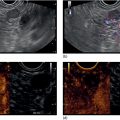
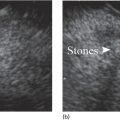
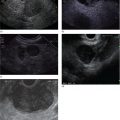
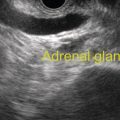
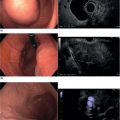
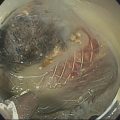
William R. Brugge Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA The frequent use of cross‐sectional radiographic imaging has led to an increase in the identification of cystic lesions of the pancreas. In asymptomatic patients, the cystic lesion most often represents a form of intraductal malignancy. Fluid collections in patients with acute pancreatitis are usually inflammatory in nature. In the management of patients with a cystic lesion, there are usually two predominant questions: “Is the cyst mucinous or non‐mucinous?” and “Is the cyst malignant or benign?” Endoscopic ultrasound (EUS) with fine needle aspiration (FNA) can provide critical data to answer these diagnostic questions. Cystic lesions of the pancreas are often readily apparent by EUS. The lesions appear as anechoic against the “salt and pepper” background of the pancreatic parenchyma. Focal fluid collections can simulate a true cyst of the pancreas, but often there are tell‐tale signs of pancreatitis. Occasionally, tumor necrosis can simulate a pancreatic cyst because of the presence of fluid in the mass. Endoscopic ultrasound can provide details of the structural characteristics of the cyst, pancreatic parenchyma, pancreatic duct as well as surrounding structures, but is not sufficient to distinguish malignant from benign lesions (Table 25.1). Fine needle aspiration of the cyst fluid and analysis of cellular material, amylase level, and tumor markers such as carcinoembryonic antigen (CEA) have been shown to assist in narrowing the differential diagnosis. In studies where surgical correlation is available, EUS with FNA and cytopathology has an accuracy of 55–91%. In particular, a cyst fluid CEA concentration above 192 ng/ml is predictive of a mucinous lesion with 79% accuracy. The presence of a KRAS mutation is also indicative of a mucinous lesion. The diagnostic techniques described are particularly pertinent in those patients who are not surgical candidates or do not have symptoms attributable to the cyst. Endoscopic ultrasound with FNA should be reserved for those cases in which the results would alter clinical management. If a cyst is found in the setting of recurrent acute pancreatitis in a patient who is fit for surgery, resection should be considered and EUS is not indicated. A pancreatic pseudocyst represents an inflammatory fluid collection. There is no epithelial lining within a pseudocyst and surrounding structures form the borders of a pseudocyst. Imaging findings of chronic pancreatitis, including calcifications, increase the likelihood that a cystic lesion is a pseudocyst. The EUS features of pseudocysts include a thick‐walled, unilocular, fluid‐filled cavity (Figure 25.1). Aspiration of a pseudocyst reveals thin, brown, amylase‐rich fluid. Cytologic examination reveals inflammatory cells and occasionally necrotic debris. The fluid CEA level should be relatively low and without evidence of mucin. Serous cystadenoma is the most common type of non‐mucinous cyst (Figure 25.2). These lesions typically have a microcystic morphology creating a honeycomb appearance. The microcysts are divided by thin septations that can become fibrotic and form the classic central stellate scar. Macrocystic morphology is seen in a minority of serous cystadenomas. Microscopically, the cyst lining is composed of cuboidal cells with glycogen‐rich cytoplasm and dense chromatin. Table 25.1 Demographics and characteristics of pancreatic cystic neoplasms. Figure 25.1 Thick‐walled cystic lesion undergoing fine needle aspiration (FNA). The lesion was resected and was a pseudocyst. Mucinous cystic neoplasms (MCNs) are a distinct type of mucinous cyst (Figure 25.3a). They occur almost exclusively in women when they are defined by the presence of ovarian stroma. These tumors are principally located in the tail of the pancreas and mucin may be visible within the cyst cavity (Figure 25.3b). Malignant MCNs may have a mural nodule or mass apparent on EUS (Figure 25.3c). Depending on the degree of cellular atypia, MCNs are classified as adenomas, borderline tumors, or carcinomas. The cysts do not communicate with the pancreatic ductal system and the cyst fluid generally contains mucin and a high concentration of CEA. Intraductal papillary mucinous neoplasm (IPMN) (Figure 25.4a) generally presents in the seventh decade of life. It can be divided into morphologic subtypes: main‐duct IPMN, branch‐duct IPMN, and mixed. Main‐duct IPMN carries a much greater likelihood of malignancy than branch‐duct IPMN. The characteristic imaging features of branch‐duct IPMN include multilocular appearance with a cluster‐of‐grapes configuration, communication with the pancreatic duct, and dilated ductal structures with filling defects representing mucus. In addition, a dilated main pancreatic duct, cyst size greater than 3.0 cm, and mural nodularity are features of IPMN that predict an increased likelihood of malignancy (Figure 25.4b,c). Figure 25.2 Serous cystadenoma. Multicystic lesion in the body of the pancreas with microcystic and macrocystic components and a central scar. The overall size is 2.8 × 3.5 cm. Solid pseudopapillary epithelial neoplasm (SPEN) usually occurs in young women. Although this lesion is rare, recognition is important given its malignant potential and very high cure rate with surgical resection. It is typically large with solid and cystic features. Fine needle aspiration cytopathology may demonstrate branching papillae and myxoid stroma. Pancreatic cystic endocrine tumors have a male to female ratio of 1 : 1 with a peak incidence in the fifth and sixth decades. Cystic neuroendocrine tumors often have a thick wall (Figure 25.5a). Given their high degree of vascularity, EUS with color flow Doppler may demonstrate increased blood flow within these lesions (Figure 25.5b). Figure 25.3 (a) Mucinous cystic neoplasm with multiple septations. The lesion was benign when surgically resected. (b) Mucinous cystic neoplasm with mucin visible within the cyst cavity. (c) A 1.5‐cm malignant cystic lesion with mucin and debris in the lumen of the cyst. Ductal adenocarcinoma can also present with cystic morphology as a result of tumor necrosis. As with solid adenocarcinoma of the pancreas, there is a male predominance, and the typical age of onset is late sixties to early seventies. Endoscopic ultrasound may demonstrate mixed solid–cystic mass with resultant ductal obstruction and possible invasion of adjacent structures.
25
Pancreatic Cystic Lesions: The Role of EUS
Introduction
Pseudocyst
Serous lesions
Cyst type
Most common location
Gender
Solitary or multicentric
Morphology
Malignant potential
Serous cystadenoma
Head
Female
Solitary
Microcystic with honeycombing, minority are macrocystic
Very low
Mucinous cystic neoplasm
Tail
Strongly female
Solitary
Macrocystic, can be septated
High
Intraductal papillary mucinous neoplasm
Multifocal
Equal
Multicentric
Main duct: dilated duct, mixed macrocystic, microcystic
Branch duct: cluster of grapes
High
Solid pseudopapillary neoplasm
Body
Strongly female
Solitary
Encapsulated, mixed solid and cystic
Moderate
Cystic endocrine tumor
Tail
Equal
Solitary
Variable
Variable based on type 
Mucinous lesions

Other cystic neoplasms

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree