliferation during infancy, which involute, regress and disappear spontaneously in most of the children. In proliferating phase of haemangioma, there are increased levels of growth factors such as FGF and VEGF with increased vascularity. During involution phase, there is an increase in levels of metalloproteinase inhibitors, thus decreasing the size and vascularity of lesion. Most common locations of haemangiomas are head, neck, chest and extremities. There is a strong association of superficial haemangiomas with intracranial and intraspinal haemangiomas. Diagnosis is by a combination of clinical history and imaging. On USG, they appear as large vascular channels with a solid component in proliferating phase of haemangiomas, thus differentiating it from AVM and showing high-velocity arterial and venous flow with absent or diminished flow in involution phase. The pulsatile flow in veins depicts A-V shunting, which is draining the lesion. On CT, isodense lobulated solid-appearing mass which has intense rapid early homogeneous enhancement after contrast administration, thus differentiating from venous malformation and enhancing solid mass differentiates from AVM. On MRI, enlargement of vessels appears as flow voids in the lesion. Isointense on T1 and hyperintense on T2 show diffuse enhancement on contrast administration. Fibro fatty replacement in involution phase gives increased SI T1. Haemangiomas do not cause any skeletal distortion and remodelling. Association of haemangiomas with certain syndromes such as PHACES necessitate MRI brain in the imaging protocol. Differentials include lymphatic and venous malformations, AVM and pyogenic granuloma. Treatment: Most haemangiomas involute in later stage of life and thus do not require initial treatment. Large haemangiomas causing airway obstruction/diminished vision require antiangiogenic treatment, corticosteroids oral (or) intralesional administration. Parotid infantile haemangiomas are most common parotid tumours of childhood which have characteristically benign course with male to female ratio of 1:3. Clinically present as an enlarging parotid mass in an infant sometimes may be associated with a cutaneous component (e.g. Strawberry lesion). On USG, it appears has homogeneous echogenic lobulated parotid mass with internal septations. On MRI, it shows T1 intermediate and T2 hyperintense with prominent flow voids. IMAGING OF PAEDIATRIC AIRWAYS Abanti Das, Ashu Seith Bhalla, Manisha Jana The imaging evaluation of airways in infants and children is inherently challenging, due to the technical difficulties of imaging paediatric patients. The small size of airways, coupled with difficulty in eliciting compliance to breath-holding manoeuvres, makes their examination particularly difficult. Quite often, children tend to breathe rapidly during the examination, probably induced by anxiety, which along with low lung volumes during tidal breathing can have significant effect on diagnostic accuracy of the study, especially high resolution CT (HRCT). Nevertheless, imaging evaluation of the airway has long been used as a screening tool, as well as a complementary examination to endoscopic evaluation. It provides non-invasive information about the structure as well as dynamic functioning of airway. Advent of newer imaging modalities and cutting edge technologies has revolutionized paediatric airway imaging, offering near-endoscopic detail of airway anatomy and its dynamic functioning. The common imaging modalities used for evaluation of paediatric airway disorders include radiography, airway fluoroscopy, upper GI contrast study/functional swallow study, ultrasound, CT (main workhorse) and MRI (emerging role). Plain radiograph forms an essential part of an imaging algorithm for evaluation of airway lesions, and are often the first investigation to be performed. Care should be taken to allay patient anxiety during image acquisition, as excessive crying can precipitate respiratory distress in patients with partial obstruction. The attending physician should accompany such children to the radiology department, ideally with preparedness for emergency intubation, should such a need arise. Radiographic views and technique: Erect soft-tissue lateral and frontal radiographs of neck are the basic projections to be acquired in a child with acute upper airway obstruction. In case of unstable patients, a single erect soft-tissue lateral radiograph of neck is usually sufficient for diagnosis. Lateral view radiographs are the most crucial and hence, utmost care should be taken for proper positioning including upright patient position and slight extension or neutral head position. The latter is particularly important to avoid ‘pseudo-thickening of retropharyngeal tissue’-normal variant as discussed later in this section. Evaluation of lower airway requires an erect frontal chest radiograph acquired during inspiration. Additional erect soft tissue lateral radiograph of neck should also be done. Paired inspiratory-expiratory radiographs in a co-operative child or decubitus chest radiographs to assess for air-trapping were obtained when foreign body aspiration was a concern, before the wide-spread use of CT. The high kilovoltage, filtered beam technique with magnification is proposed to be better than conventional radiography as it offers better contrast resolution and anatomical detail of airways while limiting radiation dose. Because plain radiographs often form the baseline investigation in evaluation of airway disorders, a sound knowledge of normal anatomical appearance and air flow dynamics during different phases of respiration is essential to interpret the abnormalities present. Normal anatomy (Fig. 3.7.2.1): The relevant anatomy/key landmarks of upper airway on lateral radiograph are summarized in Table 3.7.2.1. On a lateral radiograph of upper airway, nasopharynx, oropharynx and hypopharnx/larynx are seen as air-filled lucent structures with uvula separating the former two spaces. The false cord, laryngeal ventricle and true cords are positioned in cranio-caudal sequence followed by the subglottic cervical trachea. A properly acquired lateral soft-tissue neck radiograph on full inspiration (Fig. 3.7.2.1A) shows the hypopharynx distended with air, vertical position of thin linear epiglottis and diagonally oriented aryepiglottic folds. The supraglottic airway, however, collapses on expiration and hence, the anatomy is less discernible. The subglottis is still patent and visible on expiration. On an inspiratory frontal view of neck (Fig. 3.7.2.1B), the vocal cords move away from the midline and glottis appears almost as wide as the trachea. During expiration, when the child is crying, the vocal cords proximate in the midline and their rectangular inferior configuration is visible. The trachea also demonstrates characteristic buckling to right side as mentioned below. Normal variants: Certain normal variants that are commonly encountered in routine paediatric neck radiographs are important to be aware of (Table 3.7.2.2). While some of them are due to the normal anatomical and physiological development of paediatric airway others can result from technical and patient positioning errors. Nevertheless, their recognition is important as they can often lead to false-positive findings and misinterpretation on radiographs. Radiography: Pearls and pitfalls Airway fluoroscopy has been used as an adjunct to flexible laryngobronchoscopy historically for diagnostic evaluation of paediatric stridor. While it is still widely practiced at some institutions, several studies have questioned its diagnostic accuracy as a screening tool for the airway. Advantage and limitations: Fluoroscopy does offer certain advantages over static radiographic films. Firstly, it offers a dynamic evaluation of the airway in addition to providing static images during several time-points of the study. The examination is not very demanding in terms of patient positioning, hence requires less patient co-operation as compared to radiographs. Even a momentary patient position can be captured. It can evaluate movement of hemidiaphragms, its symmetry as well as areas of air-trapping. Both upper and lower airway extending from nasopharynx to main stem bronchi can be evaluated in multiple projections. Spot images and cine loops can be acquired to observe changes over a respiratory cycle enabling differentiation of fixed lesions from functional ones. The sensitivity and negative predictive value of fluoroscopy is as low as 18% and 35%, respectively for determining laryngotracheal pathology in infants. Hence, its role in the diagnostic algorithm of paediatric airway evaluation is controversial. Moreover, the growing concern of exposure to ionizing radiation that entails fluoroscopic examination also merits attention. Examination in infants particularly, who have small-sized airways often require longer exposure times (as compared to routine X-ray series) and higher-frame rate cine images to resolve small airways and achieve desirable, if not, high-resolution images for confident diagnosis. This in turn contributes to higher exposure rates defying the ALARA standards. Application: Airway fluoroscopy has especially been useful in suspected tracheobronchomalacia and aspirated endobronchial foreign body if CT is unavailable. Its role in assessment of obstructive sleep apnoea in children has also been actively investigated and it has been found to be a useful adjunct to endoscopy in evaluation of pediatric upper airway obstruction, when hypopharyngeal collapse or multiple sites of obstruction were suspected. Awake airway fluoroscopy was found to be more advantageous than sleep fluoroscopy (performed for upper airway obstruction in OSA) by obviating the need for sedation in an already compromised airway and allowing better evaluation of glottic lesions while the child is crying or phonating during the examination. The equivocal performance of airway fluoroscopy as a screening modality as reported in recent literature suggests that it may, at best, have some role in a sub-set of patients who have low clinical suspicion of a definitive anatomic lesion, to determine the need for rigid bronchoscopy in them. While the specificity remains high, negative fluoroscopic examinations should be viewed carefully and should always be confirmed with endoscopic examination, if clinical suspicion is high. Airway fluoroscopy: Pearls and pitfalls OSA, Obstructive sleep apnoea. Although not considered a first-line investigation in evaluation of paediatric airway disorders, upper GI contrast studies often play a useful ancillary role in suggesting a particular cause. The basic premise for the same lies in the fact that airway and oesophagus are closely placed anatomically in thorax and their embryologic development is also intimately linked. Hence, a keen observation of the kinetics and mechanism of swallowing may provide important diagnostic clues about the primary airway disorder.
3.7: Paediatric neck
Introduction
First branchial cleft anomalies
Third branchial cleft anomalies (third brachial fistula)
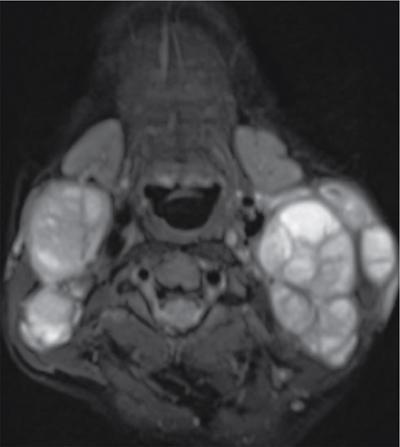
Lymphangiomas
Unilocular lymphangioma
Ranula
Dermoid
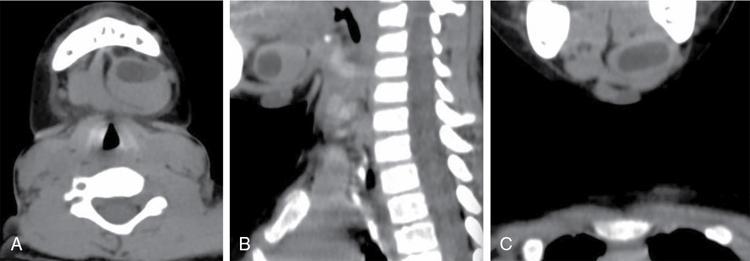
Cervicothymic cyst
Haemangioma
Rhabdomyosarcoma
Cervical neuroblastoma
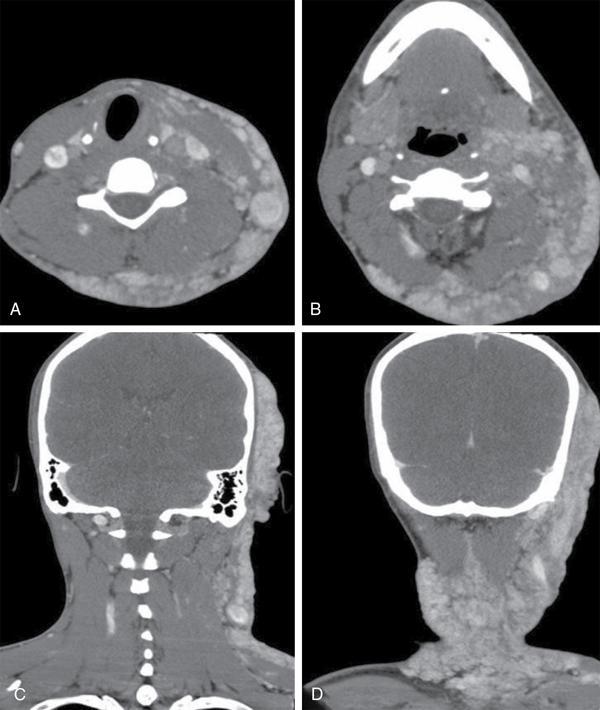
Imaging approach
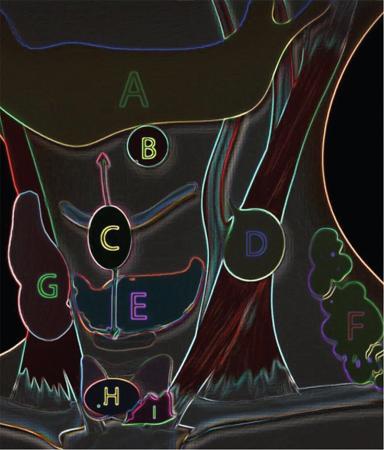
Branchial apparatus
Pouch
Derivative
First
Eustachian tube, middle ear, portions of mastoid bone
Second
Palatine tonsils, tonsillar fossa
Third
Inferior parathyroid, thymus
Fourth and sixth
Superior parathyroid, parafollicular cells of thyroid
Arch
Nerve
Muscles
Skeletal Structures
Ligaments
Artery
First (mandibular)
Second (hyoid)
Third
Fourth and sixth
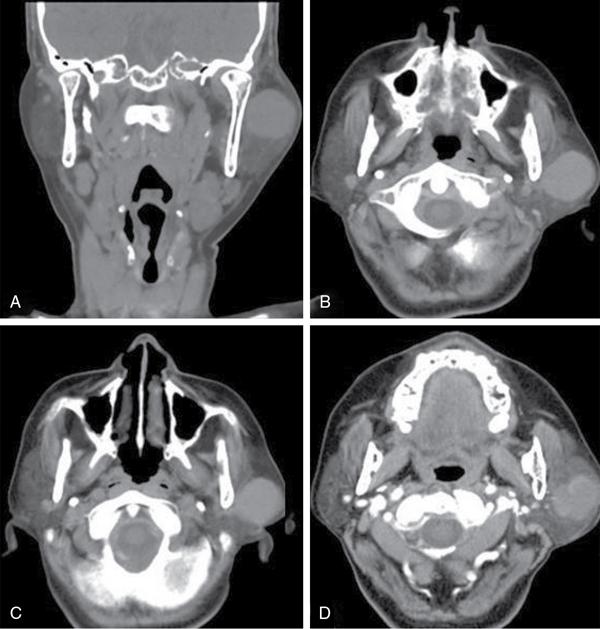
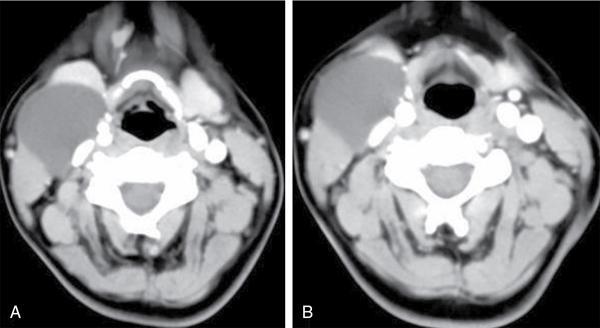
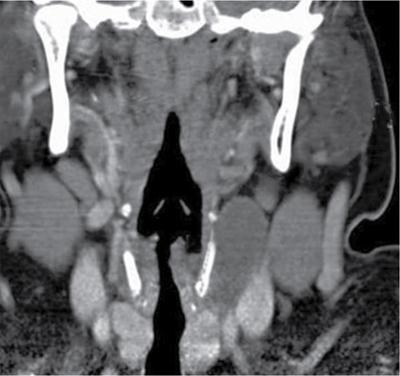
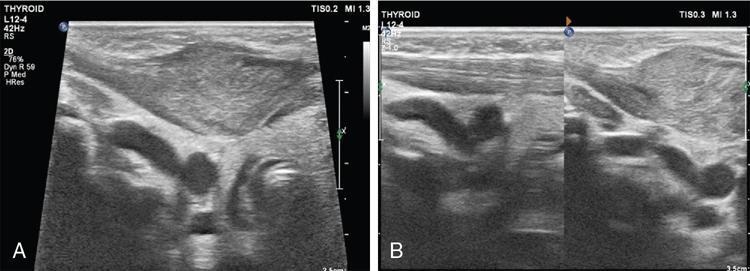
Thyroglossal duct cyst
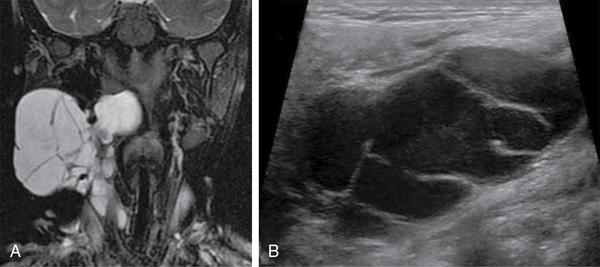
Lymphangioma
Types of lymphangioma
Imaging
Clinical features
Angiography
Differential diagnosis
Treatment
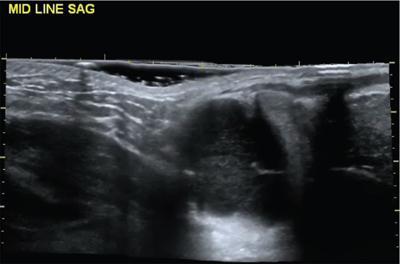
Ranula
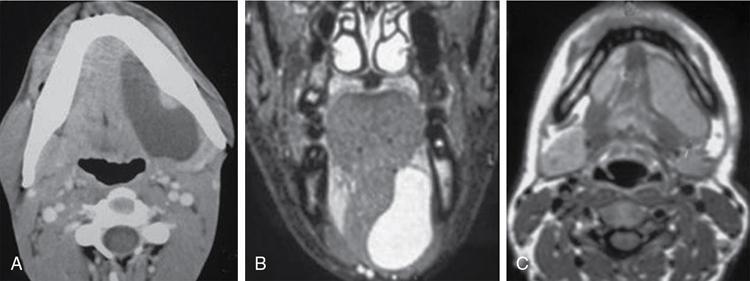
Dermoid and epidermoid
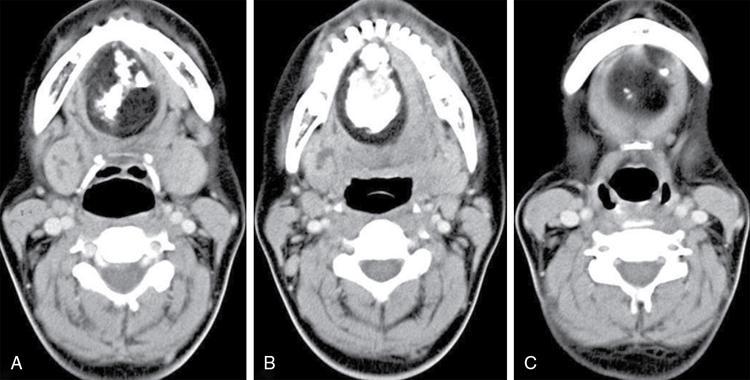
Fibromatosis colli (sternomastoid tumour/pseudotumour of infancy/congenital muscular torticollis)
Thymus/cervico – thymic cyst
Vallecular cyst
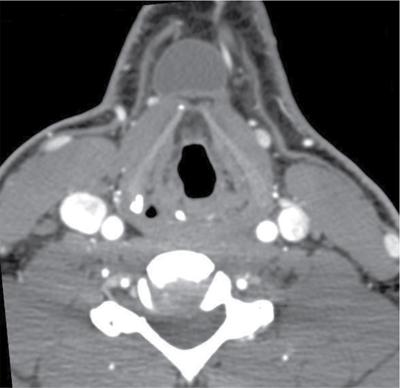
Cervical lymphadenitis
Haemangiomas
Infantile hemangioma of parotid
Low-flow vascular malformations
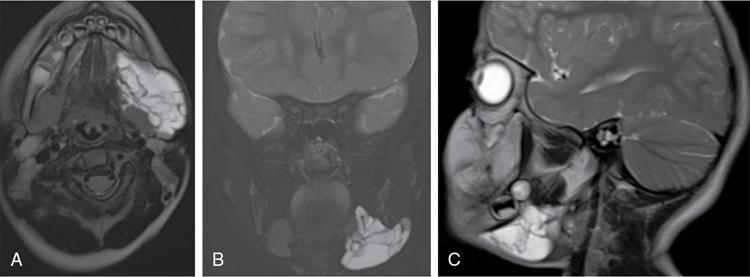
High-flow vascular malformations
Introduction
Radiography
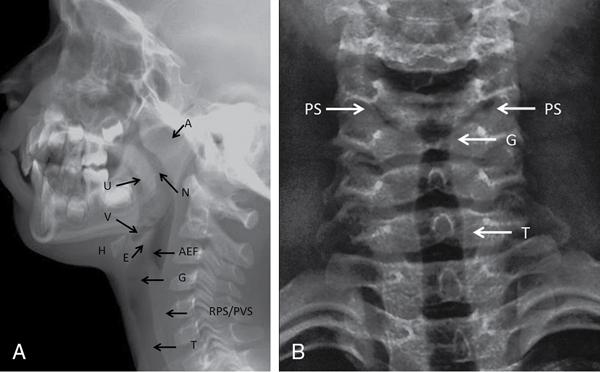
Nasopharynx, oropharynx, larynx/hypopharynx
Air-filled lucent; uvula separates nasopharynx and oropharynx
Vallecula
Depression/space between epiglottis and hyoid bone
Epiglottis
Thin soft tissue density structure extending posteriorly from back of tongue above level of hyoid
Aryepiglottic folds
Thin linear soft tissue densities from lateral aspect of epiglottis antero-inferiorly till arytenoid cartilages
Cervical trachea
Subglottic air-filled tubular lucent structure
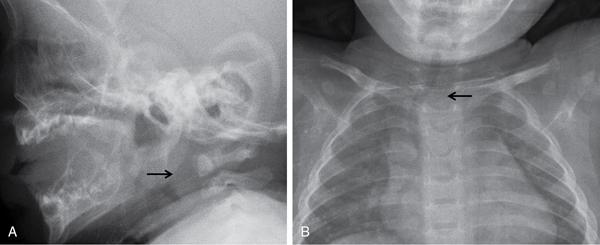
Airway fluoroscopy
Upper gastrointestinal (GI) contrast study/functional swallow study
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree