Chapter Outline
Historical Perspective
The pancreas was one of the last organs in the abdomen to receive the attention of anatomists, physiologists, physicians, and surgeons. Located in the “straggling mesenchyme” of the retroperitoneum, the pancreas has in the past been called the hermit or hidden organ of the abdomen. The pancreas was first described in the Talmud and depicted as the “finger of the liver” between 200 bc and ad 200. Ruphos named this organ the pancreas (Greek: pan, meaning “all”; kreas, meaning “flesh”) shortly thereafter. More than a millennium passed before anatomic descriptions were completed.
Wirsung of Padua demonstrated the pancreatic duct in 1642, and Santorini of Venice described the accessory duct in 1724. The papilla of Vater was described by A. Vater in 1720. In 1887, Oddi described the complex musculature of the sphincter bearing his name. The histologic structure of the pancreas was described by Langerhans in 1869. The role of the pancreas in digestion was first suggested by Bernard in 1850, and the connection between diabetes and the pancreas was established in the 1890s. Surgery for pancreatic disease was not popular until the pioneering work of Whipple in the 1930s.
The pancreas also remained a hidden organ for the radiologist for decades. Indirect signs of pancreatic disease on plain films and barium studies were usually present only in advanced disease. The 1970s brought endoscopic retrograde cholangiopancreatography (ERCP) and angiography to the fore. Computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) now routinely provide superb visualization of the gland noninvasively. Endoscopic ultrasound is a useful adjunct to cross-sectional imaging in evaluating for pancreatic disease and allows pancreatic biopsy when it is indicated.
Normal Anatomy
The pancreas is an unpaired accessory digestive gland that has both exocrine and endocrine functions. It is a slender, soft, lobulated organ that in the adult measures approximately 15 to 25 cm in length, 3 to 5 cm in height, and 1.5 to 3.5 cm in thickness and weighs 70 to 110 g. It has a pale, yellow-tan surface that is finely nodular and firm to palpation.
Topography
The pancreas lies within the anterior pararenal space ( Fig. 94-1 ). The pancreatic head has a constant relationship with the duodenum, with its right lateral border nestled in the duodenal sweep. The head is the thickest portion of the gland; it gives rise to the uncinate process, which projects like a hook, dorsal to the superior mesenteric vein. The pancreatic neck lies immediately anterior to the confluence of the splenic and superior mesenteric veins. The neck narrows behind the pylorus, then widens as it becomes the body. The body arches anteriorly and laterally to cross the spine and may be thinner than the pancreatic head and tail as it does. The body bulges up in the pancreatic tubercle almost to the level of the celiac axis. The tail is not well demarcated from the body as it extends to the splenic hilum.
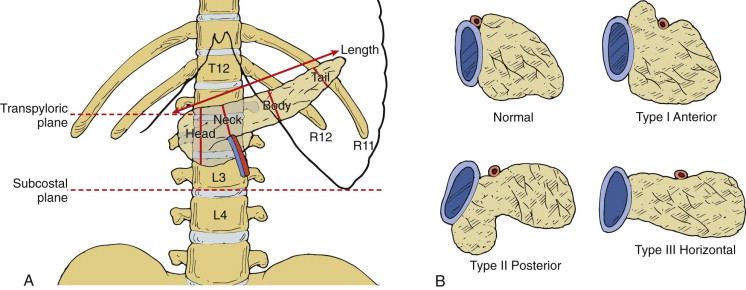
The shape, position, and axis of the pancreas are variable and are influenced by age, body habitus, previous surgery, and organomegaly. The head usually lies at the level of L1-L2, and the body crosses the spine at L1; the tail is located more superiorly in the region of the splenic hilum. The longitudinal axis of the pancreas is about 20 degrees in relationship to the transverse plane ( Fig. 94-2 ). The axis of the pancreas is occasionally transverse, and even less commonly the tail may lie caudal to the head. If the left kidney is congenitally or surgically absent, the tail often lies in a posteromedial position, adjacent to the spine. The pancreas can be shaped like an L, S, or inverted V.
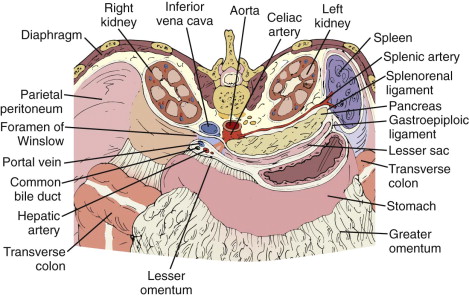
The gastric body and antrum lie anterior to the body and tail of the pancreas, and the pylorus is located ventral to the pancreatic neck. The duodenum lies along the right lateral border of the pancreatic head; it also passes inferior to the head, body, and tail. The spleen lies along the lateral and superior aspect of the pancreatic tail. The right kidney and adrenal gland are located posterior to the pancreatic head, and the left kidney and adrenal gland are dorsal and occasionally caudal to the tail. Depending on its size, the left lobe of the liver may lie anterior to the pancreatic body. The gallbladder is positioned ventral to the pancreatic head. The transverse colon lies anterior and generally inferior to the pancreas. The small bowel usually lies inferior to the level of the pancreas but occasionally can lie ventral to the tail.
Nearly all of the pancreas is retroperitoneal; a nonperitonealized bare area results from the reflection of the posterior parietal peritoneum to form the two leaves of the transverse mesocolon and the posterior inferior margin of the lesser sac ( Fig. 94-3 ). The transverse mesocolon originates where the hepatic flexure of the colon crosses ventral to the second portion of the duodenum. The bare area begins as a broad strip across the infra-ampullary portion of the descending duodenum and continues across the head, body, and tail of the pancreas. The pancreatic tail, after extending across the left kidney, is actually an intraperitoneal structure incorporated within the leaves of the splenorenal ligament. The root of the small bowel mesentery originates inferior to the pancreatic body and is contiguous with the transverse mesocolon. Therefore, pancreatic processes may affect the stomach and duodenum by direct spread and the small bowel loops and colon through the small bowel mesentery and transverse mesocolon (see Fig. 94-3 ).

Common Bile Duct
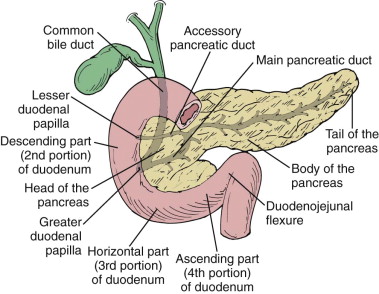
The common bile duct enters the head of the pancreas after it passes posterior to the first part of the duodenum in the hepatoduodenal ligament. It passes inferiorly and dorsally, embedded in the posterior surface of the pancreatic head, to join the pancreatic duct of Wirsung. This segment runs a short intramural course before it enters the posteromedial aspect of the duodenum through the major papilla of Vater ( Fig. 94-4 ). The common bile duct is 7 cm long and has an average diameter of 7.4 mm.
Pancreatic Duct
The pancreatic duct arises from the pancreatic tail and receives 20 to 35 short tributaries entering at right angles to its long axis as it courses toward the head. The duct lies midway between the superior and inferior margins of the pancreas and slightly more dorsally than ventrally. At the level of the major papilla, the main pancreatic duct (duct of Wirsung) courses horizontally to join the caudal surface of the common bile duct, forming the ampulla of Vater. The accessory pancreatic duct of Santorini drains the anterior and superior portion of the head of the pancreas either into the duodenum at the minor papilla or into the main pancreatic duct (see Fig. 94-4 ). The minor papilla is often not patent, and the accessory duct may be partially or completely obliterated or have an anomalous connection with the duct of Wirsung. These anatomic variants in ductal anatomy are discussed more fully in Chapter 96 .
Arterial Supply
Although the pancreatic parenchyma is well visualized on cross-sectional imaging, it is important to recognize and to define the major pancreatic vascular landmarks ( Fig. 94-5 ). The arterial blood supply of the pancreas arises from the celiac trunk and the superior mesenteric artery. After originating from the celiac artery, the common hepatic artery courses to the right in proximity to the neck and subsequently the head of the pancreas. At this point, it divides into the proper hepatic artery, which enters the free edge of the hepatoduodenal ligament, and the gastroduodenal artery, which courses caudally to lie ventral and lateral to the pancreatic head. The gastroduodenal artery gives rise to the anterior and posterior superior pancreaticoduodenal arteries, which supply the head of the pancreas. These vessels help form the pancreatic arcade when they join the anterior and posterior inferior mesenteric arteries that arise separately or as a common trunk from the proximal portion of the superior mesenteric artery.

The splenic artery arises from the celiac artery and loops like a snake above and below the superior margin of the pancreas. It becomes more tortuous with age and occasionally becomes embedded within the pancreatic parenchyma. The pancreatic body and tail are supplied by the dorsal pancreatic artery, which arises from the splenic artery or as a fourth branch of the celiac trunk, as well as by the splenic, hepatic, or superior mesenteric arteries. The pancreatica magna is the largest of the series of superior pancreatic branches of the splenic artery.
The superior mesenteric artery arises from the anterior surface of the aorta, 1 to 2 cm below the celiac trunk. It courses caudal and dorsal to the neck of the pancreas, passing anterior to the uncinate process, where it serves as a major landmark for cross-sectional imaging. Displacement of the artery to the right of the aorta is a normal variant.
The most frequent arterial anomaly of the upper abdomen is partial or complete replacement of either the right hepatic or the proper hepatic artery to the superior mesenteric artery. In these patients, the replaced hepatic artery passes cephalad and ventral to the pancreas, then anterior or posterior to the portal vein to reach the liver.
Venous Drainage
The venous drainage of the pancreas is constant; the portal system serves as an essential landmark for localizing the pancreas on cross-sectional imaging. In general, the veins of the pancreas parallel the arteries and lie inferior to them ( Fig. 94-6 ). Four pancreaticoduodenal veins form venous arcades that drain the pancreatic head and the duodenum. The inferior pancreaticoduodenal veins drain into the first jejunal branch of the superior mesenteric vein. The inferior pancreaticoduodenal veins are smaller than their superior counterparts; they are not commonly visualized on cross-sectional imaging. The posterior superior pancreaticoduodenal vein extends cephalad to join the caudal aspect of the portal vein directly. The anterior superior pancreaticoduodenal vein runs horizontally to drain into either the gastrocolic trunk or the right gastroepiploic vein, both of which extend to the superior mesenteric vein. Three to 13 small veins from the body and tail empty directly into the splenic vein, which follows a smooth arching course from the spleen to its junction with the superior mesenteric vein. The splenic vein parallels the splenic artery and lies in a groove along the dorsal and superior margin of the pancreas. It is a superb landmark for the posterior aspect of the pancreas, and long segments of this vessel can be seen on CT and ultrasound studies on a single image. Rarely, the distal tip of the pancreatic tail can lie posterior to the vein, adjacent to the adrenal gland. In these cases, it may be difficult to differentiate a mass arising in the pancreatic tail from those originating in the left adrenal gland.

The splenic vein joins the superior mesenteric vein posterior to the neck of the pancreas and is seen as a round or oval dilation to the right of the midline, posterior to the neck of the pancreas. The superior mesenteric vein courses anterior to the uncinate process just to the right of the superior mesenteric artery (see Fig. 94-6 ).
The portal vein is formed behind the pancreatic neck by the union of the splenic and superior mesenteric veins and continues superiorly and laterally toward the porta hepatis as the main extrahepatic portal vein, dorsal to the common bile duct and hepatic artery. In one third of the population, the inferior mesenteric vein enters at the confluence; in another third, it joins the splenic vein close to the junction; and in the remainder, it joins the superior mesenteric vein.
Lymphatics
The lymph nodes of the pancreas are distributed along the major vascular pathways ( Fig. 94-7 ). The lymphatic channels of the pancreas form a richly branched plexus that empties in multiple directions. The extensive network of lymphatic vessels and lymph nodes draining the pancreas provides egress to tumor cells arising from the pancreas and contributes to the fact that pancreatic cancer often presents with positive lymph nodes and a high incidence of local recurrence after resection. The anatomy of the lymphatics suggests that partial removal of the pancreas for cancer may not be sufficient because of the direct connections between different lymphatic chains.

The suprapancreatic and infrapancreatic lymphatic chains receive branches from the neck, body, and portions of the pancreatic tail. Branches from the posterior surface of the head and neck drain into the pancreaticoduodenal and juxta-aortic nodes. The posterior surface of the pancreatic body drains into the suprapancreatic and infrapancreatic nodes. The pancreatic tail drains into the nodes of the splenic hilum and gastropancreatic fold. Some drainage from the pancreatic head and proximal body enters the nodes in the porta hepatis and may extend inferiorly toward the superior mesenteric, mesocolic, and para-aortic nodal chains. The suprapancreatic nodes are closely related to the splenic artery and vein; the infrapancreatic chain is adjacent to the leaves of the transverse mesocolon.
Nerve Supply
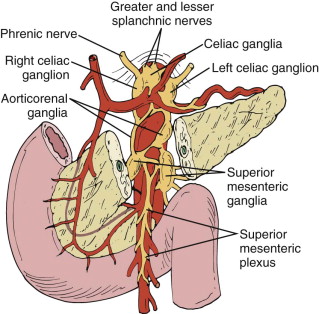
It is important to appreciate the nerve supply of the pancreas ( Fig. 94-8 ) in planning celiac nerve blocks for control of pain resulting from pancreatic carcinoma or chronic pancreatitis. The pancreas receives sympathetic innervation by way of the splanchnic nerves and parasympathetic innervation from the vagus nerve. The sympathetic nerves carry the pain (visceral afferent) fibers. They pierce the diaphragmatic crura to enter the celiac plexus and celiac ganglion that surround the celiac artery. The superior mesenteric ganglia and plexus surround the superior mesenteric artery. Chemical extirpation of the celiac ganglion interrupts afferent pain fibers from both the sympathetic and the parasympathetic systems and can be accomplished by injection of the chemical agent between the celiac artery and the superior mesenteric artery either antecrurally or retrocrurally.
Radiologic Techniques
Plain Radiographs
In patients with suspected pancreatic disease, plain radiographs are obtained chiefly to exclude other conditions, such as obstruction or a perforated duodenal ulcer that may simulate pancreatitis. Oblique views are often helpful in patients with chronic pancreatitis to detect calcifications that may be obscured by the spine on the anteroposterior view.
Contrast Studies
Before the advent of cross-sectional imaging, angiography, ERCP, and barium studies were the major means of evaluating the pancreas. Although barium studies are less important now in evaluation of the pancreas, the following areas should still be carefully evaluated in patients with suspected pancreatic disease. The posterior gastric wall, the distal duodenum, and the duodenojejunal junction can be abnormal with lesions of the pancreatic tail and body; the greater curvature of the gastric antrum and medial aspect of the descending duodenum can provide clues for lesions arising in the pancreatic head and neck. Barium enema examination may reveal abnormalities of the colon caused by disease spread through the transverse mesocolon or at the splenic flexure caused by disease carried by the phrenicocolic ligament.
Ultrasound
Sonography is a means of noninvasively evaluating the pancreas, particularly in thin patients. It is fast, safe, and inexpensive; it can be done portably; and it requires little preparation or cooperation of the patient and no administration of contrast medium. However, ultrasound can be limited by patient body habitus and is dependent on the level of experience of the operator. Ultrasound examination of the pancreas is best performed on the fasting patient to reduce the amount of gas and food in overlying bowel. Real-time equipment with the highest frequency transducer (5-8 MHz) possible should be used.
Examination Techniques
Scans of the pancreas should first be performed with the patient supine and the transducer turned into a modified transverse plane angled cephalad toward the spleen ( Fig. 94-9 ). The long axis of the pancreas is seen anterior to the splenic vein and confluence of the superior mesenteric and splenic veins. The pancreatic head and neck sweep around the superior mesenteric vein, forming the retrovenous, hooklike uncinate process. The common bile duct is often imaged axially within the pancreatic head, and the gastroduodenal artery may be seen along the anterolateral aspect of the pancreatic head. The gas-filled or fluid-filled duodenum, stomach, colon, aorta, inferior vena cava, superior mesenteric artery, and left renal vein are interrogated in this projection as well. The lesser sac, which lies between the posterior gastric wall and pancreas, is best seen in this plane and should be scanned for fluid, masses, or calcifications.
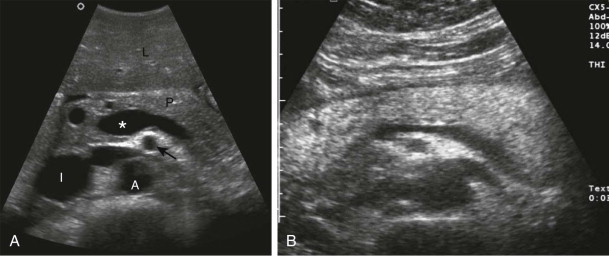
Parasagittal scans of the pancreas should begin where the portal vein merges with the longitudinally oriented superior mesenteric vein. The neck of the pancreas in transverse section is seen anterior to this confluence. The uncinate process lies posterior to the superior mesenteric vein. The pancreatic head can be seen to the right of the superior mesenteric vein, with the common bile duct located posteriorly and the gastroduodenal artery sometimes visualized anteriorly. To the left of the superior mesenteric vein, the pancreatic body and proximal portion of the tail are found anterior to the splenic vein and slightly inferior to the splenic artery.
With meticulous technique and attention to detail, diagnostic scans of the pancreas can be obtained in 90% of cases. Several maneuvers can improve sonographic visualization of the pancreas. Deep inspiration causes the liver to move inferiorly over the pancreas, caudally displacing gas-filled bowel. Having the patient drink four 6-oz cups of degassed water can provide a sonographic window for improved visualization of the body and tail.
Normal Findings on Ultrasound
Three morphologic shapes of the pancreas have been described: tadpole shaped (44%), dumbbell shaped (33%), and sausage shaped (23%). Absolute measurements of pancreatic size are controversial, but the maximal normal anteroposterior diameters of the pancreatic head and body are 2.6 cm and 2.2 cm. The tail is much more variable in shape and size and is not measured for diagnostic purposes. In a dumbbell-shaped pancreas, the tail may be 3.5 cm; when the pancreas is sausage shaped, the pancreatic head can be normal up to 3.5 cm. The pancreatic body is usually the narrowest part of the pancreas. In general, the pancreas is proportionately larger in young people; it decreases in relative size with age. The borders of the pancreas are usually smooth in youth and become somewhat irregular with age. Analysis of the shape of the uncinate process is particularly important because any rounding or enlargement implies disease.
The echogenicity of the pancreas is high throughout its substance. The pancreatic tissue texture or echo size is usually coarser, more inhomogeneous, and more echogenic than that of the liver (see Fig. 94-9 ). It is important to compare the echo patterns and amplitudes of the liver and pancreas at the same depth because dot size and echo amplitude are closely related to the transducer beam profile. The tissue texture of the pancreas is related to the extent of fatty infiltration. As the pancreas ages, it undergoes progressive fatty infiltration, and consequently it becomes more echogenic. Pancreatic parenchyma should be less echogenic than surrounding retroperitoneal fat. When the pancreas is completely replaced by fat in older individuals, it may be difficult to differentiate from the retroperitoneal fat.
Certain caveats apply in scanning the pancreas. Gas within the stomach and duodenum can cause subtle shadowing, artifactually decreasing the echogenicity of the adjacent pancreas. Different amounts of fat may be deposited in the ventral (head and uncinate process) and dorsal (body and tail) pancreas, making the head appear hypoechoic. In some cases, demarcation of the different echogenicities can be seen to correspond to the expected fusion line of the dorsal and ventral embryologic moieties ( Fig. 94-10 ).

On occasion, prepancreatic fat between the stomach and pancreas can be prominent in some patients. It is usually somewhat less echogenic than pancreas and must be differentiated from fluid in the lesser sac, lymphadenopathy, and mural thickening of the stomach. The main pancreatic duct (duct of Wirsung) is identified sonographically in approximately two thirds of patients as an anechoic space surrounded by two parallel hypoechoic lines resembling trolley tracks. The maximal inner diameter is less than 2 mm; when it is larger, an obstructing mass, stricture, or stone must be suspected. The diameter of the pancreatic duct normally increases with age.
A number of normal structures may simulate the pancreatic duct, so it is important to image the duct within the substance of the gland. The posterior wall of the stomach can mimic a dilated pancreatic duct, with normal pancreas dorsal and echogenic gastric lumen ventral, sandwiching the relatively hypoechoic gastric wall. The ingestion of water resolves this problem. A tortuous splenic artery can also simulate a dilated pancreatic duct or cystic pancreatic mass when it lies close to the pancreatic parenchyma. Doppler examination can easily resolve the confusion.
In patients with suspected choledocholithiasis or biliary obstruction, every effort should be made to visualize the distal common bile duct. It often is not visualized because it lies posterior to the second portion of the duodenum, which may be gas filled. This is especially true if the duct is scanned in the supine or left posterior oblique position. A superior method for visualizing the distal common bile duct sonographically is to scan the patient in an erect right posterior oblique position and to rely primarily on transverse rather than parasagittal images. The erect right posterior oblique position minimizes gas within the antrum and duodenum; the transverse scanning plane optimizes identification of the course of the intrapancreatic distal duct. The posterior superior pancreaticoduodenal vein can mimic the distal common bile duct; color Doppler examination can easily differentiate the two structures.
Intraoperative Ultrasound
Intraoperative sonography is a time-consuming but accurate means of localizing small islet cell tumors of the pancreas. It can also be used to guide open biopsy and aspiration. With the increased use of laparoscopic techniques, probes for use during this procedure have also been introduced.
Endoscopic Ultrasound
Ultrasound transducers have been modified so that they can be incorporated into the tip of flexible endoscopes. These transducers have the advantage of higher frequency and spatial resolution, which may be of use in diagnosing small pancreatic tumors, in demonstrating subtle pancreatitis, and in evaluating islet cell tumors preoperatively. The proximity of the stomach and duodenum to the pancreas allows high-resolution imaging of the pancreas and eliminates the limits of overlying bowel gas seen in transabdominal ultrasound. When indicated, pancreatic biopsies can also be performed by endoscopic ultrasound.
Computed Tomography
CT is the best single technique for the noninvasive imaging of the pancreas. It is unaffected by bowel gas or large body habitus, is widely available, and is relatively easily performed. CT has greatly diminished the need for diagnostic ERCP and angiography. Multidetector CT techniques have improved CT examination of the pancreas, allowing thinner contiguous images, overlapping images without increased radiation exposure, and freedom from respiratory misregistration. The rapidity of scanning allows several acquisitions to be made through the pancreas during different phases of a single bolus of contrast material.
Size, Shape, and Density on Computed Tomography
The morphologic appearance of the pancreas on CT depends on the amount of fat within the intralobular septa that separates the acinar lobules of the gland. In younger patients, the contour of the gland is smooth; the parenchyma is homogeneous, with an attenuation similar to that of spleen and muscle but less than that of liver on noncontrast scans ( Fig. 94-11 ). With age and progressive fatty deposition, the pancreas becomes lobulated, irregular, and inhomogeneous.











