201CHAPTER 13
Pancreas SBRT
Pancreatic cancer is arguably the most lethal cancer with over 53,000 new cases estimated in 2017 and over 43,000 deaths (1). It is now the third leading cause of cancer-related death in the United States with a 5-year survival of less than 10% (1, 2). More than 90% of patients diagnosed with pancreatic cancer succumb to the disease with approximately 70% of patients passing away because of extensive disease metastasis (3, 4). Although modern combination chemotherapy has resulted in improved survival, control of metastatic disease remains a challenging problem (4–6). In addition, local recurrence may also result in significant morbidity and has been shown to be an independent predictor of worse survival (7). Locoregional failure rates ranging from 28% to 63% have been reported (8–13), with locally destructive disease as the direct cause of death in up to 20% to 30% of pancreatic cancer patients on the basis of autopsy series (3, 14). Thus, with local failure contributing to poor outcomes, the addition of radiation therapy (RT) to the multidisciplinary management of pancreatic cancer is a logical choice in an attempt to decrease the risk of local recurrence.
THE ROLE OF RADIATION THERAPY IN PANCREATIC CANCER AND THE EVOLUTION OF DOSE ESCALATION
The role of conventional chemoradiation in unresectable, locally-advanced pancreatic cancer remains unclear because of conflicting results from several randomized trials. The Gastrointestinal Tumor Study Group (GITSG) conducted two randomized trials in the 1980s demonstrating a benefit to combined modality treatment compared with radiation or chemotherapy alone (15). GITSG 9273 randomized 194 unresectable pancreatic cancer patients to three arms: (a) high-dose (60 Gy) RT alone; (b) moderate-dose (40 Gy) RT with concurrent fluorouracil (5-FU); or (c) high-dose (60 Gy) RT with concurrent 5-FU. The chemoradiation arms had superior survival compared with the radiation-alone arm with no difference in overall survival (OS) observed between the 40- and 60-Gy arms with concurrent 5-FU. GITSG 9283 compared chemotherapy alone (streptozocin, mitomycin, 5-FU [SMF]) with chemoradiation (concurrent 5-FU) with adjuvant chemotherapy (SMF) in patients with unresectable disease (16). Patients treated with combined modality therapy had superior survival compared with those treated with chemotherapy alone. A survival benefit was also observed in Eastern Cooperative Oncology Group Trial (ECOG) 4201, which compared gemcitabine alone with gemcitabine with concurrent RT despite terminating early because of poor accrual (17). Higher rates of grades 4 and 5 toxicity were observed in the chemoradiation arm compared with the chemotherapy arm. In contrast, the Fédération Francophone de Cancérologie Digestive (FFCD) and the Société Francophone de Radiothérapie Oncologique (SFRO) conducted a phase III trial comparing RT with concurrent 5-FU and cisplatin followed by maintenance gemcitabine compared with gemcitabine alone (18). OS was 202shorter in the chemoradiation arm compared with the chemotherapy-alone arm with a median OS of 8.6 months versus 13.0 months, respectively. Again, higher rates of grades 3 to 4 toxicity were observed in the chemoradiation arm compared with the chemotherapy-alone arm, and were attributable to escalated doses of radiation (up to 60 Gy) with doublet chemotherapy.
Moderately hypofractionated RT with concurrent chemotherapy has been shown to be well tolerated by several studies. Small et al. conducted a multi-institutional phase II study evaluating the safety and efficacy of neoadjuvant and concurrent full-dose gemcitabine for 39 patients with localized pancreatic cancer (19). The treatment regimen consisted of three treatment cycles. In cycles 1 and 3, patients were treated with full-dose gemcitabine on days 1 and 8 of a 21-day cycle. During cycle 2, the same dose of gemcitabine was given on days 1, 8, and 15 of a 28-day cycle with RT starting on day 1 of cycle 2. The radiation dose was 36 Gy delivered in 2.4-Gy fractions and included the gross disease with a margin without elective nodal irradiation or use of intensity-modulated radiation therapy (IMRT). Grade 3 or 4 nonhematologic toxicity was observed in 48.7% of patients with 25.6% of patients experiencing grade 3 to 4 nonhematologic toxicities related to treatment. The overall response rate to treatment was 5.1% with a disease control rate of 84.6%. Following treatment, 81% of initially resectable, 33% of borderline resectable, and 7% of unresectable patients completed surgical resection. The 1-year survival rate for all patients was 73%. Attempts to dose escalate with chemoradiation have shown some improvement, but also with increased toxicity. Ben-Josef et al. conducted a phase I/II trial using IMRT with concurrent gemcitabine to determine the maximum tolerated radiation dose in 50 patients with unresectable pancreatic cancer (20). The radiation dose was escalated to 50 to 60 Gy and delivered over 25 fractions over 5 weeks. The treatment volumes included the gross tumor volume (GTV) delineated on CT and a planning target volume (PTV) consisting of the GTV plus 1 cm expansion. The dose associated with dose-limiting toxicity in 25% of the patients was 55 Gy. Dose-limiting toxicities included grade 3 or 4 gastrointestinal (GI) toxicity in seven patients, duodenal bleeding in three patients, and duodenal perforation in one patient. There were two patient deaths possibly attributed to therapy, a possible duodenal perforation and possible duodenal bleeding either secondary to tumor infiltration or treatment-related toxicity. The 2-year freedom from local progression (FFLP) was 59% with a median OS of 14.8 months. Twelve patients (24%) were able to undergo resection following neoadjuvant treatment with 83% of patients completing an R0 resection. Importantly, patients who completed resection had a FFLP of 92% with an OS of 32 months.
Most recently, the Groupe Cooperateur Multi-disciplinaire en Oncologie (GERCOR) LAP07 clinical trial was an international phase III trial designed to decisively determine the role of chemoradiation in locally-advanced pancreatic cancer (21). Patients underwent two randomizations. In the first randomization, 442 patients were randomized to either weekly gemcitabine alone for 3 weeks followed by a 1-week break for four cycles or the same dose of gemcitabine with the addition of daily erlotinib. For the second randomization, 269 patients free from progression following induction chemotherapy were randomized to the same chemotherapy or chemoradiation (54 Gy with capecitabine). For the first randomization, no difference in OS or progression-free survival was observed in patients receiving gemcitabine alone versus gemcitabine/erlotinib. For the second randomization, no difference in OS was observed between the chemotherapy-alone and chemoradiation arms with a median survival of 16.5 months versus 15.2 months (p = .83), respectively. However, in the chemoradiotherapy group, there was significantly less local progression compared with the chemotherapy-alone group, 32% versus 46% (p = .03), respectively. Similar rates of grades 3 to 4 toxicity were observed between the arms with significantly more nausea in the chemoradiation group. In addition, those patients who went to surgery had a more prolonged survival than those who did not.
STEREOTACTIC BODY RADIATION THERAPY IN PANCREATIC CANCER
As observed in these studies, the use of conventionally fractionated chemoradiation (or slightly moderate hypofractionation) for pancreatic cancer results in suboptimal outcomes, including local control, with approximately 30% to 60% of patients having local progression. Stereotactic body radiation therapy (SBRT) is an established ablative therapy with high local control rates in the lung (22, 23). Thus, there has been recent enthusiasm for testing this modality in pancreatic cancer. SBRT has several 203advantages over conventional RT. The higher dose per fraction with SBRT has the potential for “biologic” dose escalation, which may serve to reduce positive margins in the preoperative setting and may provide superior local control when compared with conventional fractionation. With such a high metastatic burden in pancreatic cancer, SBRT also provides significantly shortened treatment courses compared with conventional fractionation, thereby providing less time off of full-dose systemic doses of chemotherapy. Furthermore, shortened treatment courses improve quality of life with less time away from work and family (24). Although additional investigation is needed, there is mounting evidence demonstrating the utility of SBRT in the management of this difficult disease.
SBRT in Locally-Advanced Pancreatic Cancer
SBRT for the treatment of pancreatic cancer was first reported by Koong et al. at Stanford. This phase I dose escalation study involved 15 patients with locally-advanced pancreatic cancer (25). The patients underwent fiducial marker placement followed by CT simulation performed 1 to 2 weeks later using Alpha Cradle immobilization. Treatment was performed using a breath-hold technique on the CyberKnife® platform. The starting dose was 15 Gy, the next dose was 20 Gy, and the highest dose was 25 Gy delivered in one fraction. The maximum tolerated dose was defined as greater than 50% of patients experiencing a grade 3 or higher toxicity. During the 12-week follow-up time, no grade 3 or higher acute GI toxicity was observed. Of the evaluable patients treated at the 25-Gy dose, the median OS was 8 months. All of the patients treated at the highest dose had local control either at the last follow-up or until death. A phase II study was conducted by the same group evaluating the efficacy of concurrent 5-FU with IMRT followed by an SBRT boost in 19 patients with locally-advanced pancreatic cancer (26). Patients in the study were treated with IMRT to the primary tumor and regional lymph nodes receiving a dose of 45 Gy in 1.8-Gy fractions with either concurrent 5-FU or capecitabine. Within 1 month of finishing chemoradiation, the patients completed an SBRT boost to the pancreatic tumor. Sixteen of the 19 patients completed the planned treatment with three patients developing distant disease prior to the SBRT boost. Grade 3 toxicity was reported in 12.5% of the patients, with additional patients developing duodenal ulcers 4 to 6 months after completing treatment. The median OS for the entire cohort was 33 weeks with a 93.8% local control rate prior to death. OS in the study was poor overall because of rapid systemic progression.
A phase II study performed in Denmark was conducted by Hoyer et al. and showed high toxicity with poor treatment outcomes (27). This study involved 22 patients, 19 with inoperable primary pancreatic cancer and 3 with local recurrence following Whipple resection, with all tumors measuring less than 6 cm in diameter. The GTV was delineated and the clinical target volume (CTV) included the GTV with surrounding edema. A 5-mm radial and 10-mm superior–inferior margin was used to create the PTV. Treatment was delivered using standard linear accelerators with a planned dose of 45 Gy in three fractions. Of note, the median volume receiving above 67% of the prescription dose was 136 cm3, which was relatively large, thought to be attributed to uncertainty in defining the CTV because of reaction and edema of the adjacent tissues. The study did not include CT- or fiducial-based image guidance. On the basis of imaging response that was unclear because of treatment-related fibrosis, 9% of patients had a partial response with the remaining patients having stable or progressive disease. Local progression was observed in six patients (27%) with only one patient having an isolated local failure. The median survival time was 5.4 months and the 1-year OS was 5%. Following treatment, there was a significant decrease in performance status as well as increased pain and nausea. Four patients (18%) experienced severe mucositis or stomach/duodenum ulceration and one patient (5%) required surgery for an ulcer with perforation of the stomach. This study concluded that SBRT for pancreatic cancer was of questionable benefit with severe toxicity.
Because of the high metastatic disease burden associated with pancreatic cancer, subsequent studies incorporated systemic therapy with SBRT. In a follow-up prospective study from the Stanford group, the efficacy of a single fraction of SBRT interdigitated with full-dose gemcitabine for patients with locally-advanced disease was investigated (28). Patients were treated with gemcitabine 1,000 mg/m2 weekly on days 1, 8, and 15 followed by a single fraction of 25 Gy of SBRT delivered on day 29. Following 2 or more weeks after RT, weekly gemcitabine was restarted with a 3-week-on, 1-week-off schedule until disease progression or treatment intolerance. The GTV was delineated 204using a PET scan as guidance and the internal target volume (ITV) was created on the basis of the respiratory-gated CT scans. An additional 2- to 3-mm uniform expansion was created for the PTV. The median target volume was 48 cm3, compared with 136 cm3 in the Danish study (27). Acute toxicities included pain and gastritis in 19% of patients with one patient with gastric outlet obstruction symptoms requiring J-tube placement. Late toxicities occurred in 47% of patients surviving greater than 4 months after RT. Five patients were treated for ulcers (grade 2); one patient required a duodenal stent for a stricture (grade 3); and one patient required surgery for a duodenal perforation. The median survival was 11.4 months with 50% of patients alive at 1 year and local disease progression in 19%.
The Stanford group conducted a separate phase II trial evaluating toxicity, local control, and OS in patients treated with sequential gemcitabine and SBRT delivered using a linear accelerator rather than CyberKnife (on which their previous studies were based) (29). The chemotherapy and radiation schedule and dose were the same as the prior study with a minimum of 2 weeks required between SBRT and restarting gemcitabine (28). Following SBRT, three to five cycles of additional gemcitabine were recommended. Target delineation was performed in the same manner as on the prior study (28). All 20 patients in the study completed RT and a median of five chemotherapy cycles following SBRT. No acute grade 3 or greater toxicities were observed and only one patient experienced a late grade 3 or higher toxicity, which was a duodenal perforation requiring surgery 5.4 months after SBRT. Three additional patients developed grade 2 toxicity (two gastric ulcers, one duodenal ulcer), which was treated medically. The median survival was 11.8 months with a 1-year survival of 50%. The median time to progression was 9.2 months and 10% of patients had local recurrence as the first site of progression.
A retrospective study from Beth Israel Deaconess Medical Center conducted by Mahadevan et al. reported on 36 locally-advanced, unresectable pancreatic cancer patients treated using a three-fraction SBRT approach followed by gemcitabine (30). The CTV was defined as the gross disease with a 5-mm or less margin except where the tumor was in contact with the bowel. It is important to note that this was not the PTV, but rather was used to prevent any areas of decreased dose adjacent to the tumor directly outside the prescription volume. The radiation dose was determined based on the proximity of the tumor to the duodenum with a maximum dose of 36 Gy, an intermediate dose of 30 Gy, and a minimum dose of 24 Gy to the PTV delivered in three fractions using the CyberKnife platform. Patients were recommended to start chemotherapy 1 month after completing SBRT. Treatment was well tolerated with 25% of patients experiencing grade 2 toxicity, primarily nausea. Grade 3 toxicity was observed in 14% of patients. There were two patients who developed GI bleeding following treatment that required transfusion. At a median follow-up of 24 months, the local control rate was high at 78% with a median OS of 14.3 months. As expected, 78% of patients eventually developed distant metastasis.
A follow-up retrospective study from the same group reported on the use of SBRT between cycles of chemotherapy for 47 patients with locally-advanced pancreatic cancer (31). Patients completed two cycles of gemcitabine 1,000 mg/m2 delivered weekly with 3 weeks on and 1 week off. Following the second cycle, patients were restaged. Patients without metastatic disease received a third dose of gemcitabine and completed fiducial placement followed by SBRT planning. After SBRT completion, patients received gemcitabine until progression or intolerance. SBRT was performed in the same manner as in the prior study (30). Grade 2 toxicity was observed in 23% of the patients and consisted of nausea requiring antiemetics. Although no acute grade 3 toxicities were observed, 9% of patients experienced late grade 3 toxicity including two duodenal bleeds requiring transfusions and endoscopy and one patient with gastric outlet obstruction. In one of the duodenal bleeds and the gastric outlet obstruction, it was uncertain whether the toxicity was from treatment or related to tumor progression. Of the 47 patients included in the study, eight developed metastatic disease after two cycles of gemcitabine. The median follow-up was 21 months and the median OS was 20 months for patients who completed SBRT. Of the patients who were treated with SBRT, the local control rate was quite high at 85%, whereas 54% of the patients developed metastatic disease.
A prospective Italian study also evaluated treating patients with locally-advanced pancreatic cancer with SBRT and gemcitabine (32). Twenty-three patients were treated with gemcitabine 1,000 mg/m2 delivered weekly for 6 weeks prior to SBRT. Treatment volumes were planned according to Koong et al. (25). A dose of 30 Gy in three fractions 205was delivered using the CyberKnife system. Following treatment, patients resumed gemcitabine. No grade 2 or greater toxicities were observed. At a median follow-up of 9 months, the median OS was 10.6 months. Ultimately, 8% of patients became resectable.
In a separate prospective study from Georgetown, Gurka et al. evaluated the safety of delivering SBRT for 10 unresectable pancreatic cancer patients who were receiving concurrent gemcitabine (33). Following gold marker placement, patients were treated with gemcitabine 1,000 mg/m2 delivered on days 1, 8, and 15. SBRT was delivered during cycle 1, week 4. Cycle 2 of gemcitabine was then administered without any planned delay. Patients also underwent esophagogastroduodenoscopy (EGD) prior to any treatment and at 2 and 6 months following treatment to assess mucosal toxicity. Patients who were tolerating gemcitabine without any issues continued for a maximum of six cycles. The GTV included the pancreatic tumor and the PTV encompassed the GTV plus adjacent vasculature without additional margin. The PTV was planned to receive 25 Gy delivered in five fractions over 5 days. Of note, the mean PTV was relatively large with a mean volume of 360 cm3. However, patients tolerated treatment well with no grade 3 acute toxicities and no delays in initiating cycle 2 of gemcitabine. On serial EGDs, grade 1 mucosal ulcerations were seen in 60% of patients at 2 months after treatment, all of whom were asymptomatic. At 6 months, there were no grade 2 or higher mucosal toxicities. Local control was suboptimal with six patients (60%) developing a local progression before death. The median OS was 12.2 months. In a follow-up retrospective study from the same group, 38 patients treated with SBRT and chemotherapy were reviewed, which included locally-advanced, borderline resectable, and medically inoperable patients (34). SBRT planning and delivery was performed in the same manner as in the pilot study; however, chemotherapy regimens on the study also included modified FOLFOX (fluorouracil, leucovorin, and oxaliplatin), 5-FU, and capecitabine in addition to gemcitabine (33). Patients were treated with either 25 or 30 Gy delivered in five fractions. There were three grade 3 or greater late toxicities including one grade 5 toxicity because of gastric hemorrhage. At a median follow-up of 7.2 months, the local control rate was 79% with a trend toward significance for improved local control with 30 Gy versus 25 Gy. The median OS was 14.3 months.
Multiple other groups have explored the use of multifraction regimens. Goyal et al. reported on 20 patients with locally-advanced pancreatic cancer treated on a prospective database at University Hospitals-Case Medical Center (35). The median prescription dose was 25 Gy (range, 20–30 Gy) delivered in one to three fractions. Grades 1 to 2 toxicity were observed in 11% of patients and 16% of patients developed GI ulcers that were managed medically. FFLP at 6 months and 1 year were 88% and 65%, respectively, and the median OS was 14.4 months. The overall local control rate was 81%. Pollom et al. conducted a retrospective review comparing single-fraction with five-fraction SBRT in 167 patients with unresectable pancreatic cancer treated at Stanford (36). No differences in cumulative incidence rates (CIRs) of local recurrence or survival were observed between the single-fraction and multifraction groups. At 1 year, the CIR of local recurrence for patients treated with a single-fraction and multifraction regimen was 9.5% and 11.7%, respectively. The survival rates at 1 year for the single-fraction and multifraction regimens were 30.8% and 34.9%, respectively. However, there was a difference in toxicity with significantly fewer occurrences of grade 2 or higher toxicity with the multifraction regimen.
A retrospective study from Johns Hopkins reported on the use of a five-fraction regimen for both locally-advanced and borderline resectable pancreatic cancer (37). A total of 88 patients were reviewed with 84% of the patients with locally-advanced disease. The PTV was defined as the GTV with a 2- to 3-mm margin to account for microscopic disease and setup error with exclusion of critical structures that was primarily bowel. The PTV received a dose of 25 to 33 Gy in five fractions. Treatment was well tolerated with an acute grade 3 or higher GI toxicity rate of 3.4% with one case of grade 4 GI bleeding. Late grade 2 or higher GI toxicity was observed in 5.7% of patients with one grade 5 toxicity. The median OS was very promising at 18.4 months. Of note, 21.6% of patients underwent surgical resection (84% of these had R0 resection), with 79% of those patients having locally-advanced disease at presentation. Moffitt Cancer Center also published their experience using a five-fraction SBRT regimen following induction chemotherapy (38, 39). In an updated review consisting of 159 patients, all patients reviewed received at least one dose of induction chemotherapy with SBRT delivery at least 1 week following the last chemotherapy dose (39). RT was 206delivered using IMRT with a planned dose of 28 to 30 Gy to the PTV with use of dose painting up to 50 Gy to areas of vessel involvement. Treatment was well tolerated with 7% of patients experiencing a grade 3 or higher acute and/or late toxicity with the most common toxicity consisting of a GI bleed. With a median follow-up of 14 months, the median OS was also high at 18.1 months. For locally-advanced patients treated with FOLFIRINOX (oxaliplatin, irinotecan, 5-fluorouracil), 24% were able to undergo an R0 resection. A trend for improved survival was seen in the locally-advanced patients able to undergo resection.
A recent Italian study reported promising results using a six-fraction regimen (40). This phase II study reported by Comito et al. used volumetric-modulated arc therapy (VMAT) to deliver a dose of 45 Gy in six fractions to patients with locally-advanced pancreatic cancer (40). The PTV was created by adding a 5-mm radial and 10-mm superior–inferior margin. A total of 45 patients were enrolled in the study with 43 patients evaluable. The majority (71%) of patients received pre-SBRT chemotherapy completed at least 2 weeks prior to SBRT. There were no acute or late grade 3 or higher toxicities reported. At a median follow-up of 13.5 months, FFLP was 87% at 1 year with a median OS from diagnosis of 19 months.
