Chapter Outline
Multidetector Computed Tomography Protocols
Magnetic Resonance Imaging Protocols
Multidetector Computed Tomography Findings
Magnetic Resonance Imaging Findings
Changes in the Pancreatic Duct
Pancreatic Neuroendocrine Tumors
The World Health Organization classification of pancreatic neoplasms is remarkable for the wide variety and type. This is because benign, borderline, and malignant tumors can arise from exocrine, neuroendocrine, intraductal, and stromal elements. In addition, secondary (metastatic) neoplasms and non-neoplastic tumor-like conditions (e.g., mass-forming chronic pancreatitis) affect the pancreas. However, in clinical practice, only a few of these (adenocarcinoma, cystic neoplasms, pancreatic neuroendocrine tumors, and metastases) are encountered with any frequency. This chapter attempts to review the protocol details that are necessary to be used for patients thought to have a pancreatic neoplasm; the roles of additional imaging, such as positron emission tomography (PET) and endoscopic ultrasound (EUS); and the imaging appearances of these tumors.
Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDA) is the fourth most common cause of cancer deaths for both men and women in the United States. The American Cancer Society estimates approximately 45,000 new cases with 38,000 deaths for 2014. This is a striking increase from the 32,000 expected new cases of ductal cancers predicted in 2005; a true increase in incidence is documented between 1998 and 2008. Despite improvements in imaging, surgery, radiation, and chemotherapy and rapidly emerging molecular understanding, mortality remains high; 23% of patients will be alive at 1 year from diagnosis, whereas 4% will be alive at 5 years. Established risk factors for development of PDA include cigarette smoking, male African American, BRCA2 gene positivity, hereditary pancreatitis, cirrhosis, diabetes mellitus, chronic pancreatitis, hypercholesterolemia, obesity, Peutz-Jeghers syndrome, Lynch syndrome, vitamin D deficiency, certain occupations, and carcinogens. Common symptoms include weight loss, jaundice, floating stool, pain, dyspepsia, nausea, and depression. New-onset type 2 diabetes in patients older than 50 years may trigger investigation for PDA.
The American Joint Committee on Cancer has developed a TNM-based staging system for pancreatic adenocarcinoma ; however, because only 15% to 20% of patients are candidates for surgery at the time of diagnosis, the majority of patients will not be able to be formally assigned to a stage. For all patients who do not undergo surgery, clinical staging is solely based on imaging. The quality of imaging can directly affect the accuracy of staging; patients deemed to have unresectable tumor at non–pancreatic-directed imaging have been shown to actually be surgical candidates.
Ductal adenocarcinoma accounts for 85% to 90% of all pancreatic tumors; 60% to 70% arise in the head, 5% to 10% in the body, and 10% to 15% in the tail of the gland. Up to 22% of tumors can affect multiple regions of the gland (diffuse). By the time the tumors are discovered, they usually measure 2 to 3 cm in diameter. Pancreatic adenocarcinoma has scant cellular elements and elicits an intense desmoplastic response within the stroma surrounding the tumor. The stromal reaction encases intrapancreatic blood vessels, explaining why 90% of these masses are hypodense compared with background pancreas. The upstream main pancreatic duct may be obstructed, and the surrounding parenchyma is atrophic. Tumors in the head of the pancreas will also eventually obstruct the common bile duct. The tumor rapidly grows through lymphatics, along peripancreatic vessels, and along nerves; it may also grow along tissue planes into the surrounding duodenum and posterior wall of the stomach. Lesions in the tail of the pancreas infiltrate into the splenic hilum and into the left renal hilum. Distant metastases are most frequently found in the liver and peritoneal cavity. These can be present even in cases in which the tumor does not appear locally advanced.
The major blood vessels involved by PDA are the superior mesenteric artery, celiac axis, and branches. More frequently, the tumor will involve the superior mesenteric, splenic, and portal veins. Once the major arterial branches are involved with tumor, most surgeons agree that they are unresectable. However, experienced surgeons can achieve a negative-margin resection if the degree of contact between the tumor and the vessel is minimal. Most lymph node disease in pancreatic cancer is found in unenlarged lymph nodes ; lymphadenopathy is unusual. Most surgeons will operate on patients who have lymph node disease within the field of resection. Distant lymph node disease is a contraindication to resection.
Goals of Imaging
A high-quality imaging study is the first part of the evaluation of all patients thought to have pancreatic cancer. In most cases, multidetector computed tomography (MDCT), magnetic resonance imaging (MRI), or EUS, with needle aspiration biopsy, will serve as that procedure. Other imaging modalities, such as transabdominal ultrasound, diagnostic endoscopic retrograde cholangiopancreatography (ERCP), 18 F-fluorodeoxyglucose PET (FDG PET), and receptor (somatostatin)–specific nuclear medicine studies, are secondary procedures. Examinations such as upper gastrointestinal series, hypotonic duodenography, and selective catheter angiography no longer have any role in the diagnostic work-up.
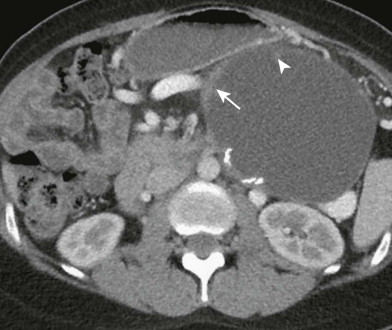
The choice of imaging study must take into account safety, patient comfort, and affordability combined with a sufficient sensitivity, specificity, and accuracy. The protocol for the imaging test is designed to detect the presence of a pancreatic neoplasm and to determine whether an individual patient has truly resectable disease. Timing of the imaging acquisition must be optimized to maximize lesion-to-background pancreas contrast differences, to evaluate the integrity of major peripancreatic arteries and veins, and to detect extrapancreatic metastases ( Fig. 98-1 ). There is a high level of agreement that high-quality MDCT using a multiphase acquisition protocol is the procedure of choice for these patients.

Multidetector Computed Tomography Protocols
Image data from MDCT examination is collected by use of the narrowest detector configuration. From the data acquisition, two sets of image data are reconstructed. The first set is reconstructed at familiar CT slice widths (3 or 4 mm); these images are sent to a picture archiving and communications system (PACS) or film. The second set is reconstructed at the thinnest possible slice width as determined by the detector (e.g., 0.75-mm sections from a 0.6-mm detector configuration). These isotropic data sets are sent to a three-dimensional (3D) workstation, allowing multiplanar reformatting, 3D volume rendering, CT angiography, and CT cholangiopancreatography (CTCP). Utility of 3D imaging is enhanced by the use of neutral (close to water density) oral contrast material.
Proper delivery of intravenous contrast material and timing of the acquisition are critical for successful pancreatic CT. A low-osmolality intravenous contrast agent with high iodine concentration (370 mg I/mL) at a dose of 1.5 mg I/kg provides optimal pancreatic parenchymal and hepatic enhancement. The contrast agent should be administered by power injector at a minimum rate of 3 mL but preferably between 4 and 5 mL/s. All investigators use a dual acquisition protocol. The first image set is acquired during the pancreatic phase (approximately 40 to 50 seconds), and a second image set is acquired during the portal venous phase (70 to 80 seconds) ( Fig. 98-1 ). Some advocate a three-phase protocol that includes an unenhanced acquisition; in our practice, we incorporate an unenhanced acquisition only when pancreatic neuroendocrine tumor is suspected.
Dual-energy CT acquisitions have the advantage of allowing the radiologist to view images acquired at two different energies (kVp). Viewing the images acquired at the lower energy or by simulating the contrast that might have been acquired at a lower beam energy (monoenergetic imaging) enhances tissue contrast by maximizing photoelectric interactions. This results in an increase in the attenuation of background pancreas with resultant increase in lesion-to-background conspicuity.
Magnetic Resonance Imaging Protocols
A comprehensive (“one-stop shopping”) MRI evaluation of the pancreas and biliary tree can be performed by use of multiple acquisitions at multiple pulse sequences. In our practice, a pancreas-dedicated MRI evaluation includes an axial fat-suppressed fast (turbo) T2 sequence; two-dimensional (2D) axial in-phase and opposed-phase noncontrast T1-weighted sequences; axial and coronal single-shot fast spin-echo (HASTE or spoiled GRASS) T2-weighted acquisition; and multiphasic gadolinium-enhanced, 3D T1-weighted gradient recalled acquisition with frequency selected fat suppression (VIBE) sequences during the phase of pancreatic enhancement and the portal phase of hepatic enhancement. Current MRI scanners can obtain diffusion-weighted sequences; however, these have been shown not to be reliable for pancreatic adenocarcinoma.
Magnetic resonance cholangiopancreatography (MRCP) is also performed with a heavily weighted T2 acquisition either in 2D with thick slab or 3D respiratory-triggered acquisition. The 2D pancreaticobiliary duct evaluation can also be obtained from the HASTE acquisitions.
Multidetector Computed Tomography Findings
PDA appears as a hypodense mass with poorly defined borders compared with the background parenchyma during the pancreatic phase. With current MDCT technology, non–contour-deforming tumors will be frequently encountered, underscoring the critical importance of imaging the pancreas during the phase of maximal background pancreatic enhancement, in which the tumor-to-gland attenuation difference is greatest. In 11% of cases, pancreatic adenocarcinoma may be isoattenuating ( Fig. 98-2 ). Secondary findings include dilation of the upstream pancreatic duct and dilation of the common bile duct when the tumor is located within the pancreatic head. Use of curved multiplanar reformatting or 3D volume rendering can improve detection of ductal dilation.

CT findings of major arterial encasement include obliteration of the normal fat between the pancreatic margin and the adjacent vessel; more than 180-degree contact between the tumor and the vessel; and morphologic changes in the artery, including narrowing or encasement of the affected artery. With 3D volume rendering, images of the pancreatic arterial supply can be created that rival conventional catheter angiography, making recognition of the vascular changes straightforward ( Fig. 98-2 ). CT angiographic images have been shown to be more accurate for detection of arterial involvement from pancreatic adenocarcinoma than by looking at traditional axial slices alone ( Fig. 98-3 ).

With improving MDCT technology, there is increasing appreciation of local spread of PDA before it actually encases major vessels. Predictable pathways for perilymphatic and perineural tumor infiltration have been correlated with detailed anatomic dissections. High-quality thin-section imaging is needed to view these changes. When spread along these pathways is suspected, the surgeon should be alerted, and neoadjuvant therapy may be appropriate before attempt at resection.
Criteria for venous invasion include more than 180-degree contact with a soft tissue mass and the vein. When the superior mesenteric vein is involved with tumor, it may display a “teardrop” configuration ( Fig. 98-4 ). Although soft tissue contact with the venous system has a high predictive value for nonresectability, significant involvement of the vein may be found at surgery when the imaging study fails to reveal any direct contact with the vein. It is therefore critical to evaluate the presence and pattern of collateral venous channels surrounding the pancreatic head. In advanced cases, collateral venous channels are easily recognized. Common collateral channels include prominent short gastric varices, gastrohepatic ligament varices, and gastroepiploic to gastrocolic trunk. It is important to look for the small posterior pancreaticoduodenal veins; when these collaterals are present, there is a high likelihood that the tumor has involved the superior mesenteric vein to a degree that would preclude the ability to obtain a negative tumor margin ( Fig. 98-4 ).

Magnetic Resonance Imaging Findings
The appearance of the pancreatic mass will depend on the acquisition sequence used ( Fig. 98-5 ). As outlined in the preceding section, a multisequence approach is used for a comprehensive evaluation. Because the pancreatic mass elicits a dense desmoplastic stromal response, most masses will have a shorter relaxation time than the surrounding normal parenchyma and therefore appear as a “dark” region. Contrast-enhanced pancreatic phase T1-weighted sequences are most effective in delineating the mass. Fat suppression will increase the conspicuity of the mass by significantly decreasing the signal from intraperitoneal and retroperitoneal fat, thereby increasing the signal from the pancreas, heightening contrast between normal parenchyma, the mass, and the pancreatic duct. PDA is hypointense compared with background pancreatic parenchyma, similar to CT findings. Liver metastases are best seen against a densely enhanced liver as occurs in the portal venous phase. Metastases possess a more rapid relaxation time than the background liver on both T1- and T2-weighted sequences and therefore will appear relatively bright. Detection of liver metastases is improved when diffusion-weighted sequences are obtained in addition to contrast-enhanced acquisitions.

Because the gadolinium-enhanced imaging sequences are acquired as a 3D volume, the arterial and venous anatomy can be displayed as MR angiographic images with imaging features of vascular involvement and tumor extension similar to those displayed on MDCT. Familiar 3D techniques, such as maximum intensity projection and volume rendering, can be used to create displays of the pathologic process in any useful plane.
Endoscopic Ultrasound
The accuracy of EUS in the diagnosis of pancreatic and periampullary neoplasm is well established. However, with rapid improvement in both MDCT and MRI, EUS use is predominantly complementary to cross-sectional techniques and for case-by-case problem solving. EUS is an excellent guide for fine-needle aspiration biopsy, with positive results in 75% of cases. It has been shown that there is a statistically significant lower chance for development of peritoneal carcinomatosis after EUS biopsy as opposed to percutaneous fine-needle aspiration biopsy.
Accuracies of Multidetector Computed Tomography, Magnetic Resonance Imaging, and Endoscopic Ultrasound
There is relatively uniform consensus at the time of this writing that MDCT ranges in overall accuracy between 86% and 99%. The positive predictive value of CT for predicting resectability ranges between 45% and 79% because the criteria for diagnosis of vascular invasion favor specificity over sensitivity. However, there has been overall improvement coincident with improving technology. This trend has continued; high-quality MDCT restaged 81 of 88 patients considered unresectable to resectable, with 94% having R0 resections. Centers that see a high volume of pancreatic cancer patients will obtain a repeated pancreas-dedicated CT study if outside imaging is considered inadequate.
Reported accuracy for MDCT in detection of arterial involvement is reported as high as 99%, with a negative predictive value of 100%. Confirmation of venous involvement is best assessed on hepatic phase images, and the negative predictive value approaches 100%. Detection of lymph node metastasis is limited. With use of a short-axis diameter of more than 10 mm as the criterion for nodal involvement, CT has a reported sensitivity of 14%, a specificity of 85%, a negative predictive value of 82%, and an overall accuracy of 73%.
Reported accuracies for high-quality multiacquisition MRI range between 76% and 89%. A meta-analysis of eight studies found the pooled sensitivity, specificity, and positive and negative likelihood ratio to be identical for CT and MRI for diagnosis of vascular invasion. Some centers find the improved contrast sensitivity of MRI over CT useful for planning radiation therapy (see Fig. 98-5 ).
The most frequent cause of understaging of pancreatic adenocarcinoma is the failure of the detection of small metastases in the liver and peritoneal cavity. Imaging is poor in the detection of these small lesions. Small peritoneal implants may be present without ascites or other more flagrant signs of peritoneal carcinomatosis, making them extremely difficult to detect. A combination of CT scanning supplemented by laparoscopic ultrasound immediately before surgery in those patients deemed to have resectable tumor is an imaging strategy that has accuracy as high as that of more expensive tests, with preservation of the highest levels of quality-adjusted life-years. In most clinical practice, MDCT is the primary imaging modality, with MRI reserved for patients with allergy to contrast material. EUS is used in cases in which ductal obstruction is visualized without a mass visible on the cross-sectional study and is the best method for obtaining tissue to confirm malignant disease.
With reported successes of newer neoadjuvant treatment options and increasing skill in surgical removal of tumor, consistent detailed reporting that specifically addresses each of the components of the status of a given tumor around which appropriate therapeutic decisions are made is desirable. Such reports guarantee that the examination has been adequately performed and carefully reviewed. Furthermore, these reports can serve as a record by which institutional accuracy in image interpretation can be measured as well as provide a uniform database for larger interinstitutional trials. The Society of Abdominal Radiology and the American Pancreatic Association called together a panel of experts in imaging, gastroenterology, and surgery to create a template for reporting of imaging findings in patients with pancreatic adenocarcinoma. The results have been published in both the radiology and gastroenterology literatures.
Changes in the Pancreatic Duct
The main pancreatic duct is routinely visualized on MRCP performed with most current MR systems in clinical use. Rapid T2-weighted sequences and respiratory-gated long T2 3D sequences can show the normal duct in almost all cases. The duct can be visualized on high-quality pancreatic phase MDCT and with moderate frequency during the portal phase. Minimum intensity projections and curved multiplanar reformatting facilitate recognition of the duct.
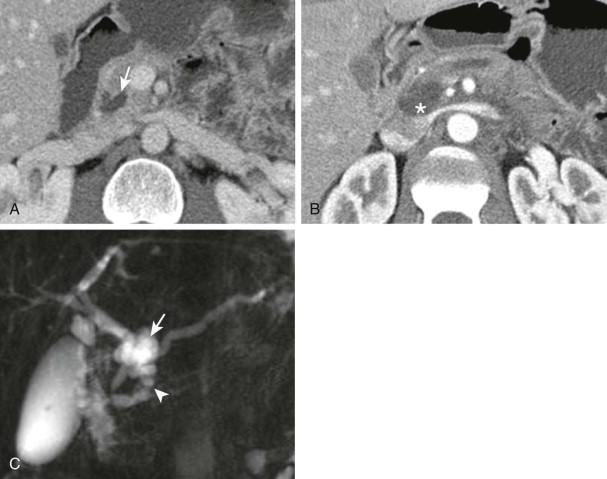
When this finding is observed on an imaging study, patients should undergo an intensive work-up to exclude malignant disease as the cause ( Fig. 98-6 ). In one series, 71 of 86 isolated duct strictures were due to a malignant disease. Pancreatic adenocarcinoma and neuroendocrine tumor are the most common tumor causes. Differential diagnosis includes main duct intraductal papillary mucinous neoplasm. When the entire duct is dilated, chronic pancreatitis cannot be excluded.

Isolated duct strictures may predate the typical imaging appearance of PDA and can be thought of as a sign of “early” pancreatic cancer. Several studies have documented that retrospective review of prior imaging studies in patients presenting with PDA will reveal abnormalities in the pancreas in nearly half of patients, the most common being segmental dilation of the main pancreatic duct. This finding can predate the clinical appearance of pancreatic neoplasm by as long as 18 months. In our practice, when we detect segmental pancreatic duct obstruction, the patient will be referred for EUS and biopsy; the high sensitivity and specificity for diagnosis of the underlying neoplasm are validated in the literature. Small cysts (>5 mm) associated with a dilated pancreatic duct appear to be a predictor of future development of PDA.
Other Imaging Modalities
Several other imaging modalities are in current use for imaging of pancreatic adenocarcinoma, including transabdominal ultrasound, ERCP, and FDG PET. Several reports have shown promise of FDG PET scanning to detect pancreatic cancer ; however, with improved MDCT and MRI balanced against the cost, most believe that PET-CT is complementary and best used on a case-by-case basis.
Transabdominal ultrasound will often be used in jaundiced patients to rapidly identify a dilated biliary tree. Whereas a dilated intrahepatic biliary tree is easily demonstrated, the specific cause of extrahepatic bile duct dilation is frequently difficult to establish. The most frequent reason for this is bowel gas obscuring the region of the pancreatic head and common bile duct. In selected patients, however, careful transabdominal technique can demonstrate the echogenic mass obstructing either the pancreatic or common bile duct. Contrast-enhanced transabdominal ultrasound has become an established technique to detect pancreatic lesions and to measure organ perfusion.
ERCP is often used preoperatively to place a drainage stent within an obstructed common bile duct. Several studies have shown that this preoperative stent placement should not be performed before surgical resection as there is a clear increase in the complication rate. Most leading pancreatic surgeons will request common bile duct stenting when surgery will be delayed more than 14 days after diagnostic evaluation. Expandable metallic endoscopically placed endobiliary stents should be reserved for those patients with unresectable disease needing palliation.
Cystic Neoplasms
Cystic pancreatic neoplasms are frequently encountered on imaging studies. Despite a large accumulating clinical and imaging experience with these lesions, their behavior in individual patients is difficult to predict. Therefore, when they are detected on an imaging study, there will be uncertainty about the diagnosis and varying recommendations for follow-up or treatment despite the fact that there has been improving ability to correctly characterize individual lesions on imaging studies.
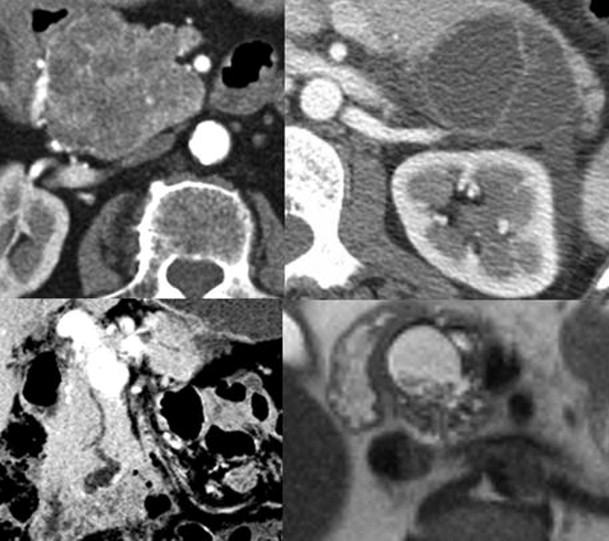
Cystic pancreatic neoplasms can be divided into four distinct categories: serous cystadenoma (SCA); mucinous cystic neoplasm; intraductal papillary mucinous neoplasm (IPMN); and “other” ( Fig. 98-7 ). This classification helps in focusing of the imaging search for diagnostic features that allow differentiation of benign from malignant lesions, paralleling the management that is distinct within each category.
Serous Cystadenoma
SCA accounts for approximately 1% to 2% of all exocrine pancreatic neoplasms. The World Health Organization classification of pancreatic tumors considers SCA a benign lesion. At the time of diagnosis, 25% to 50% of patients will be symptomatic. The tumor occurs more frequently in women (mean age of 57 years) and most often in the pancreatic head. The tumor contains glycogen-rich periodic acid–Schiff–positive epithelial cells delimiting cysts separated by variable amounts of fibrous septations. They most frequently occur sporadically; 60% to 80% of patients with von Hippel–Lindau syndrome will have pancreatic SCA.
SCA has two morphologic appearances: the microcystic or classic type, and the macrocystic or oligocystic type. The microcystic form, accounting for two thirds of SCAs, is a spongelike lesion formed from innumerable cysts containing clear watery fluid. The cysts range from 1 to 5 mm in the center of the mass; larger cysts (up to 2 cm) are present in the periphery. A central fibrous stellate nidus is present giving rise to radially oriented fibrous bands. This central nidus frequently calcifies. The oligocystic type has scant locules or can be unilocular. There is no central scar; the fluid may be clear but often is hemorrhagic. Because of the recognition of the oligocystic type of SCA, the term microcystic serous cystadenoma is no longer used to describe these lesions.
The imaging features of SCA reflect the morphologic appearance of the mass. The classic variety is manifested as a solitary mass that displays central calcification and a radial arrangement of dense fibrous tissue delimiting a variable number of cysts ( Fig. 98-8 ). In some tumors, the cysts are so small and the fibrous component so dense that the lesion may actually appear “solid.” As one might imagine, on MDCT, the cysts are near water density, and the surrounding fibrous network appears dense; on MRI, the appearance is dependent on the pulse sequence of the acquisition. Central calcification establishes the diagnosis of SCA, but it is present, at most, in 30% of cases, best detected by MDCT. The tumors may encase vessels and obstruct the pancreatic or biliary duct system. Nevertheless, even these seemingly aggressive tumors may remain indolent.
On T1-weighted fat-suppressed sequences, the fluid component is darker than the fibrous matrix; on T2-weighted acquisitions, the fluid component becomes more conspicuous, appearing bright because of the longer T2 relaxation time. EUS may be particularly useful in displaying the honeycombed internal structure in lesions smaller than 2 cm. EUS provides an excellent means by which to sample cyst fluid.
The oligocystic variety can be suspected when there is a unilocular nonenhancing cystic mass in the pancreatic head with a lobulated contour. Despite the high reported specificity of this constellation of findings, differentiation of this lesion from mucinous cystic tumor or pseudocyst may be impossible.
Mucinous Cystic Tumor
These rare neoplasms are thought to be potentially malignant; therefore, the terms mucinous cystadenoma and mucinous cystadenocarcinoma should not be used; rather, the lesion is properly referred to as mucinous cystic tumor. Mucinous cystic tumors are formed from variably atypical epithelial cells that produce mucin and are supported by an ovarian-type stroma, which does not communicate with the pancreatic duct system. They account for 2% to 6% of all exocrine pancreatic neoplasms. These tumors occur almost exclusively in women, with peak incidence in the fifth decade. The female preponderance is similar to that of mucinous tumors of the hepatobiliary system, retroperitoneum, and ovary. The stromal component of the mucinous cystic tumor stains for cytokeratin markers, indicative of cellular luteinization, the ovarian-type stroma characteristic of these lesions. It is important to remember this fact because the diagnosis of mucinous cystic tumor should probably not be considered in a male patient.
At MDCT, the lesions will display large cysts with thin septa, best seen after intravenous administration of contrast material. When calcification occurs, it is lamellated (as opposed to the starburst pattern seen in SCA) and in the periphery of the lesion (as opposed to the central location of calcification in SCA). Lesions with a higher degree of epithelial atypia will display nodules on the wall, peripheral calcification, and a more disorganized internal architecture ( Fig. 98-9 ). Malignant lesions tend to be larger than benign lesions. On MRI, the lesion will appear bright on T2-weighted sequences. On T1-weighted sequences, intravenous administration of gadolinium is necessary to image the septations, which become more apparent the longer the imaging sequence is carried out ( Fig. 98-10 ). Mucin itself can produce decreased signal within the center of the lesion that should not be confused with the radiating septa seen in SCA. On EUS, the mural nodularity is easily recognized and can be differentiated from the honeycombed appearance of SCA. EUS is particularly valuable for aspiration of cyst fluid. Presence of carcinoembryonic antigen in aspirated cyst fluid has a high predictive value for mucinous cystic tumor.