Herman et al. conducted a single-arm phase II multi-institutional study to determine whether gemcitabine delivered with more prolonged fractionated SBRT would reduce late grades 2 to 4 GI toxicity when compared with the early Stanford experience of gemcitabine and a single 25-Gy fraction of SBRT (41). A total of 49 patients with locally-advanced pancreatic cancer (<7.5 cm) received up to three doses of 1,000 mg/m2 of gemcitabine followed by a 1-week break and then SBRT with additional gemcitabine until progression or toxicity. The SBRT dose was 33 Gy delivered in five fractions. At a median follow-up from SBRT of 13.9 months, 2% of patients experienced acute (within 90 days of SBRT) grade 2 or higher GI toxicity, whereas 11% experienced late (>90 days from SBRT) grade 2 or higher GI toxicity. The 1- and 2-year OS rates were 59% and 18%, respectively, and the 1-year FFLP rate was 78%. Following treatment, five patients were deemed to be resectable and four underwent surgery with all of the patients completing a margin- and lymph node–negative resection.
A summary of selected prospective studies is provided in Table 13.1. Given the reasonable local control and acceptable toxicity rates, a multifraction regimen has evolved as the preferred SBRT regimen for locally-advanced pancreatic cancer. Indeed, the dosing regimen of 33 Gy in five fractions from the aforementioned single-arm phase II trial now serves as the preferred dose in patients randomized to SBRT for the borderline resectable Alliance A021501 randomized phase II trial (42). A021501 is the first trial to prospectively test SBRT in patients with pancreatic cancer in a cooperative group setting in North America.
SBRT in the Preoperative Setting
Surgical resection offers the only possibility of cure for localized pancreatic cancer (4). However, only 15% to 20% of patients are able to undergo resection at initial diagnosis (43). Neoadjuvant therapy including RT has the potential to select patients who develop early metastasis and would not benefit from surgical resection (43). It also allows for tumor downstaging, increases the possibility of an R0 resection, and eradicates occult micrometastatic disease (43).
Data about the use of SBRT for borderline resectable pancreatic patients is currently limited, but is an area of increased interest. Rajagopalan et al. reported on the University of Pittsburgh experience using neoadjuvant chemotherapy and SBRT prior to surgery for borderline resectable and locally-advanced pancreatic cancer patients (44). Twelve patients were included in the study, with 92% receiving chemotherapy prior to SBRT treatment. Patients were treated with either 24 Gy in a single fraction or 30 to 36 Gy in three fractions. Patients completed surgery at a mean time of 3.3 months following SBRT. Two patients developed pseudoaneurysms felt to be related to SBRT with one patient dying from complications related to this toxicity. An R0 resection was achieved in 91.7% of patients with 25% achieving a complete pathologic response to treatment. The median OS for the study was 47.2 months with a 1-year OS of 92%. The retrospective series from Moffitt Cancer Center by Mellon et al. included 110 patients with borderline resectable disease (39). A total of 51% of the patients underwent curative intent resection with the remaining 49% developing local or distant progression or a decline in performance status that prohibited resection. An R0 resection was achieved in 96% of the borderline resectable group with a median OS of 19.2 months. A small number of borderline resectable patients were included in the review of the Johns Hopkins experience with SBRT by Moningi et al. (37). Of the 14 borderline resectable patients included in the study, four underwent surgery. Overall, 84% of patients had R0 resections with a completed pathologic response observed in 16% of patients and a partial response in 74%. Patients who were able to complete resection had a median OS of 20.2 months compared with those unable to complete surgery, 12.3 months.
207
A dose escalation phase 1 study was performed at Emory University for patients with borderline resectable disease (45). Patients were treated with modified FOLFIRINOX (mFOLFIRINOX) administered every 14 days for four treatments. SBRT was then started 2 weeks after completing chemotherapy. The PTV included any gross disease and areas of at-risk microscopic spread with a uniform 5-mm margin. Lymph nodes were not included in the volume. The posterior margin, the area at the highest risk of a positive margin, was defined as the volume between the posterior 1 cm of the GTV and either the mesenteric vessels or the soft tissues in the retroperitoneum, or both. There were four dose levels for the study. Dose level 1 for the PTV was 30 Gy in three fractions and for dose levels 2 to 4, the PTV dose was 36 Gy in three fractions. For the posterior (retroperitoneal) margin, a simultaneous integrated boost (SIB) was used and varied by dose level. For dose levels 1 and 2, the SIB was 6 Gy. For dose level 3, it was 7.5 Gy and for dose level 4, it was 9 Gy. RT was planned and delivered using VMAT. A total of 13 patients were accrued with 12 patients who completed the planned treatment course with three patients treated at each dose level. No dose-limiting or grades 3 to 4 toxicities were observed on the study. Five patients (42%) in the study were not able to complete surgical resection because of progressive disease with one local progression and four distant progressions reported. The remaining eight patients underwent an R0 resection with 63% of the patients requiring vessel reconstruction. The postoperative adverse events were as expected. Of the eight patients who completed resection, only one local recurrence was reported. At a median follow-up of 18 months, the median OS was 11 months.
Although there are limited data on the use of SBRT in the preoperative setting, the available studies show that it is a useful neoadjuvant therapy with a high rate of margin-negative resections and 208no apparent increase in treatment-related toxicity, although additional larger prospective studies are needed. A currently enrolling study in the United Kingdom, the “SPARC” trial, is a phase I dose escalation study with borderline resectable pancreatic cancer patients using five-fraction SBRT with an SIB to the area at the highest risk of a positive margin followed by resection (46). In the United States, the Alliance A021501 randomized phase II clinical trial is testing the role of hypofractionated RT in borderline resectable patients by randomizing patients to preoperative mFOLFIRINOX versus mFOLFIRINOX followed by hypofractionated RT (ranging from 5 or 6.6 Gy per fraction in five fractions with up to 8 Gy per fraction allowed to the tumor–vessel interface) (42). Additional prospective studies including the use of neoadjuvant chemotherapy with SBRT in borderline resectable patients are also being conducted at the University of Maryland and University of Pittsburgh (47, 48).
SBRT in the Postoperative Setting
SBRT can also be considered after resection for close or positive margins; however, data regarding the use of SBRT as adjuvant therapy following resection is even more limited. A study by Rwigema et al. from the University of Pittsburgh published a retrospective series consisting of 24 patients treated using primarily a single-fraction regimen (49). The majority (66.7%) of patients treated had a positive margin, whereas the balance (33.3%) had margins that were 1 to 2.5 mm from the retroperitoneal, vascular structures, and periduodenal adipose tissue. Patients were treated with a median dose of 24 Gy in one fraction to a relatively small median volume of 11 cm3. The majority of patients were treated with chemotherapy both before and after SBRT. The overall local control rates were 66% and 44% at 1 and 2 years, respectively, with 87.5% with close margins and 62.5% of those patients with positive margins not progressing locally. The median OS was 26.7 months with 1- and 2-year OS rates of 80.4% and 57.2%, respectively. Overall, 79% of patients were able to start or continue with chemotherapy at a median of 18 days post-SBRT. There were no grade 3 or higher toxicities reported. Additional studies including prospective trials need to be performed to determine the efficacy and safety of SBRT as adjuvant therapy, particularly in the setting of a close or positive resection margin.
SBRT in the Recurrent Setting
Although distant failure remains the predominant cause of death in pancreatic cancer, local recurrence is the cause of death in approximately 30% of patients (3, 4). Treatment options for patients with an isolated local recurrence are limited, particularly in patients who have had prior adjuvant or definitive RT (50). A systematic review by Groot et al. found that mortality for treatment of an isolated local recurrence with re-resection, chemoradiation, and SBRT was quite low (0%–1%), but morbidity could be quite high reaching approximately 30% with re-resection and 50% with chemoradiation, but only 3% with SBRT (51). Furthermore, in patients with likely incurable disease, a repeat laparotomy can adversely affect patient quality of life (51). SBRT is a noninvasive method of delivering a high radiation dose to an area of local recurrence with the goal of improved local control.
Several studies have reported on the use of SBRT in the reirradiation setting for a local recurrence. A pooled retrospective review of patients treated at Johns Hopkins and Stanford with reirradiation for an isolated locally recurrent pancreatic cancer was reported by Wild et al. (52). A total of 18 patients were included in the study with 15 receiving resection earlier with adjuvant or neoadjuvant chemoradiation and three patients treated with definitive chemoradiation. The median dose for the first course of RT was 50.4 Gy. Patients were treated using a five-fraction regimen with a median dose of 25 Gy. Median survival following SBRT was 8.8 months. FFLP at 6 and 12 months following SBRT was 78% and 62%, respectively. Treatment was well tolerated with no acute grade 3 or higher toxicity and only one patient (6%) who developed late toxicity with a small bowel obstruction. A retrospective study from Harvard also reported on 30 patients reirradiated with SBRT for recurrent pancreatic cancer (50). Patients included in the study either completed adjuvant RT after resection or were treated with definitive conventional chemoradiation or SBRT. All patients were felt to be unresectable at the time of recurrence and received systemic therapy both before and after SBRT delivery. Patients were treated to a total SBRT dose of 24 to 36 Gy (median 25 Gy) in three to five fractions (median 5). The median OS following SBRT for recurrence was 14 months with local control rates of 78% at 1 and 2 years. Treatment was well tolerated with a rate of acute and late grade 3 or higher toxicity of 11% and 2097%, respectively. In the difficult setting of a local recurrence after either resection or treatment with adjuvant or definitive radiation/chemoradiation, SBRT offers a potential therapeutic option to gain local control with acceptable toxicity. Additional studies are needed, however, particularly in the prospective setting, to further define the role of SBRT for recurrent pancreatic cancer.
TREATMENT PLANNING AND DELIVERY
Patient Selection
Ideally, patients with pancreatic cancer should be evaluated by a multidisciplinary team specializing in GI malignancies including surgical oncology, medical oncology, radiation oncology, radiology, and pathology in addition to other supportive staff such as palliative medicine and nutrition (53). As part of the staging workup, patients should undergo endoscopy for tissue diagnosis and possible stent placement in the setting of a biliary obstruction, and to evaluate the presence or extent of local invasion. A relative contraindication to SBRT is direct invasion of the tumor into the duodenum or stomach (53). Although patients with positive lymph nodes are not necessarily excluded given that the regional lymph nodes can sometimes be included in the target volume as long as dose constraints are still met, extensive regional lymphadenopathy is a relative contraindication to SBRT (41, 53). Given the high risk of distant metastasis, SBRT delivery following induction chemotherapy with either mFOLFIRINOX or gemcitabine/nab-paclitaxel is preferred and has been shown to be feasible and well tolerated in multiple studies (28, 29, 31–33, 39, 41, 45). Ideally, patients should undergo restaging studies prior to delivery of SBRT to rule out the presence of metastatic disease.
Treatment Simulation
Prior to completing CT simulation, patients should undergo either CT or endoscopic ultrasonography (EUS)-guided fiducial marker placement either into or adjacent to the tumor approximately 1 day prior to CT simulation because of possible seed migration (53, 54). Because tumors cannot be seen on kV imaging or cone-beam CT, fiducial markers allow for precise alignment with the treatment planning CT and for fluoroscopic verification of the tumor motion during respiration. CT simulation should be performed with the patient in the supine position with arms up using an Alpha Cradle with/without a wing board or equivalent custom immobilization device to allow reproducible setup during treatment. The patient should ideally fast 2 hours prior to the scan and both intravenous (100 mL) and oral (240 mL) Omnipaque contrast should be used, if possible, for the simulation scan to better delineate the stomach, small bowel, and vasculature (41).
A four-dimensional CT should be performed to evaluate tumor motion during the respiratory cycle. For patients with more than 3 to 5 mm of motion, motion management techniques should be implemented that may include active breathing control, respiratory gating, and end-expiration or end-inspiration breath hold (53, 55). Treatment with expiratory breath hold has been shown to decrease the dose to the duodenum compared with treatment during the inspiratory phase, likely because of inferior displacement of the diaphragm that approximates the bowel closer to the PTV (56). Likewise, abdominal compression is felt to be less optimal because of possible movement of the duodenum and stomach toward the PTV during compression (53, 55). Patients with less than 3 to 5 mm of motion can be treated using an ITV on the basis of the tumor motion during the respiratory cycle.
Target Delineation
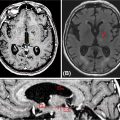
Delineation of the GTV can be accomplished using the CT simulation scan or other diagnostic imaging including MRI or PET/CT. For the CT simulation scan, dual-phase imaging with both arterial and portal venous phases can be useful for accurately delineating the GTV. The arterial phase helps determine the extent of vascular involvement, whereas the tumor is typically best visualized in the venous phase. PET has been used in multiple studies for treatment volume definition (28, 29, 36, 37, 41). If PET is used, obtaining the PET/CT in the treatment position to more accurately define the GTV is preferred (54). For patients treated prior to resection, both the tumor–vessel interface and regional lymph nodes greater than 1.5 cm should be included in the GTV when possible (53). The ITV can then be determined based on tumor motion during the respiratory cycle and a 0- to 5-mm uniform expansion is made to create the PTV on the basis of institutional preference and setup (53). The PTV can then be modified based on adjacent organs at risk including the duodenum, stomach, and bowel, but should always include both the GTV and the ITV (55). The organs at risk that should be contoured include the duodenum, stomach, bowel, liver, spinal cord, and kidneys. An atlas is available from the Radiation Therapy Oncology Group to standardize and to assist with contouring of these structures (57). An example of SBRT target and organ-at-risk delineation for the treatment of a pancreatic head lesion is shown in Figure 13.1.
210
FIGURE 13.1 Targets and organs at risk for stereotactic body radiation therapy for treatment of pancreatic head cancer. Axial (A) and coronal (B) views. This patient was treated using respiratory gating with fiducials that are not visible in the CT slices shown. The targets include the GTV (teal) and PTV (red). The organs at risk include stomach (purple), liver (green), duodenum (orange), right kidney (blue), left kidney (green), bowel (brown), and spinal cord (pink).
GTV, gross tumor volume; PTV, planning target volume.
Dose Fractionation and Dose Constraints for Treatment Planning
Both single-fraction and multifraction regimens have been investigated and shown to be effective. On the basis of retrospective data from Stanford, although the local control and OS rates between the two regimens were similar, there was significantly more GI toxicity in the single-fraction group (36). Numerous studies have used a multifraction regimen consisting of three to six fractions (31, 32, 34, 37, 39, 40, 45). The multi-institutional phase II study by Herman et al. used a dose of 33 Gy in five fractions following induction gemcitabine with good local control and acceptable toxicity (41). In the preoperative setting, dose painting has been used to deliver a higher dose to areas at risk for a positive margin with favorable results (45).
The dose that can be safely delivered to the PTV is dependent on the location of the tumor. Tumors of the pancreatic head may be limited by the dose to the adjacent duodenum, whereas doses to pancreatic body or tail tumors may be limited by the small bowel or stomach tolerance. Ideally, 95% of the PTV receives the prescribed dose while meeting the objectives for the organs at risk. However, dose heterogeneity may be greater than 15% to 20% because of strict dose constraints. Dose constraints used in the multi-institution phase II study by Herman et al. are included in Table 13.2 (41). Murphy et al. used the Stanford single-fraction experience to determine dose–volume histogram end points and maximum doses to 1 cm3 (Dmax) that were predictive of duodenal toxicity (58). A Dmax value less than 23 Gy, V15 value less than 9.1 cm3, and V20 value less than 3.3 cm3 were all predictive of lower rates of duodenal toxicity. Duodenal and bowel toxicities of concern include hemorrhage, ulcer, stricture, and perforation.
TABLE 13.2 Dose Constraints for Pancreas Stereotactic Body Radiation Therapy
Organ at Risk | Constraints |
Proximal duodenuma | V15 <9 mL V20 <3 mL |
Bowel | V15 <9 mL V20 <3 mL |
Proximal bowela | V33 <1 mL |
Stomach | V15 <9 mL V20 <3 mL V12 <50% |
Proximal stomacha | V33 <1 mL |
Liver | V12 <50% |
Combined kidneys | V12 <75% |
Spinal cord | V8 <1 mL |
aProximal structure includes 1 cm superior and inferior to the planning target volume.
211Treatment Planning and Delivery
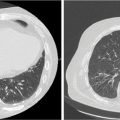
SBRT delivery can be performed with either linear accelerator-based or CyberKnife-based platforms. For treatment using linear accelerators, either IMRT or VMAT can be used. Daily image guidance should be used with kV or fluoroscopic imaging, or cone-beam CT (53). The use of high-definition multileaf collimators and flattening filter-free beams permits delivery of a highly conformal treatment with reduced treatment time (59). An example of an acceptable and an unacceptable treatment plan is shown in Figure 13.2. Treatment can be delivered on consecutive days. Physicians should consider starting patients on a proton pump inhibitor prior to starting SBRT and should continue for at least 6 months following treatment. Prophylactic antiemetics should also be considered for the patient.
TREATMENT FOLLOW-UP
Follow-Up Imaging
Follow-up imaging with CT is limited because of posttreatment inflammatory changes making it difficult to differentiate between anticipated post-SBRT changes and progressive disease. Toesca et al. reported on the use of PET to evaluate local progression following SBRT for pancreatic cancer (60). Both fluorodeoxyglucose (FDG)-PET and a pancreatic protocol CT were obtained 3 to 6 months following SBRT and local progression was defined based on the Response Evaluation Criteria in Solid Tumors (RECIST) and the FDG-PET Response Evaluation Criteria in Solid Tumors (PERCIST). PET was superior in determining local progression with CT alone diagnosing approximately 50% of cases of local progression. Evaluating the treatment response to SBRT with imaging remains an area of active investigation and standardization is critical for comparison with future studies.
Outcome Data
Numerous prospective and retrospective studies of SBRT for pancreatic cancer have been published with a range of reported outcomes. The largest prospective study to date reported a 1-year local control rate of 78% with a median OS of 13.9 months (41). On the basis of a systematic review and pooled analysis of 19 studies consisting of 1,009 patients treated with SBRT for locally-advanced pancreatic cancer, the pooled 1-year OS was 51.6% with a median OS of 17 months (5.7–47 months; 61). The locoregional control rate at 1 year was 72.3%.
Quality of Life
As part of the multi-institutional trial by Herman et al., quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and pancreatic cancer-specific QLQ-PAN26 module pre-SBRT and at 4 to 6 weeks and 4 months post-SBRT (62). No changes in quality of life from pre-SBRT to either follow-up time point were observed. Patients reported a significant improvement in pancreatic pain at 1 month, but this returned to the pre-SBRT levels by 2 months. Role functioning at 2 months was noted to be significantly decreased compared with 212baseline, although it is uncertain whether this decrease is secondary to the disease or the treatment. For patients diagnosed with pancreatic cancer, which remains largely an incurable disease, quality of life assessment is an essential part of future studies.

FIGURE 13.2 Example of stereotactic body radiation therapy plans for treatment of pancreatic head cancer. This patient was treated using respiratory gating with fiducials. The targets include the GTV (teal) and PTV (red). The organs at risk include stomach (purple), liver (green), duodenum (orange), right kidney (blue), bowel (brown), and spinal cord (pink). For each plan, the 3,300-cGy (yellow), 3,135-cGy (dark blue), 2,000-cGy (cyan), 1,650-cGy (light green), and 1,500-cGy (pink) isodose lines are shown. (A) Dynamic conformal arc planning was used with 6-MV photons. This plan was not acceptable because of stomach V15 >9 cm3. As seen, the 1,500-cGy isodose line extends far into the stomach. (B) Volumetric-modulated arc therapy planning was used with 6-MV photons. This was an acceptable plan with all dose constraints and acceptable coverage of the target.
GTV, gross tumor volume; PTV, planning target volume.
CONCLUSIONS
Pancreatic cancer remains a deadly disease that is resistant to currently available therapies. The use of RT with conventional fractionation provides some local control, but rates of local failure remain high. SBRT is an emerging therapy for the treatment of pancreatic cancer that allows for expedited treatment of radiation over a shorter time interval. Data for pancreas SBRT are accumulating for the definitive treatment of locally-advanced disease as well as for the preoperative, adjuvant, and recurrent settings. Overall, treatment outcomes with pancreas SBRT have been favorable with acceptable rates of toxicity. Although additional studies are needed, pancreas SBRT appears to be a promising new therapy in the management of this lethal disease.
SUMMARY OF DOSE CONSTRAINTS (TABLE 13.3)
TABLE 13.3 SBRT-Required OARs and Dose Constraints
Description | Planning System Name | Constraints |
(iGTV + iTVI) + 3 mm | PTV1 | PTV1: 25 Gy, Dmin >22.5 Gy |
PTVV1 PRVGi | PTV2 | PTV2: 33 Gy, Dmin >29.7 Gy |
(iTVI + 3 mm) – PRVGi | PTV3 | PTV3: 36 Gy, Dmin >32.4 (maximum of 40 Gy) |
OAR |
| Constraints |
Duodenum | Duodenum | V20 <20 mL |
|
| V35 <1 mL |
Small bowel (other) | Bowel | V20 <20 mL |
|
| V35 <1 mL |
Stomach | Stomach | V20 <20 mL |
|
| V35 <1 mL |
Liver | Liver | V12 <50% |
Combined kidneys | Kidneys | V12 <25% |
Spinal cord | Spinal cord | V20 <1 mL |
Spleen | Spleen | No constraint |
OAR, organ at risk; SBRT, stereotactic body radiation therapy.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387
2. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694-9705. doi: 10.3748/wjg.v22.i44.9694
3. Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 Gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806-1813. doi: 10.1200/JCO.2008.17.7188
4. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140-2141. doi: 10.1056/NEJMc1412266
5. Conroy T, Desseigne F, Ychou M, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923
6. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369
7. Merrell KW, Haddock MG, Quevedo JF, et al. Predictors of locoregional failure and impact on overall survival in patients with resected exocrine pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):561-570. doi: 10.1016/j.ijrobp.2015.11.003
8. Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899-903. PubMed PMID: 4015380.
9. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776-782. PubMed PMID: 10615932.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree