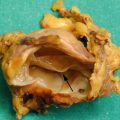
Fig. 1
IPMN branch-duct type: morphology. (a) Surgical specimen (pancreaticoduodenectomy, particular): uncinate process of the pancreas with a small multicystic grape-like lesion (arrow) involving the branch ducts. (b) Surgical specimen (pancreaticoduodenectomy): unicystic lesion (arrow) involving the branch duct filled by thick tenacious mucoid material. Probe in the common bile duct (Courtesy of Piccin Editore, Milan, Italy)
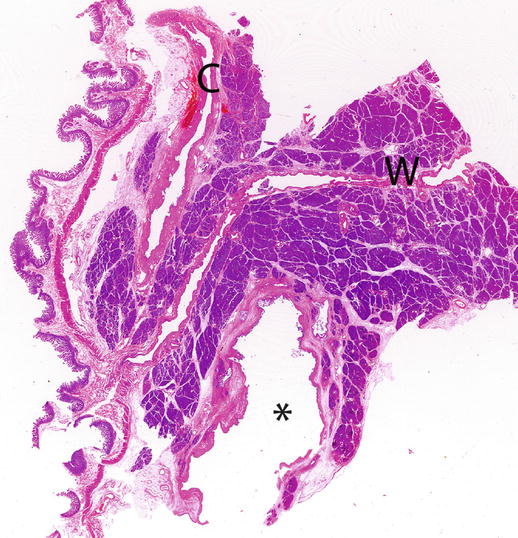
Fig. 2
IPMN branch-duct type: site. Whole mount macrosection, surgical specimen (pancreaticoduodenectomy): small unicystic BD-IPMN (asterisk) involving the uncinate process of the pancreas. Wirsung duct (W). Common bile duct (C)
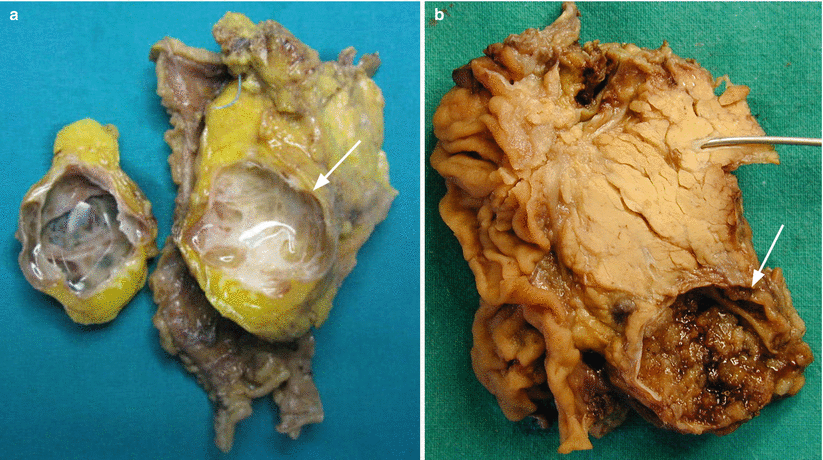
Fig. 3
IPMN branch-duct type: content. (a) Surgical specimen (pancreaticoduodenectomy): unicystic lesion (arrow) with viscous translucent fluid content. (b) Surgical specimen (pancreaticoduodenectomy): unicystic lesion (arrow) with solid content in the form of papillary vegetations. Probe in the Wirsung duct
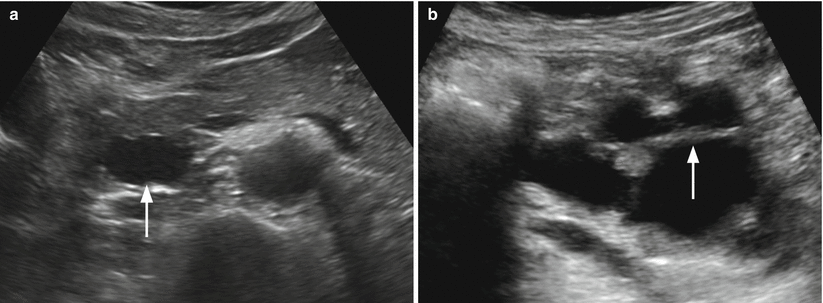
Fig. 4
IPMN branch-duct type. (a) Ultrasound study: small unicystic lesion (arrow) in the uncinate process of the pancreas. (b) Ultrasound study: multicystic lesion with thick septa (arrow) and lobulated contours
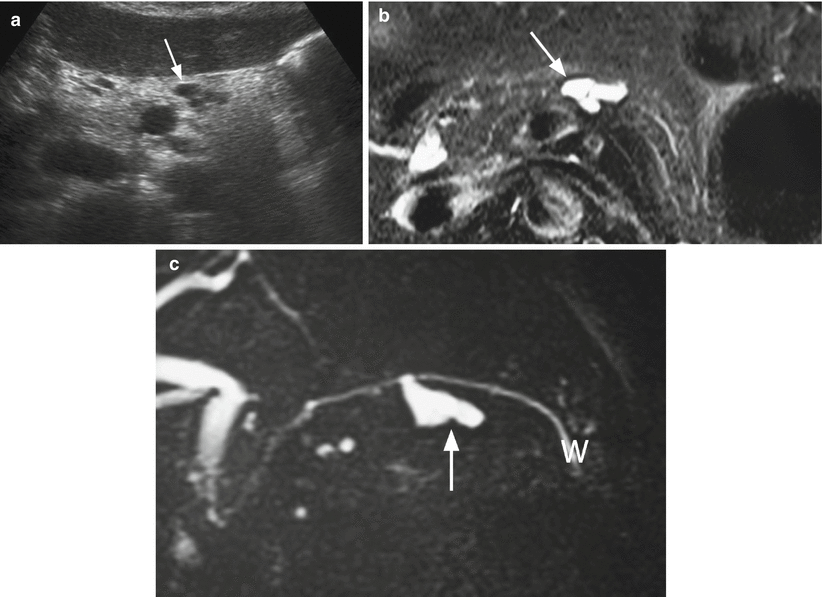
Fig. 5
IPMN branch-duct type: multicystic appearance. (a) Ultrasound study: multicystic branch-duct IPMN (arrow). (b, c) MRI study: branch-duct IPMN with typical cystic hyperintense appearance on T2-weighted images (b) and lobulated contours (arrow in b). On MRCP (c), the cystic lesion (arrow in c) is connected with the main pancreatic duct (W in c)
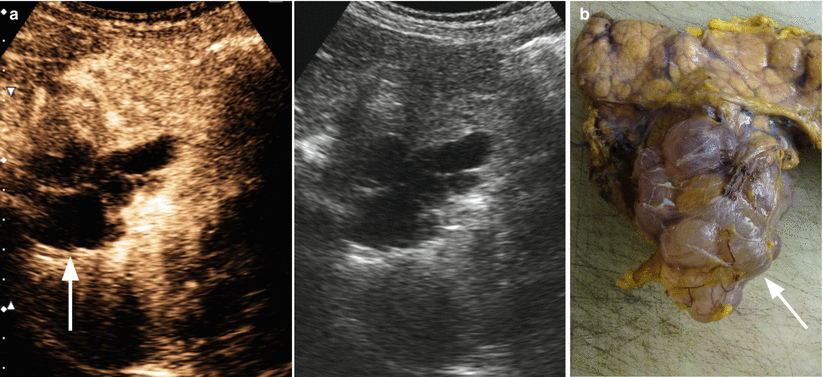
Fig. 6
IPMN branch-duct type. (a) CEUS study: pancreatic body–tail small multicystic lesion with lobulated contours (arrow) and multiple vascularized septa. (b) Surgical specimen (distal pancreatectomy): small multicystic grape-like lesion (arrow) with lobulated contours bulging the pancreatic profile
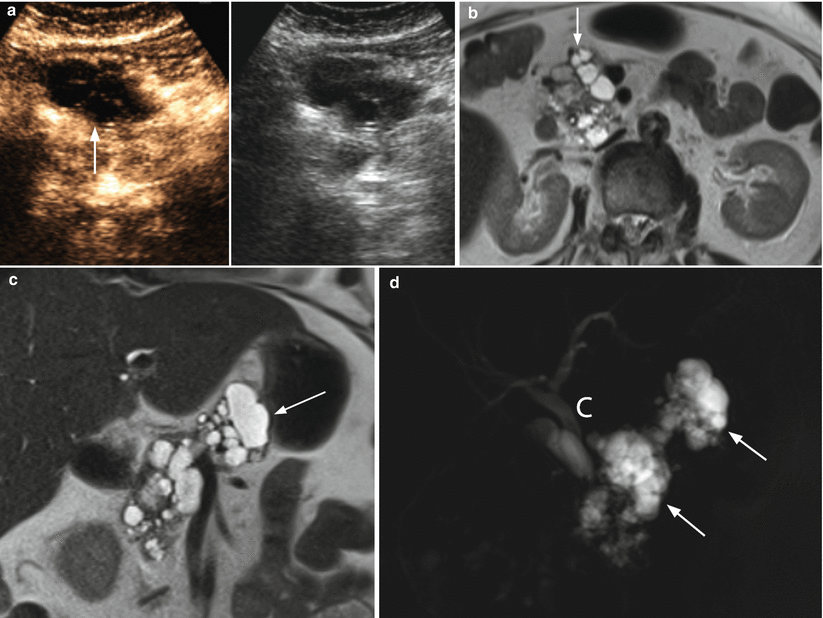
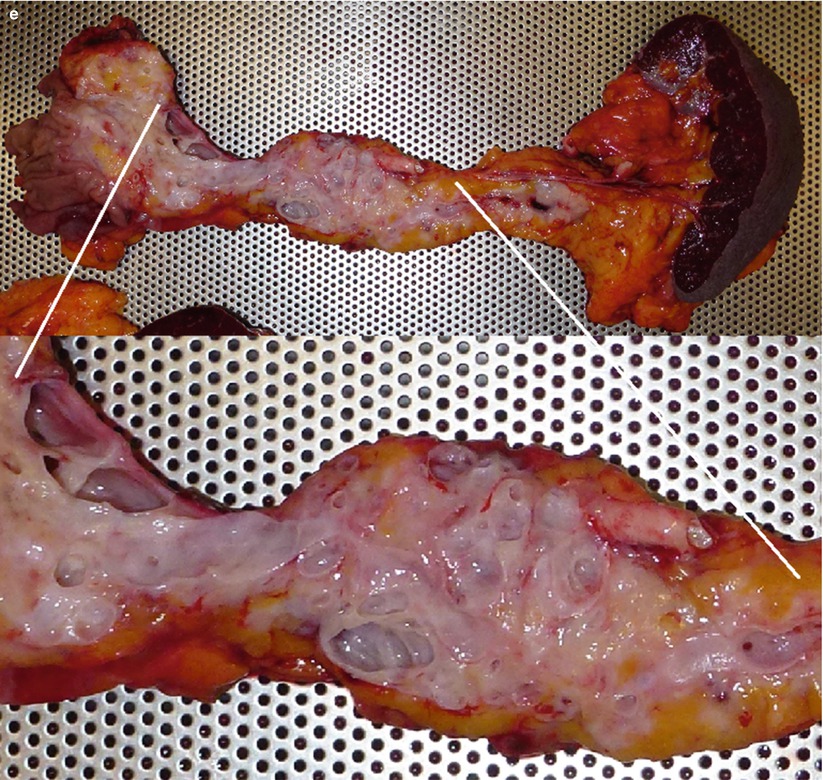
Fig. 7
IPMN mixed type. (a) CEUS study: pancreatic head multicystic lesion with lobulated contours (arrow) and multiple thin centrally orientated vascularized septa. (b–d) MRI study: axial T2-weighted (b) images show pancreatic head complex multicystic lesion with lobulated contours (arrow in b) and multiple thin septa. Coronal T2-weighted (c) images show also a complex multicystic lesion with lobulated contours (arrow in c) in the body of the pancreas with a dilated Wirsung duct in between. On MRCP (d) the two lesions (arrows in d) with dilated Wirsung duct in between are perfectly shown. Initial dilation of the common bile duct (C in d) is also appreciable. (e) Surgical specimen (total pancreatectomy): multifocal IPMN; multiple cystic lesions are visible on the cut section (zoomed particular)
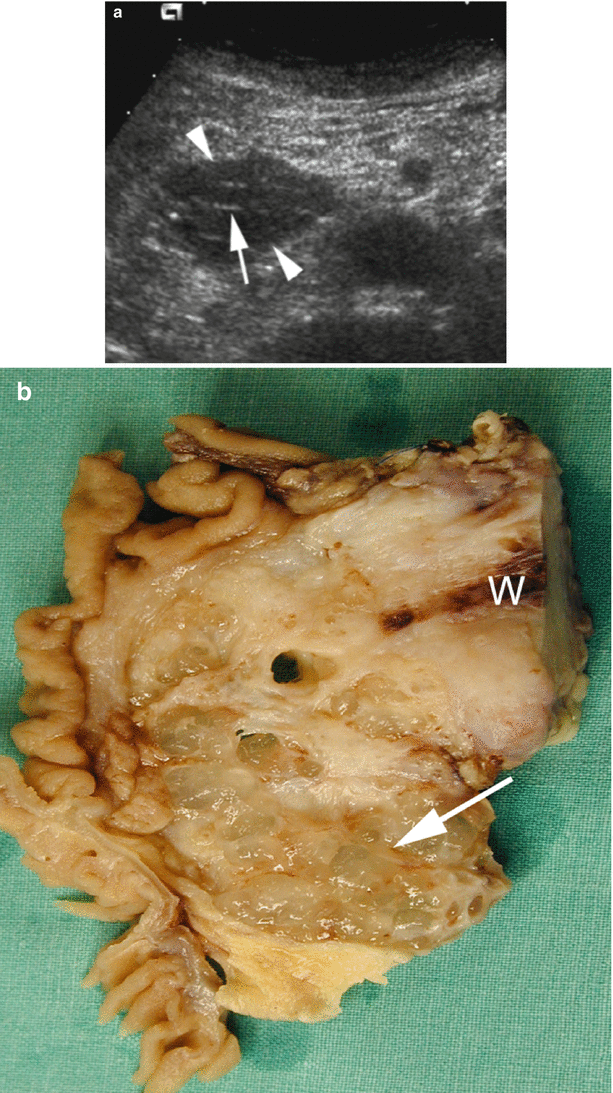
Fig. 8
IPMN with invasive carcinoma. (a) Ultrasound study: pancreatic hypoechoic mass (arrowheads) in the uncinate process of the pancreas with very thin hyperechoic strings (arrow). (b) Surgical specimen (pancreaticoduodenectomy): mucoid material dissociates the uncinate process of the pancreas. Residual fibrous pancreatic parenchyma (arrow) is present. Wirsung duct (W)
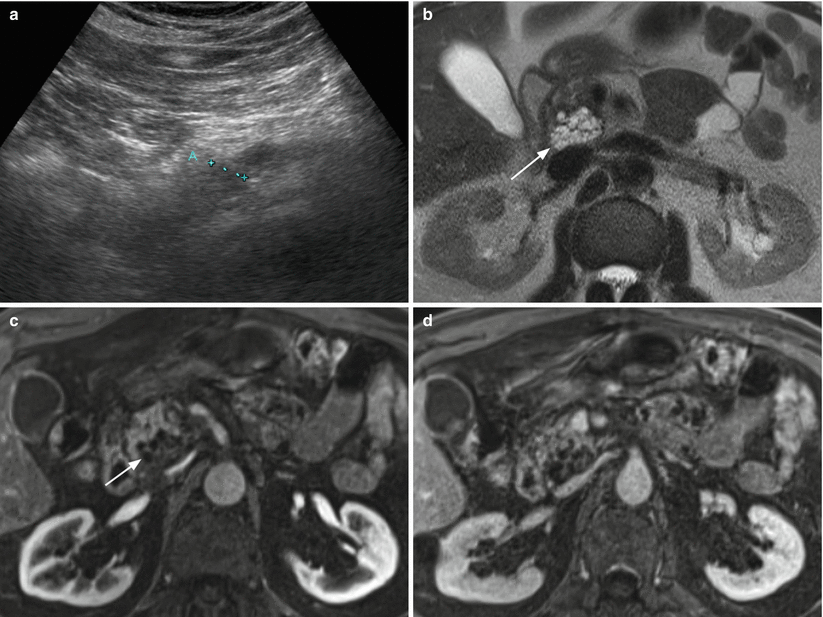
Fig. 9
IPMN branch-duct type. (a) Ultrasound study: pancreatic hypoechoic small nodule (calipers). (b–d) MRI study: branch-duct IPMN with typical multicystic hyperintense appearance on T2-weighted images (b) and lobulated contours (arrow in b). On dynamic phases (c, d), multiple very thin vascularized septa (arrow in c) are detectable delimitating the small lesion cystic cavity
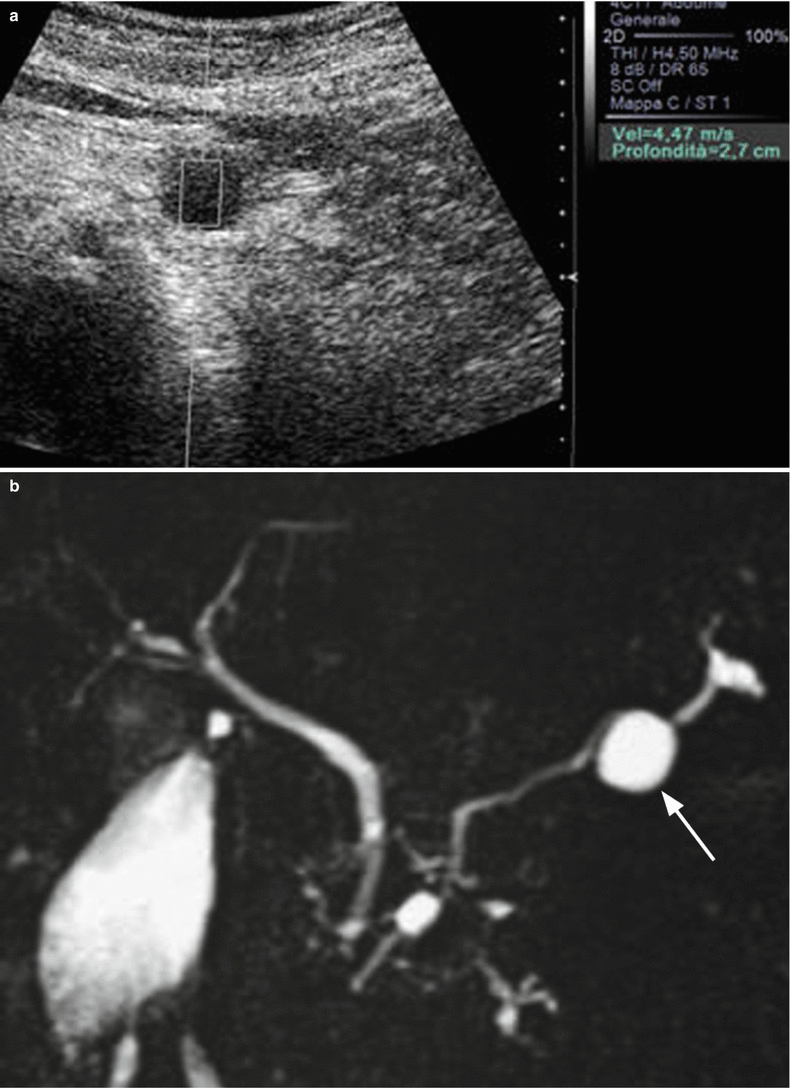
Fig. 10
IPMN branch-duct type. (a) Ultrasound study: unique unicystic lesion (ROI) at ultrasound studied by means of acoustic radiation force impulse ultrasound. (b) MRCP study: the cystic lesion (arrow) is confirmed, but other cystic lesions are visible connected with the main pancreatic duct in the pancreatic tail and in the pancreatic body
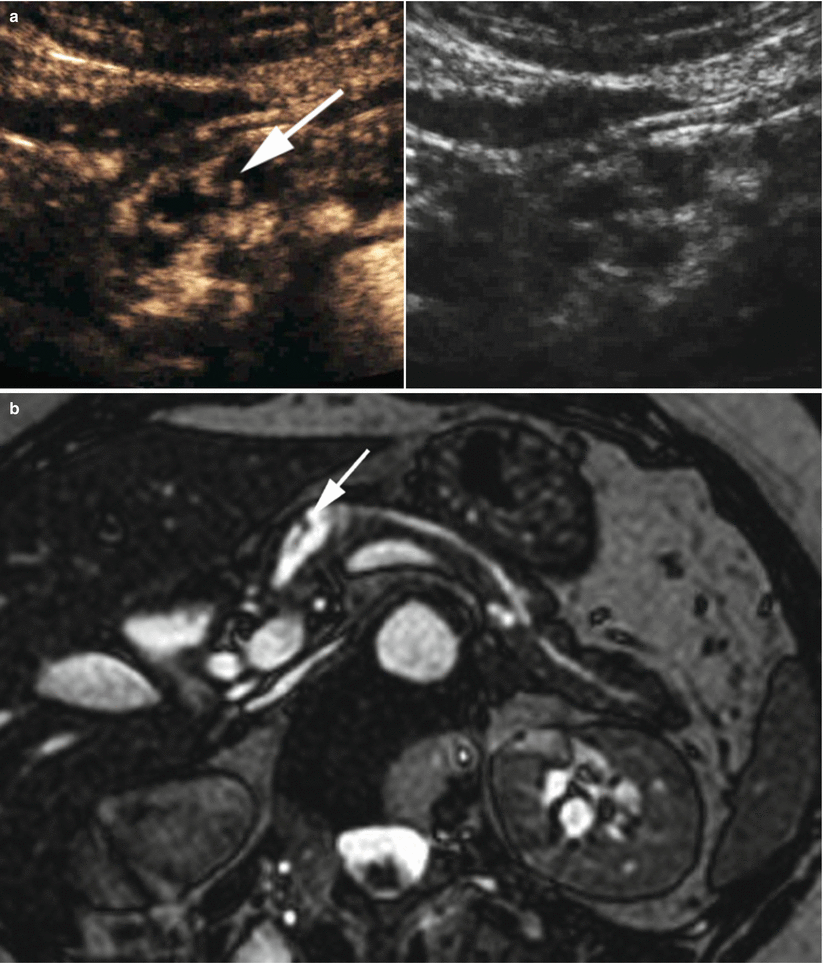
Fig. 11
IPMN branch-duct type: content. (a) CEUS study: unicystic lesion with elongated morphology and vascularized hyperechoic intralesional papillary vegetation (arrow) during the dynamic phases. (b) MRI study: the cystic lesion is confirmed with typical hyperintense appearance on T2-weighted images and with clear vegetation defect (arrow) in the lumen
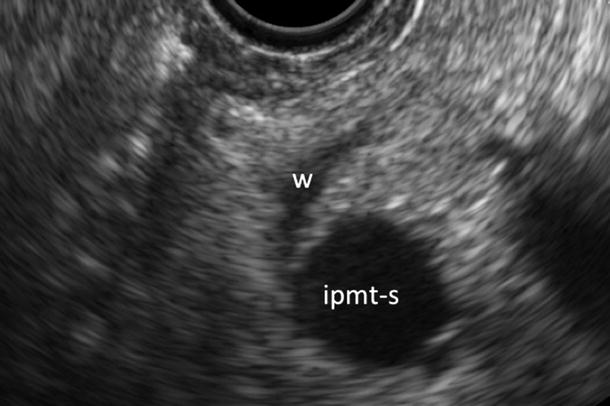
Fig. 12
IPMN branch-duct type. EUS study: small cystic lesion (ipmt-s) of the pancreatic isthmus with a normal sized Wirsung duct (W)
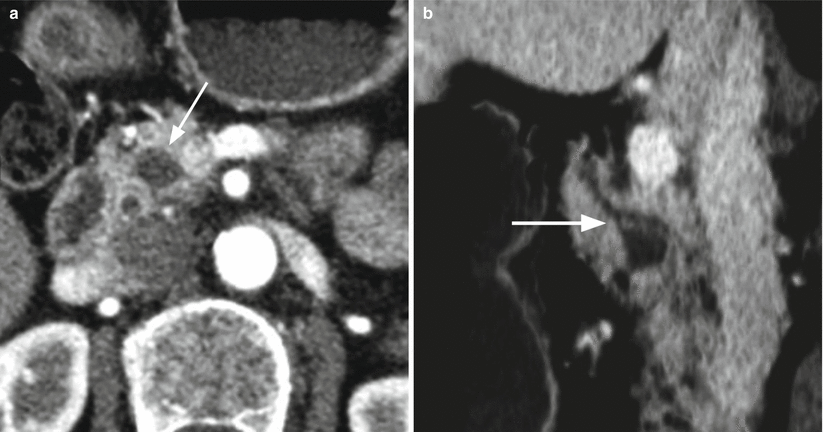
Fig. 13
IPMN branch-duct type. (a, b) CT study: on dynamic phase (a) a small cystic hypodense lesion is visible (arrow in a). On minimum intensity projection reconstructions (b), a connection (arrow in b) between the cystic lesion and the main pancreatic duct is clearly visible
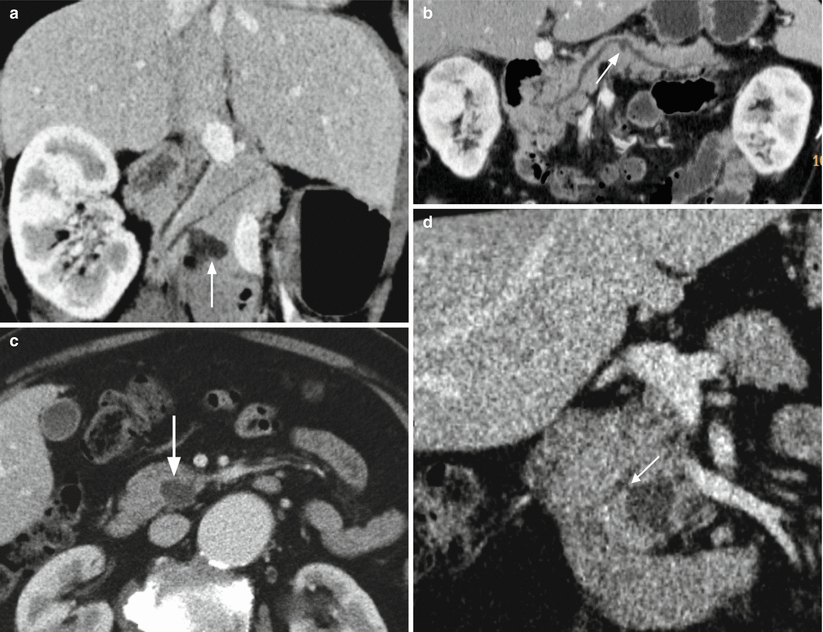
Fig. 14
IPMN branch-duct type. (a) CT study: minimum intensity projection reconstructions showing a cystic lesion with lobulated contours (arrow) connected with the main pancreatic duct. (b) CT study: minimum intensity projection reconstructions showing a small cystic lesion (arrow) connected with the main pancreatic duct. (c, d) CT study: in the dynamic phase (c), a small cystic hypodense lesion is visible (arrow in c) in the uncinate process of the pancreas. On minimum intensity projection reconstructions (d), a connection (arrow in d) between the cystic lesion and the main pancreatic duct is visible
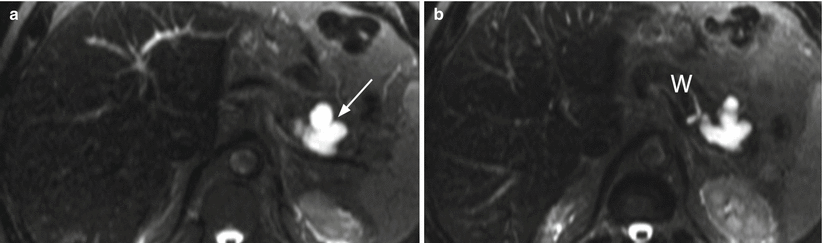
Fig. 15
IPMN branch-duct type. (a, b) MRI study: branch-duct IPMN with typical cystic hyperintense appearance on T2-weighted images (a) and lobulated contours (arrow in a). On T2-weighted more caudal axial image (b), the lesion is clearly in connection with the main pancreatic duct (W in b)
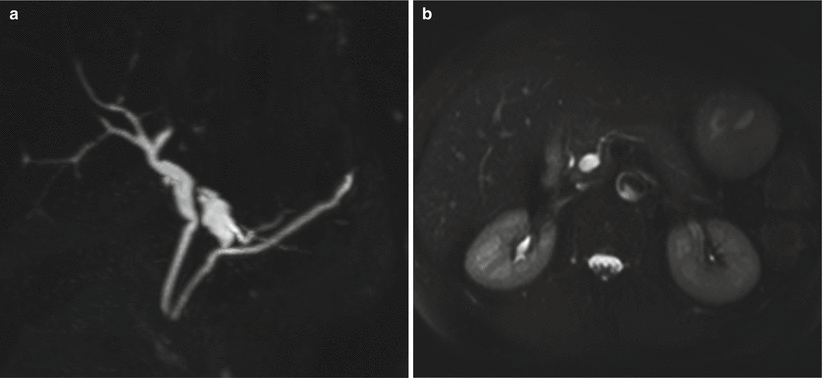
Fig. 16
IPMN branch-duct type. (a, b) MRCP (a) and T2-weighted HASTE (b). A monofocal unilocular finger-like dilation of a branch duct is visible in the neck of the pancreas
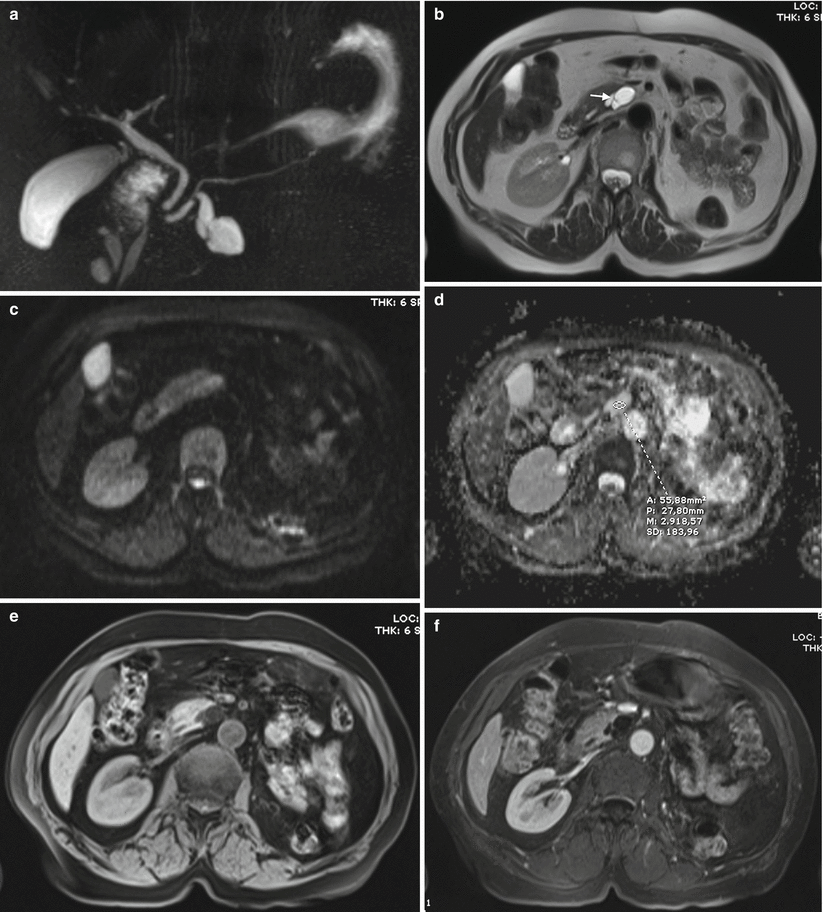
Fig. 17
IPMN branch-duct type. (a–f) MRI study: a monofocal multilocular dilation of a branch duct in the uncinate process is appreciated with MRCP (a). The main pancreatic duct downstream is slightly dilated. With T2 HASTE (b), no evidence of solid nodules. Thin septa are visible (white arrow in b). With DWI, at b 600 (c), no evidence of solid nodules. With ADC map (d), the signal intensity is high (n = 2.9). With GE T1-weighted fat-sat (e), the septa are slightly visible. With Gd-enhanced MRI (f), no pathological enhancement of solid nodules is appreciable; slight enhancement of the septa. At surgical resection, no involvement of main pancreatic duct was observed, whose dilation was due to the overproduction of mucin by the lesion in the branch duct
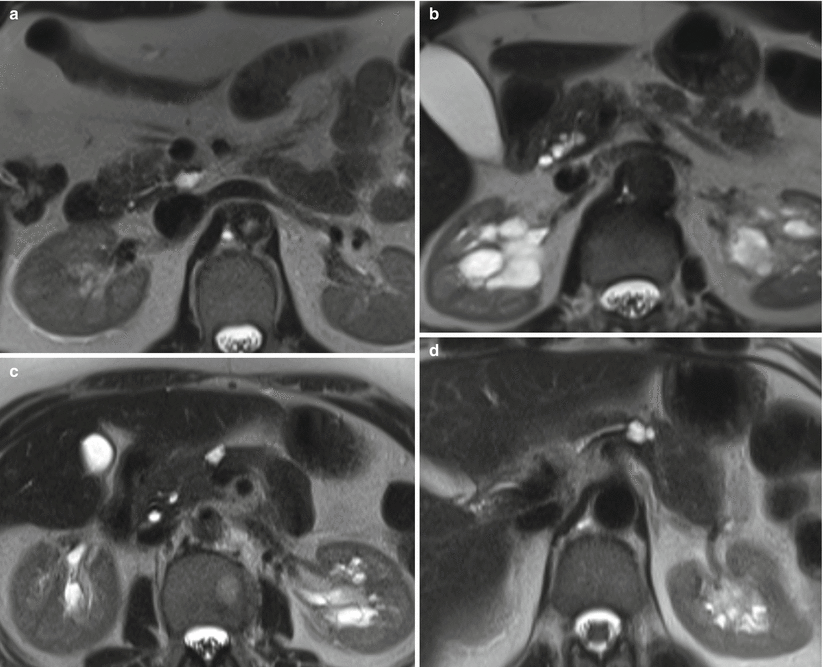
Fig. 18
IPMN branch-duct type: ubiquity. (a) MRI study: small branch-duct IPMN in the uncinate process of the pancreas. (b) MRI study: small branch-duct IPMN in the pancreatic head. (c) MRI study: small branch-duct IPMN in the isthmus of the pancreas. (d) MRI study: small branch-duct IPMN in the body of the pancreas
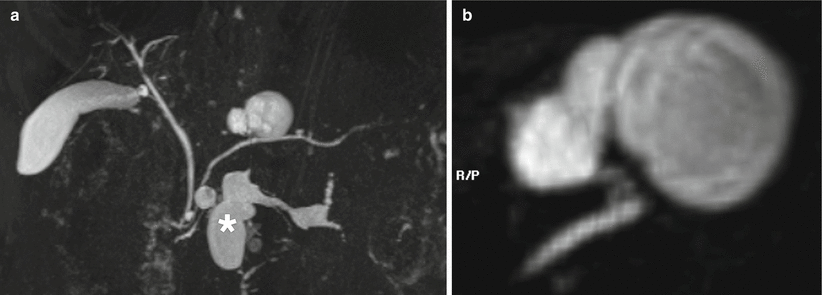
Fig. 19
IPMN branch-duct type. (a, b) MRI study: large cystic lesion very close to the main pancreatic duct is appreciated in the body of the pancreas at MRCP (a). Dilation of cisterna chyli (* in a) is appreciable. With sub-MIP reconstruction (b), the communication between the cystic lesion and the main pancreatic duct is clearly visible
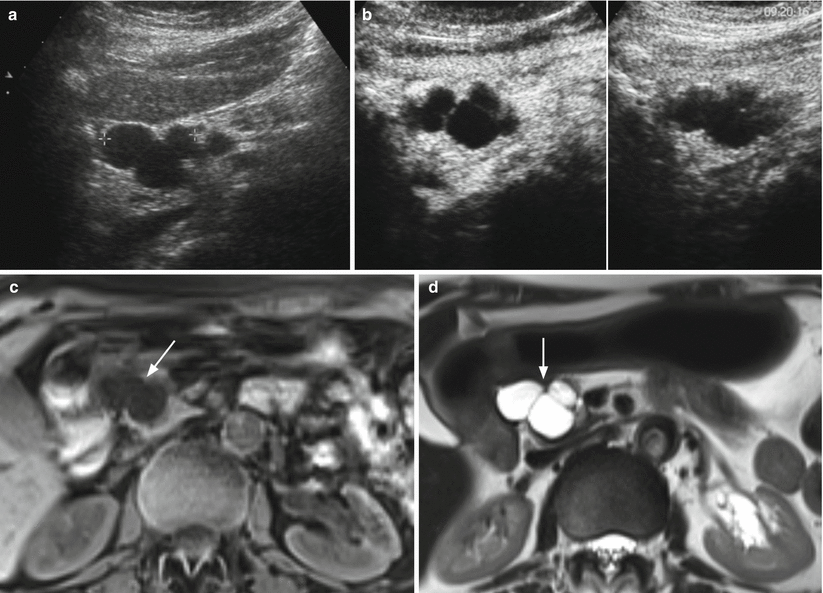
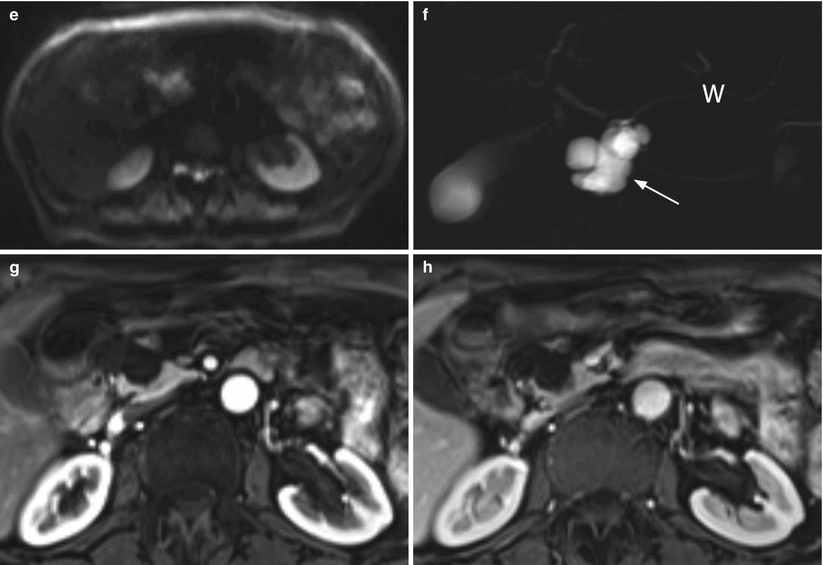
Fig. 20
IPMN branch-duct type. (a, b) Ultrasound study: lobulated cystic lesion (calipers in a) of the pancreatic head. At CEUS (b) the lesion shows thin wall and vascularized septa. (c–h) MRI study: branch-duct IPMN with typical cystic appearance hypointense on T1-weighted images (c) and hyperintense on T2-weighted images (d) and lobulated contours (arrow in c, d). On DWI (e), the lesion is slightly hyperintense on high b-value evaluation (T2 shine-through artifact). On MRCP (f), the cystic lesion (arrow in f) is connected with the main pancreatic duct (W in f). On dynamic phases (g, h), very thin vascularized intralesional septa are visible
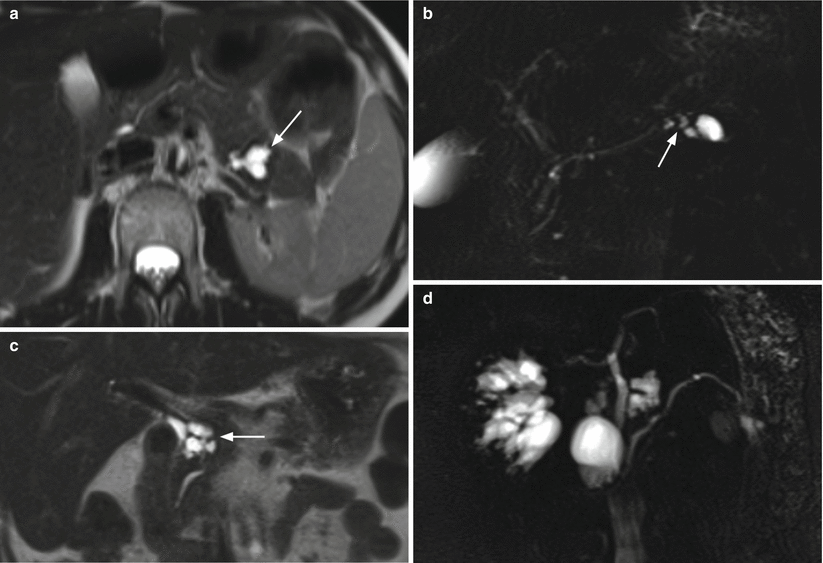
Fig. 21
IPMN branch-duct type. (a, b) MRI study: axial T2-weighted image (a) in which a lobulated cystic lesion (arrow in a) is visible in the pancreatic tail. At MRCP (b), a connection (arrow in b) between the lesion and the main pancreatic duct is demonstrated. (c, d) MRI study: coronal T2-weighted image (c) in which a multicystic grape-like lesion (arrow in c) is visible in the pancreatic head. At MRCP (d), a very thin connection between the lesion and the main pancreatic duct is visible
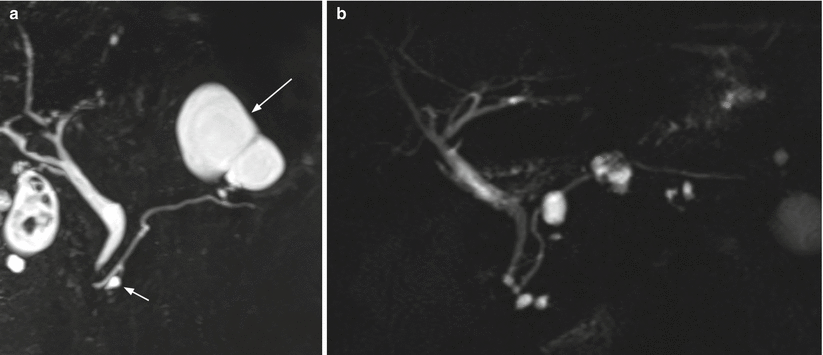
Fig. 22
IPMN branch-duct type: multifocal aspect. (a) MRCP study: large cystic lesion (long arrow) in the pancreatic tail in connection with the main pancreatic duct. Other small cystic lesion (small arrow) is clearly detectable in the pancreatic head. (b) MRCP study: multifocal branch-duct IPMN
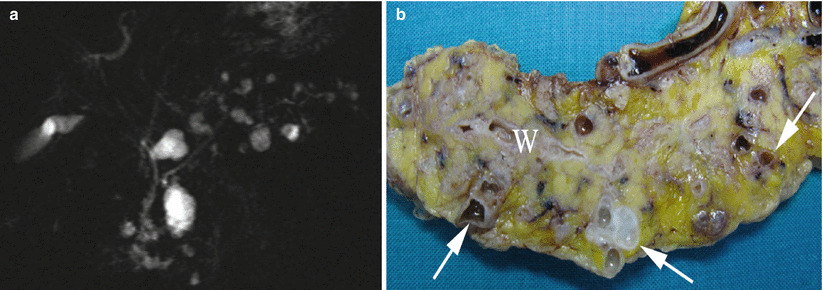
Fig. 23
Multifocal branch-duct type IPMN. (a) MRCP study: multiple small hyperintense cystic lesions with a Wirsung duct regular in caliber. (b) Surgical specimen (distal pancreatectomy): multifocal (arrows) branch-duct IPMN. Main pancreatic duct (W) is normal
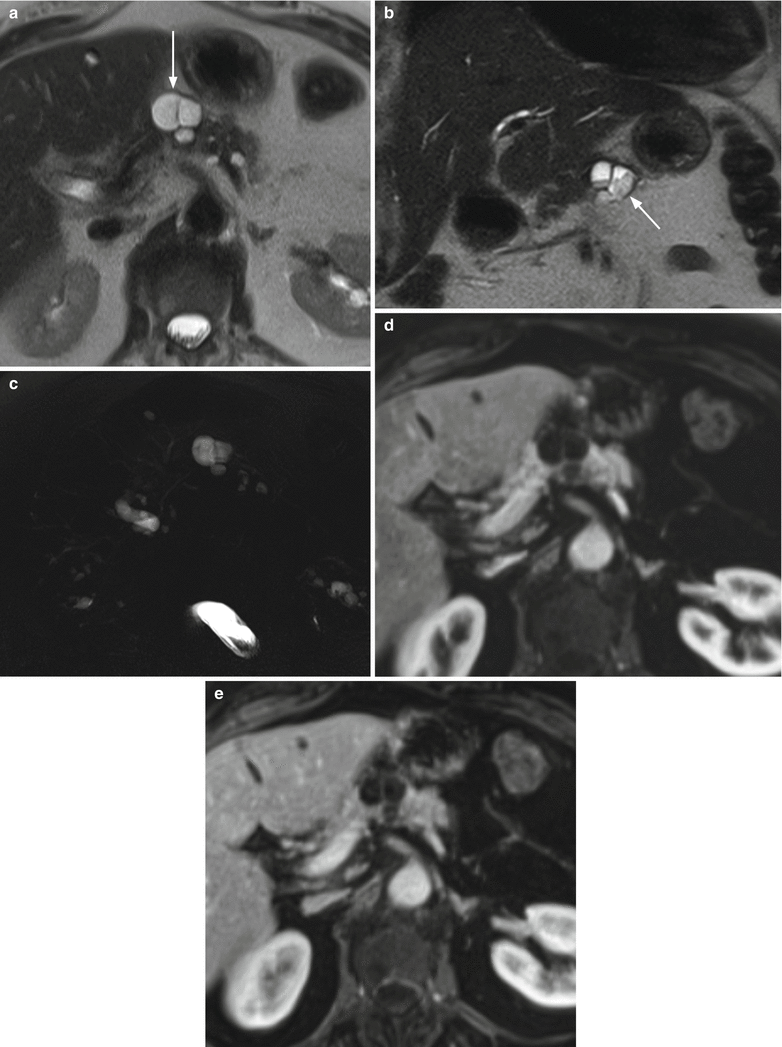
Fig. 24
Multifocal branch-duct type IPMN: intralesional deposits. (a–e) MRI study: lobulated cystic lesion (arrow in a) with thin septa is visible in the pancreatic body hyperintense on axial T2-weighted images (a) and with deposits and fluid–fluid level on coronal T2-weighted images (b) within the lesion (arrow in b). On MRCP (c), multiple cystic lesions are easily detected. In the dynamic phases (d, e), the lesion shows intralesional vascularized thin septa
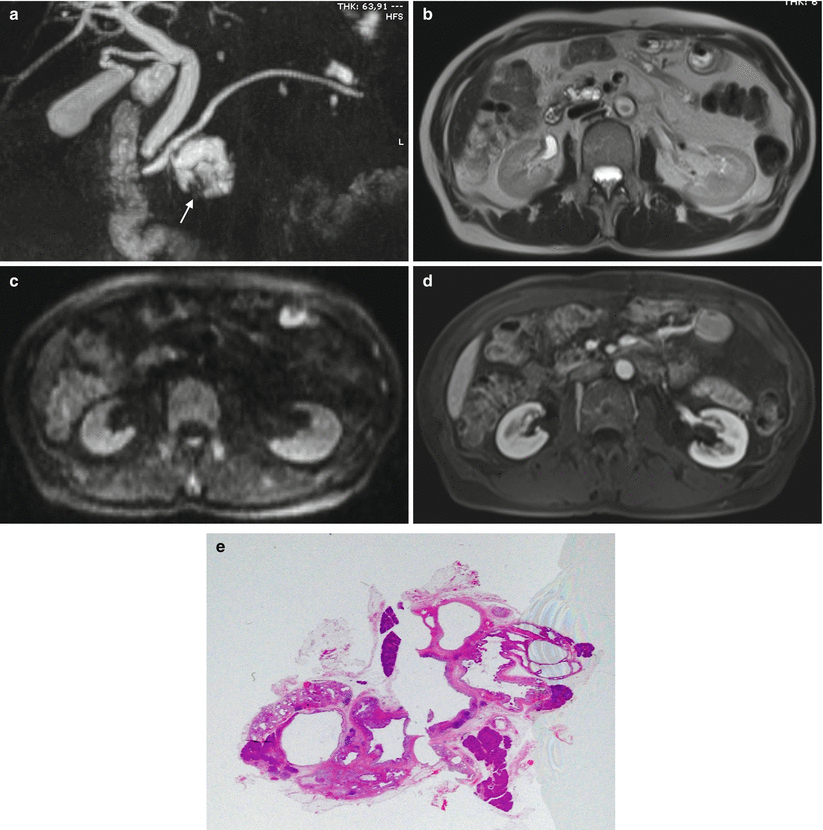
Fig. 25
Branch-duct type IPMN with pseudonodule. (a–c) MRI study: a large multiloculate cystic lesion communicating with the main pancreatic duct is visible in the uncinate process at MRCP (a). In the infero-lateral portion of the cyst a defect is recognizable (arrow in a). With T2-weighted HASTE (b), DWI b 800 (c), and Gd-enhanced MRI (d) no nodules are recognized in the lesion. Due to previous Billroth II surgical procedure, an EUS was not feasible. (e) Macrosection: multiloculated cystic lesion with no evidence of solid nodules
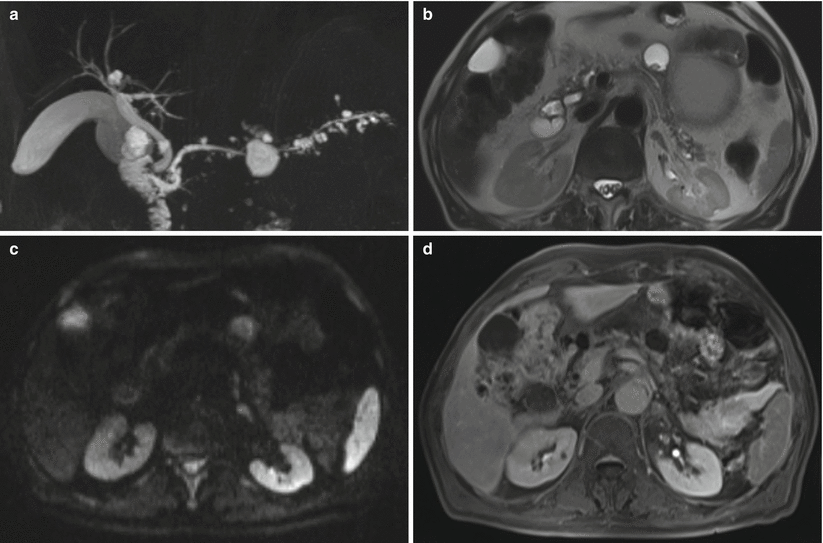
Fig. 26
Multifocal branch-duct type IPMN and pseudonodule. (a–d) MRI study: multifocal BD-IPMN with non-enhancing nodule. At MRCP (a), a multifocal BD-IPMN is recognizable. With T2-weighted HASTE (b), a solid nodule is visible in the largest lesion. With DWI at b 600 (c), no diffusion restriction is appreciated within the lesion. With Gd-enhanced MRI (d), no enhancement can be appreciated in the nodule
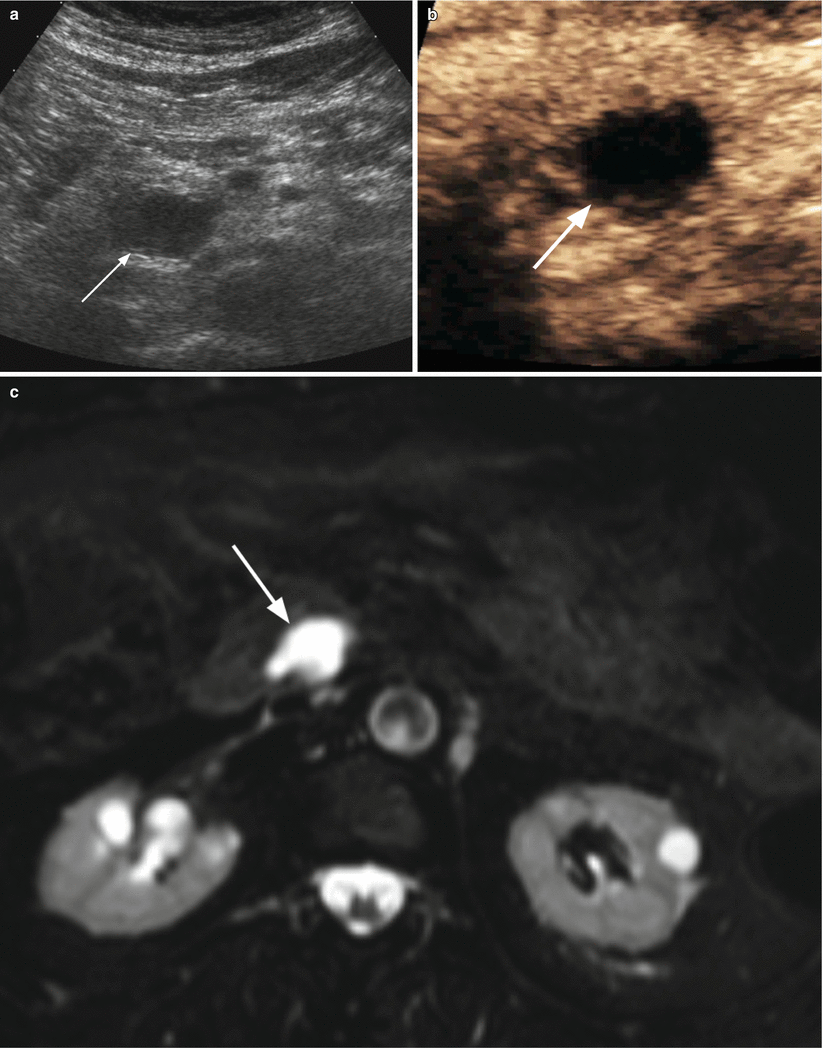
Fig. 27
IPMN branch-duct type: pseudo-solid ultrasound appearance. (a, b) Ultrasound study: at conventional ultrasound (a) hypoechoic mass (arrow in a) is detected in the uncinate process of the pancreatic. At CEUS (b) the pancreatic mass is completely avascular (arrow in b). (c) MRI study: branch-duct IPMN with typical cystic appearance hyperintense on T2-weighted images (arrow)
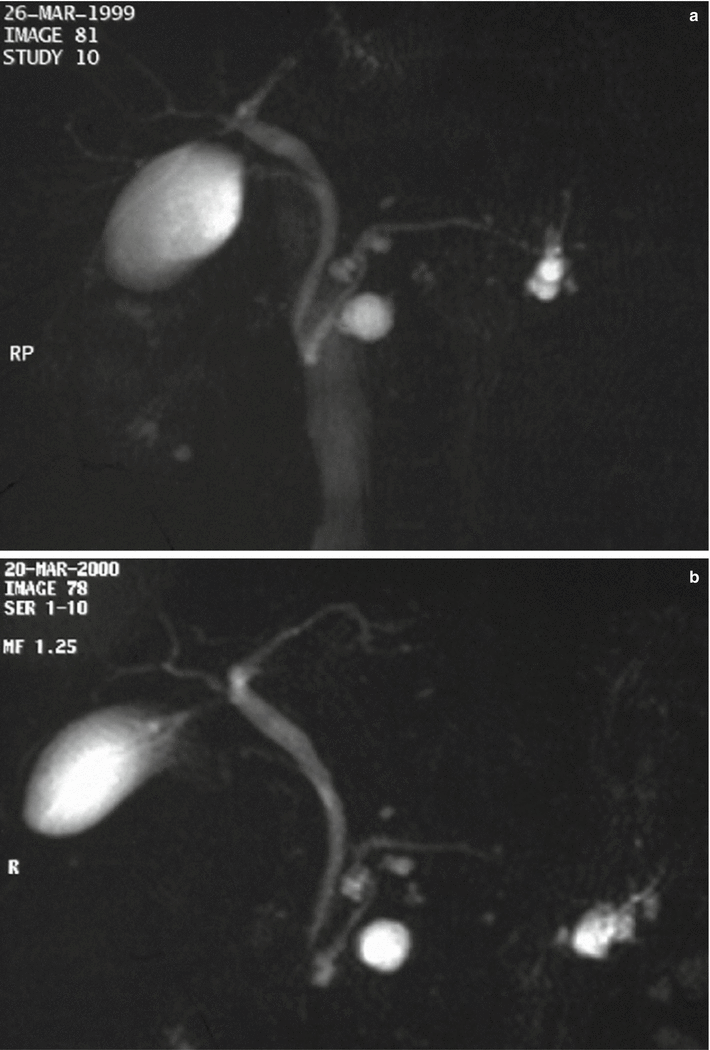
Fig. 28
IPMN branch-duct type: stable at follow-up. (a) MRCP study (Mar 1999): multiple small branch-duct IPMNs. (b) MRCP study (Mar 2000): essentially unchanged multiple small branch-duct IPMNs
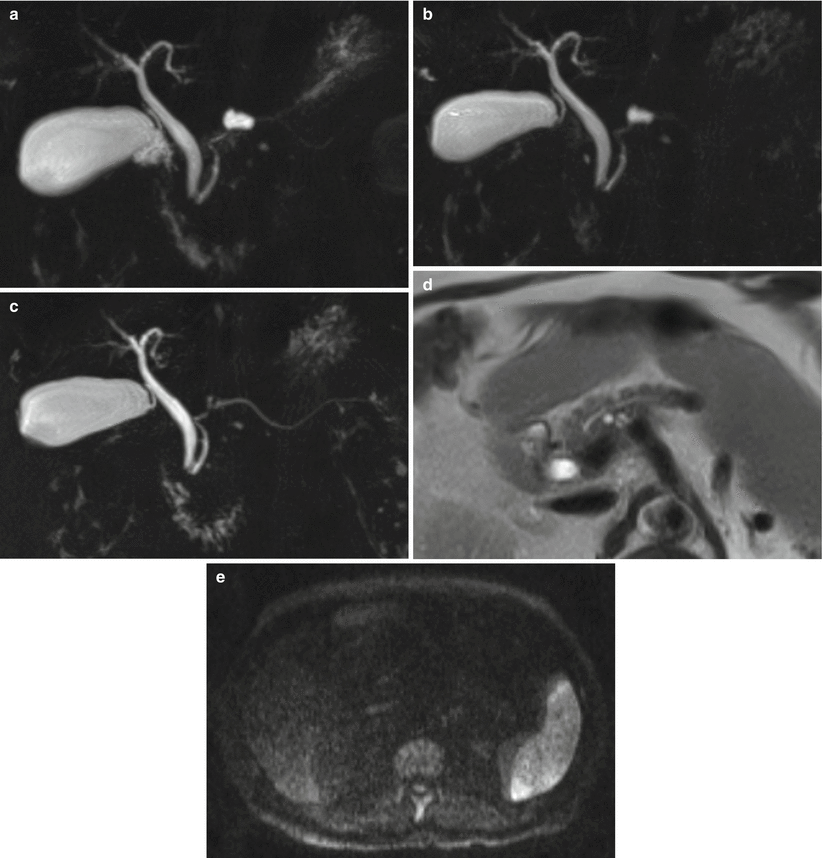
Fig. 29
IPMN branch-duct type. (a) MRCP: small cystic dilation of a branch duct. (b) MRCP 1 year later: unchanged. (c–e) MRI study 4 years later: on MRCP (c), the lesion is markedly decreased with no significant alterations of the pancreatic parenchyma at T2-weighted HASTE (d) and DWI b 800 (e)
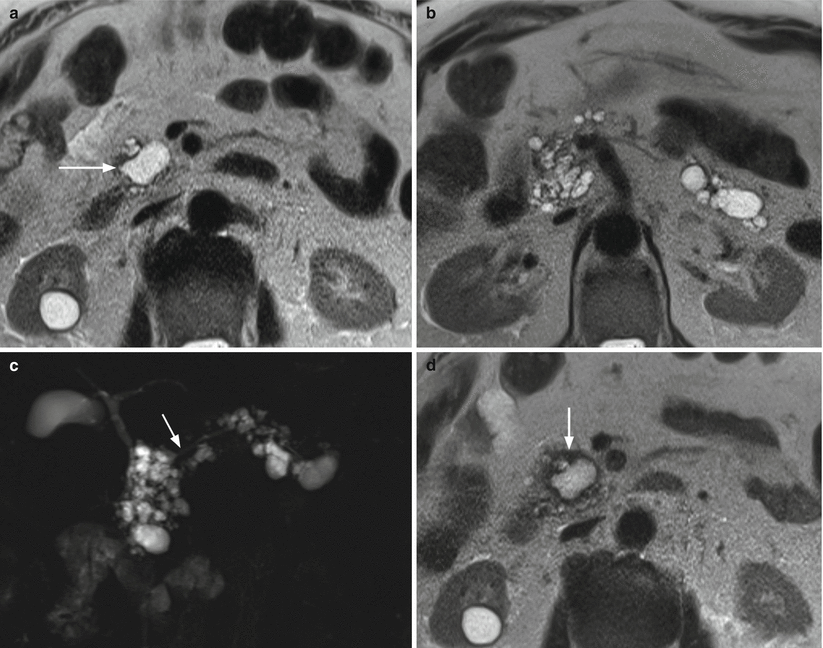
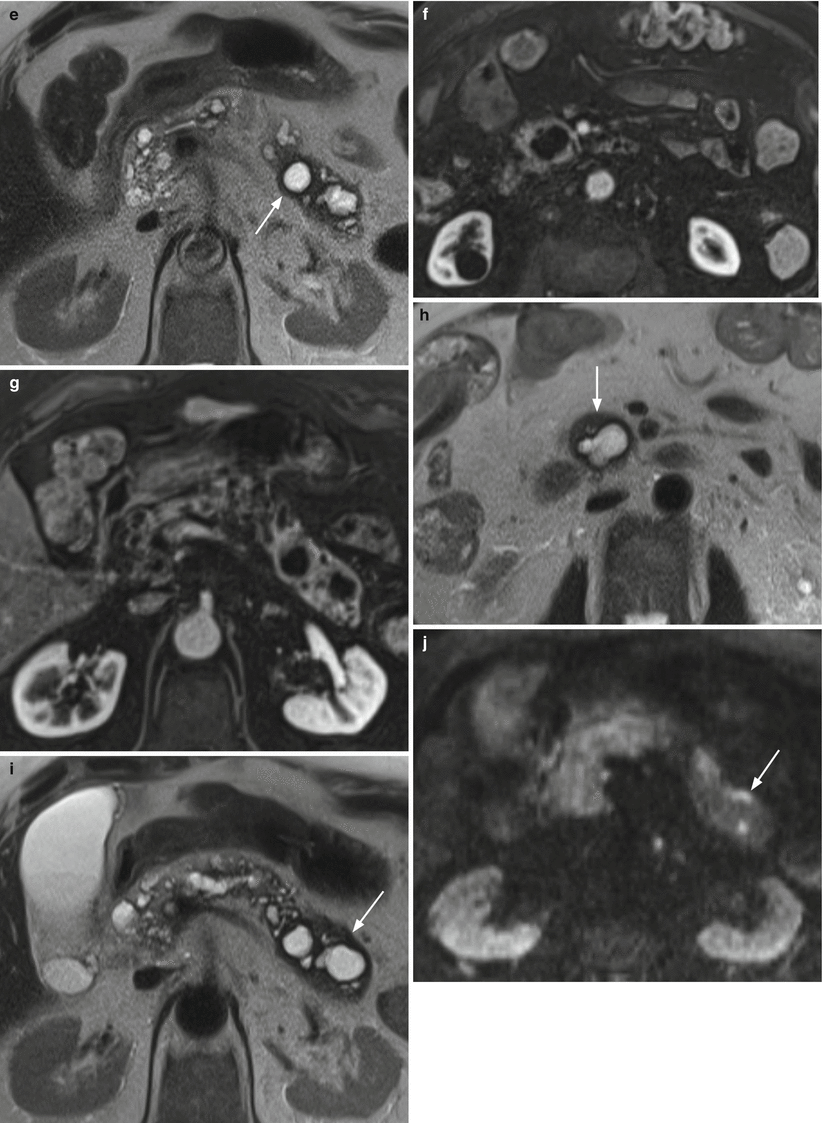
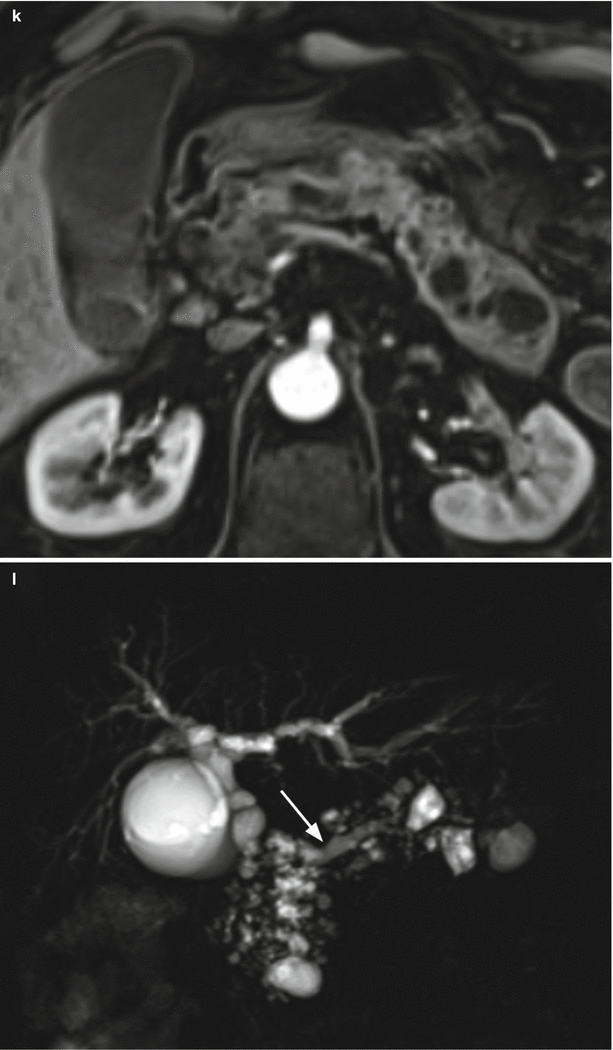
Fig. 30
Multifocal branch-duct type IPMN: progression. (a–c) MRI study: in the uncinate process of the pancreas, cystic lesion (arrow in a) hyperintense on axial T2-weighted images (a). Other multiple cystic lesions are easily detected in the head and in the body–tail of the gland hyperintense on axial T2-weighted images (b). MRCP (c) panoramically shows the evident multifocal aspect of the disease. The main pancreatic duct (arrow in c) is regular in caliber. (d–g) MRI study (after 6 months): on axial T2-weighted images (d, e), the known cystic lesions at the uncinate process of the pancreas (arrow in d) and in the pancreatic tail (arrow in e) show irregular thick wall with inhomogeneous enhancing tissue around the cystic cavity on dynamic images (f, g). (h–l) MRI study (after 3 months): on axial T2-weighted images (h, i), the known cystic lesions in the uncinate process of the pancreas (arrow in h) and in the pancreatic tail (arrow in i) show thicker wall. On DWI (j), in the lesion of the tail of the pancreas small nodules (arrow in j) with clear diffusion restriction at high b-value are detected. On dynamic phases (k), the lesion in the tail of the pancreas is enlarged and the enhancement of cystic wall is clearly more irregular and thick. MRCP (l) shows dilated main pancreatic duct (arrow in l)
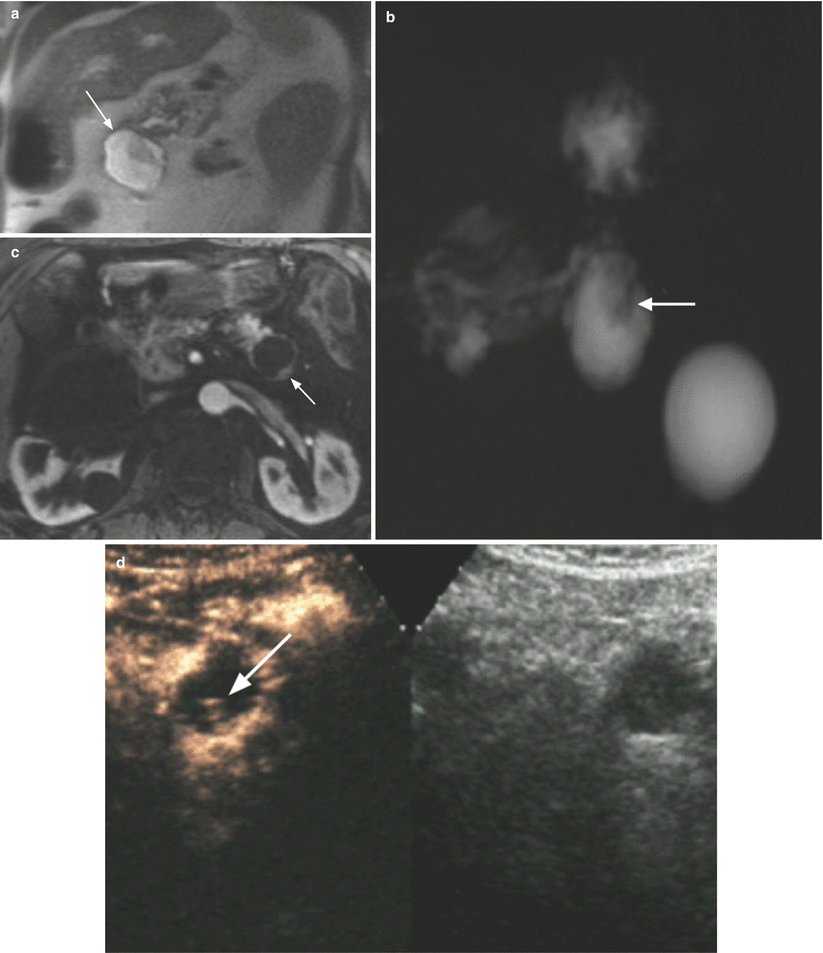
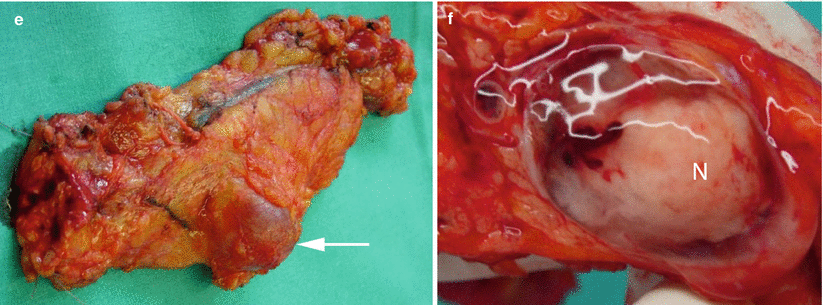
Fig. 31
Branch-duct type IPMN with nodule. (a–c) MRI study: coronal T2-weighted images (a) showing a pancreatic body cystic lesion (arrow in a) with hypointense vegetation that is confirmed at MRCP (b) as an intracystic nodule (arrow in b). In the dynamic phase (c), focal enhancing thickening of the wall is detected (arrow in c). (d) CEUS: cystic pancreatic body lesion with intralesional vascularized nodule (arrow). (e, f) Surgical specimen (distal pancreatectomy): cystic lesion (arrow in e) bulging the inferior profile of the pancreatic body. At the cut section (f), the presence of intralesional solid nodule (N in f) is confirmed
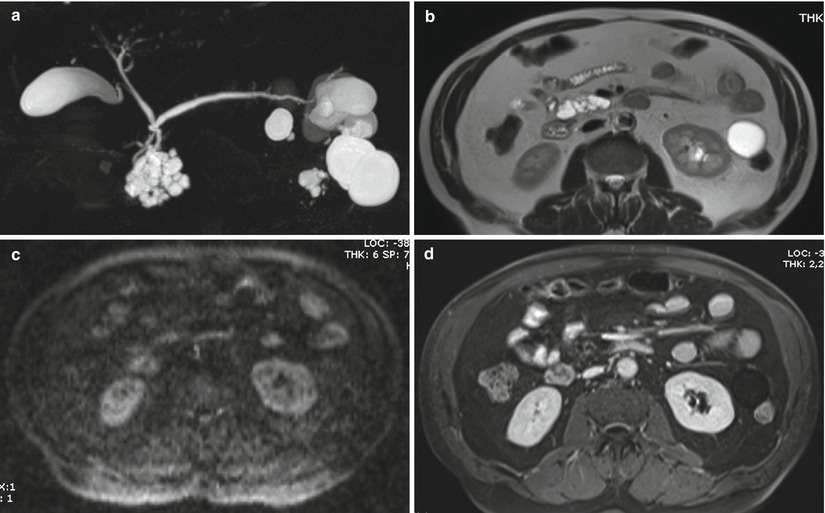
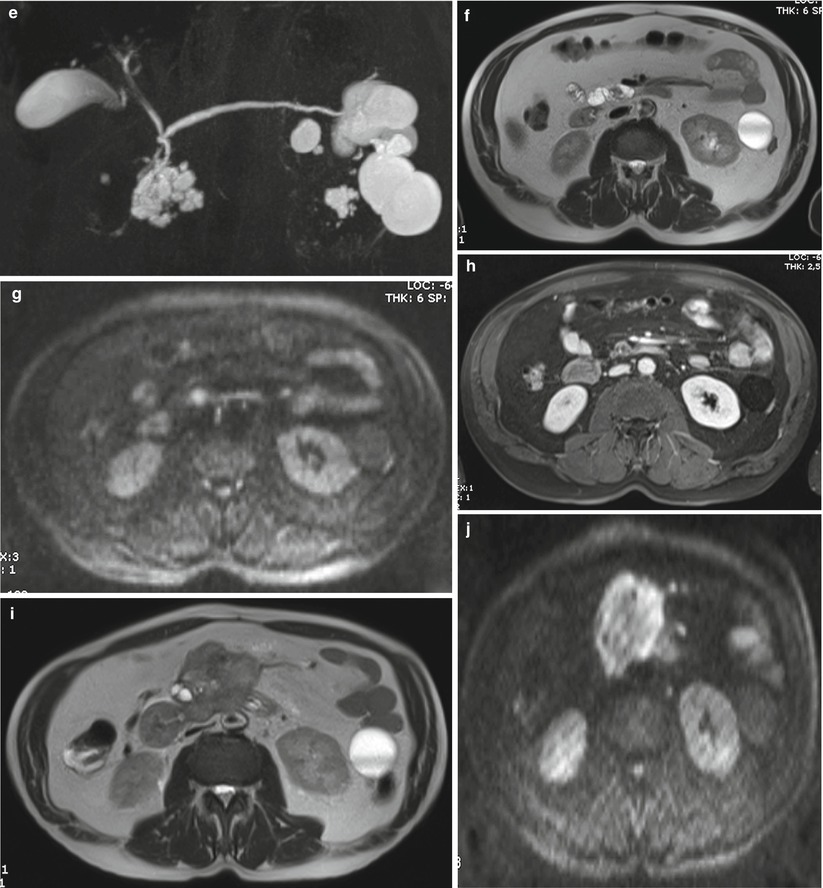
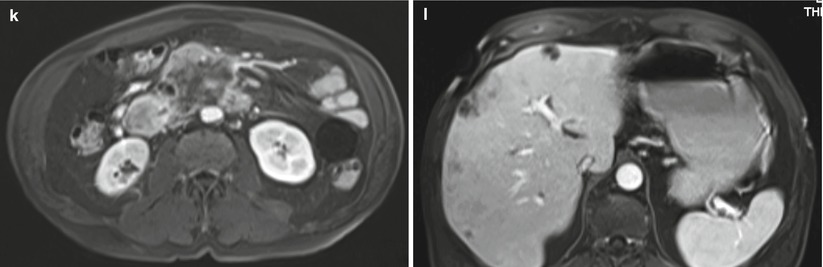
Fig. 32
IPMN branch-duct type: progression. (a–d) MRI study: on MRCP (a) a unifocal multilocular 4 cm BD-IPMN is visible in the uncinate process. With T2 HASTE (b), DWI b 800 (c), and Gd-enhanced MRI (d), no solid nodules are appreciated. (e–h) MRI study at 4-month follow-up: a solid nodule appears visible as a defect at MRCP (e ), hypointense on T2-weighted HASTE (f), hyperintense on DWI b 800 (g), and enhancing after Gd administration (h). The patient refused surgery. (i–l) MRI study after 6 months: a large mass is appreciated on T2-weighted HASTE (i), markedly hyperintense on DWI b 800 (j), hypoenhancing after Gd administration (k), with liver metastases (l)
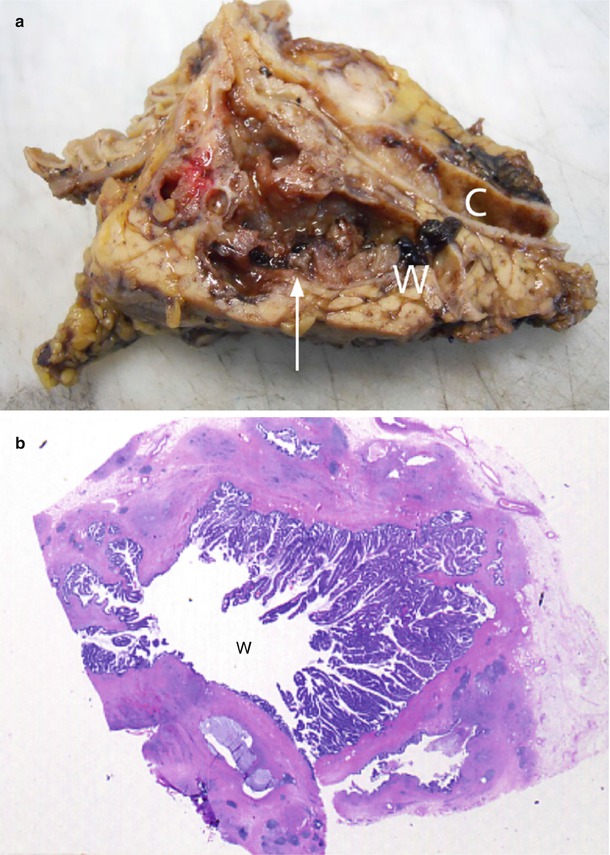
Fig. 33
IPMN main-duct type. (a) Surgical specimen (pancreaticoduodenectomy): the main pancreatic duct (W) is enlarged and filled by multiple small papillary vegetations giving to the lesion an irregular profile (arrow). Common bile duct (C) is dilated. (b) Macrosection of the Wirsung duct: the main pancreatic duct (W) is dilated and with multiple papillary projections along the wall
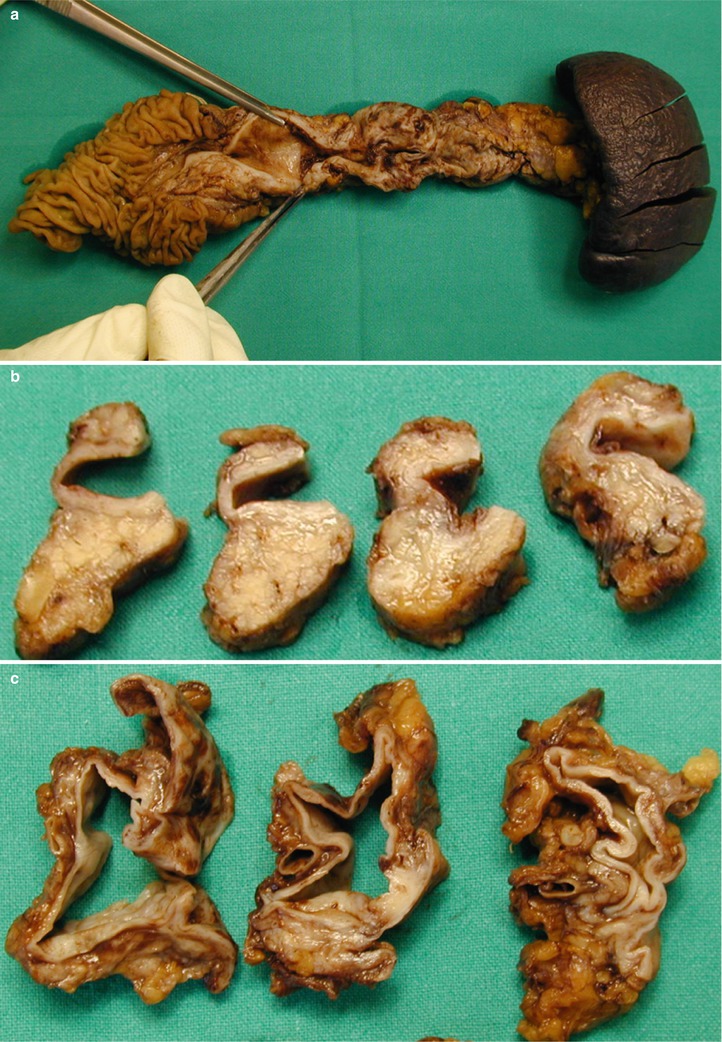
Fig. 34
IPMN main-duct type. (a–c) Surgical specimen (total pancreatectomy): the main pancreatic duct is dilated and after a longitudinal cut (a) the internal surface is smooth. Downstream (b) and upstream (c) at transverse cut the main pancreatic duct is confirmed to be dilated and with regular smooth internal surface. Distally the main pancreatic duct is extremely more dilated appearing as a cystic lesion collapsed after cut
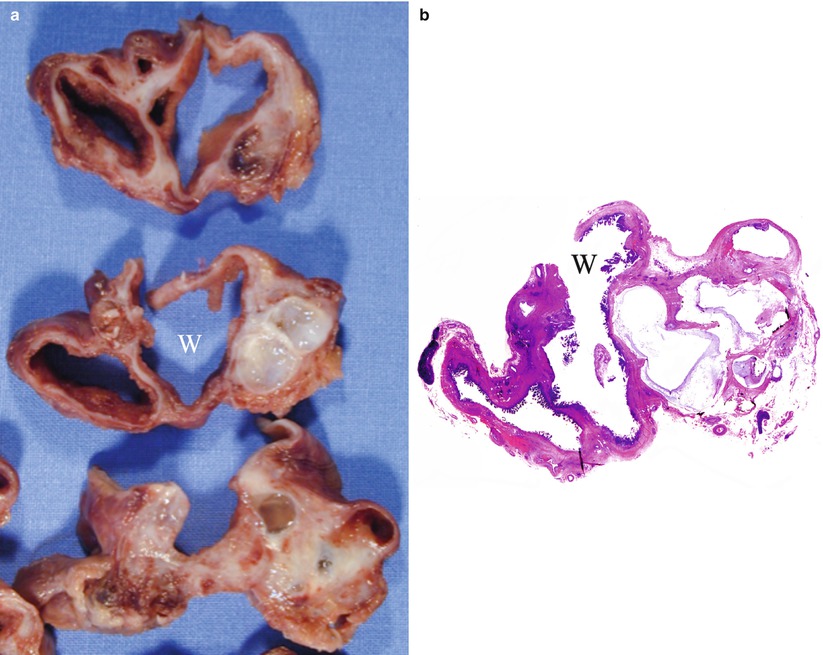
Fig. 35
IPMN main-duct and branch-duct type: content. (a) Surgical specimen (distal pancreatectomy): the main pancreatic duct (W) is surrounded by multiple dilated branch ducts. (b) Macrosection: multiple small papillary projections are visible along the internal wall of the dilated main pancreatic duct (W) and into the dilated branch ducts
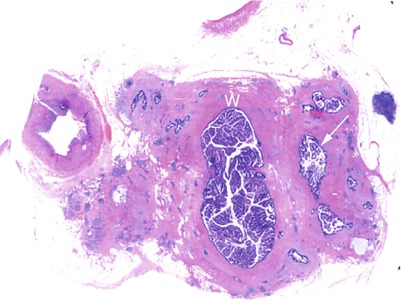
Fig. 36
IPMN main-duct and branch-duct type: content. Macrosection: the main pancreatic duct (W) is dilated and filled by multiple grossly visible papillary vegetations. Also the branch ducts (arrow) are dilated and filled by multiple papillary vegetations
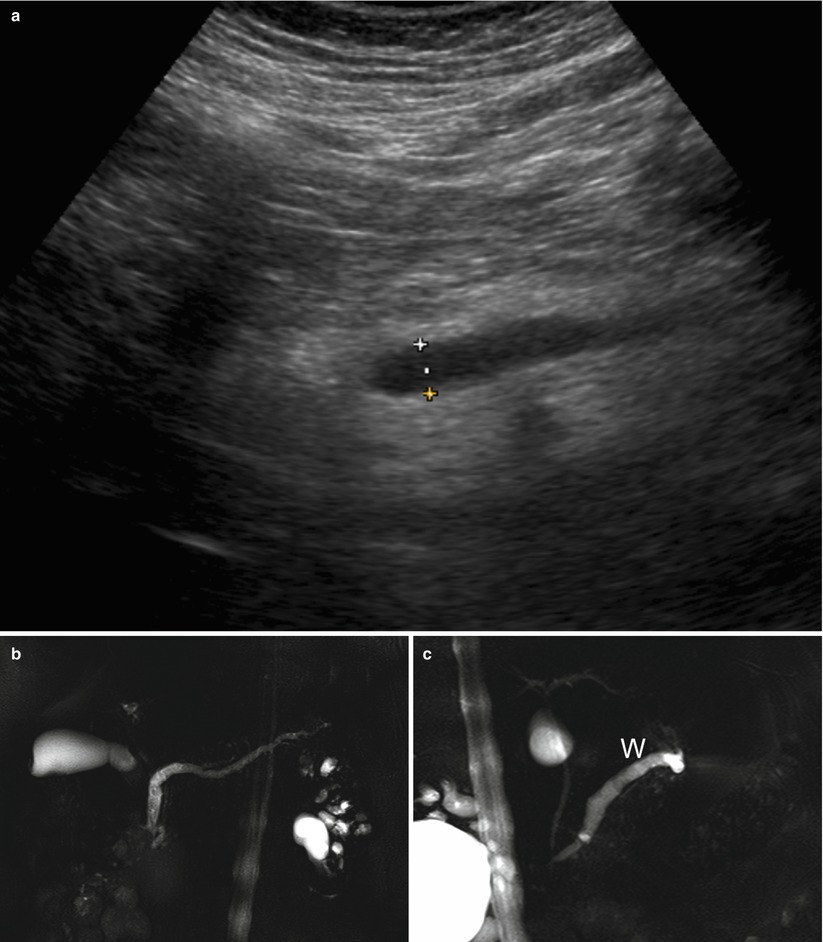
Fig. 37
IPMN main-duct type. (a) Ultrasound study: dilated (calipers) main pancreatic duct. (b, c) MRCP: main-duct IPMN with typical uniform dilation of the main pancreatic duct (W in c) from the tail to the major papilla
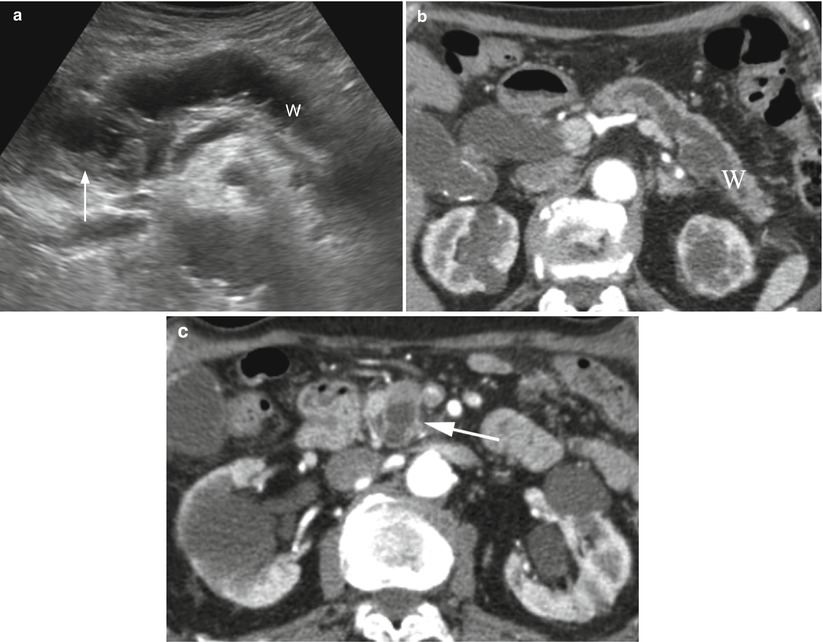
Fig. 38
IPMN main-duct type. (a) Ultrasound study: dilated main pancreatic duct (W) ending in an inhomogeneously hypoechoic mass (arrow) only partially cystic. (b, c) CT study: dilated main pancreatic duct (W in b) ending in an inhomogeneously hypodense cystic mass (arrow in c)
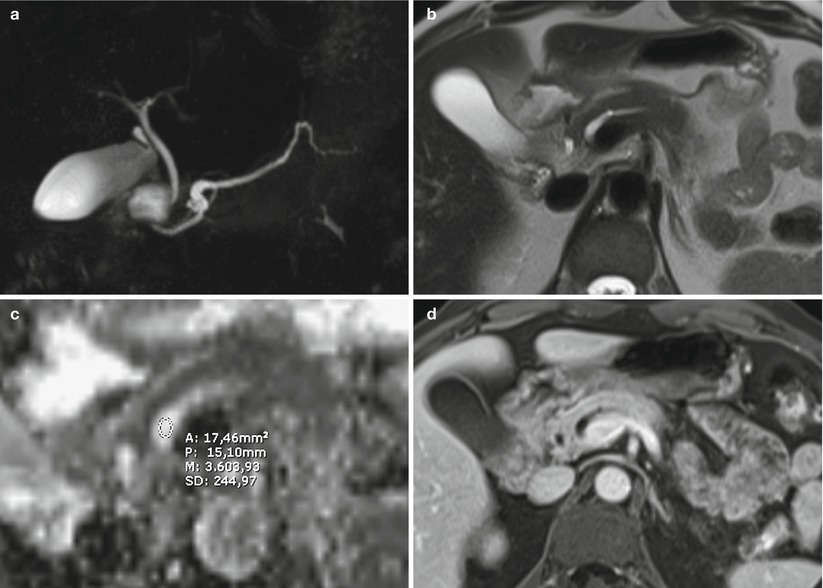
Fig. 39
IPMN main-duct type. (a–d) MRI study: segmental dilation of main pancreatic duct in the head of the pancreas is visible at MRCP (a). With T2 HASTE (b) no evidence of solid nodules. With DWI, at ADC map (c), the ROI shows no restricted diffusion of water molecules in the pancreatic lumen (n = 3.6). With Gd-enhanced MRI (d), no pathological enhancement of solid nodules is appreciable
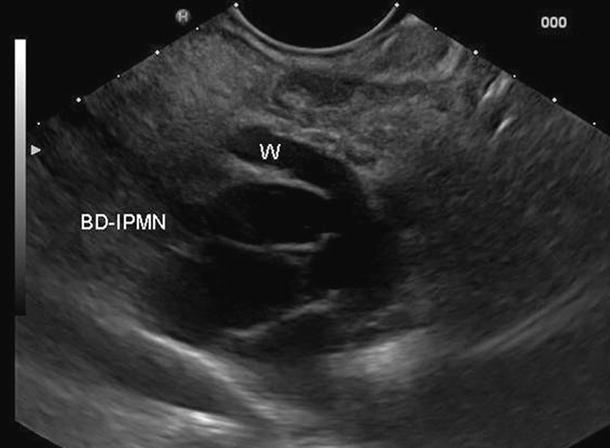
Fig. 40
IPMN. EUS study: branch-duct IPMN (BD-IPMN) in communication with the Wirsung duct (W) slightly dilated
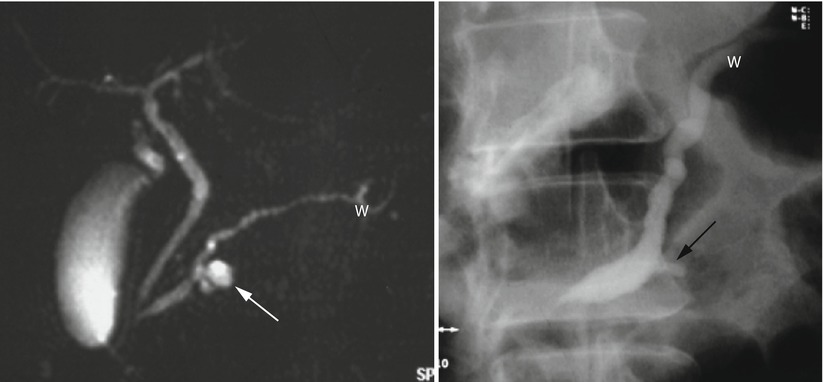
Fig. 41
IPMN. MRCP (left) and ERCP (right) studies: on MRCP, branch-duct IPMN (white arrow) is visible in communication with the Wirsung duct (W) slightly dilated. On ERCP, only the dilated Wirsung duct (W) and the lesion communication (black arrow) are injected
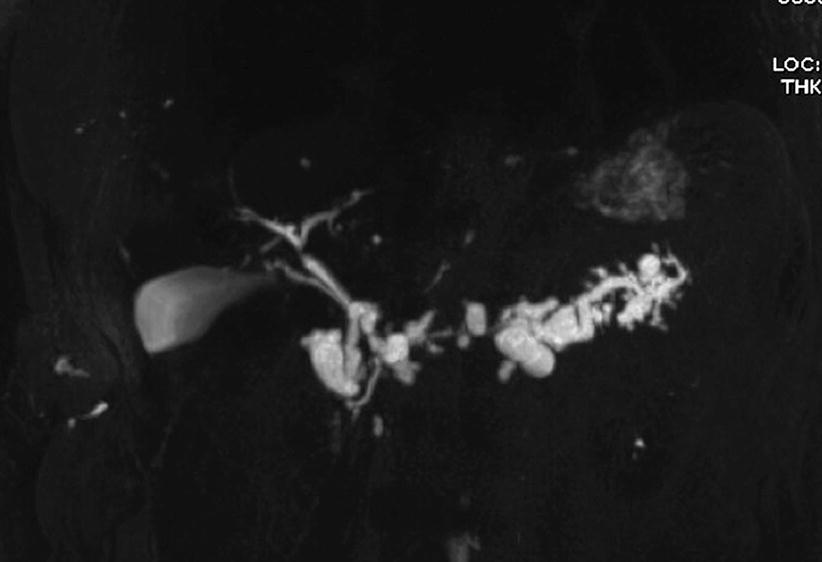
Fig. 42
IPMN mixed type. MRCP: multiple cystic dilation of branch ducts and main pancreatic duct dilation in the body–tail
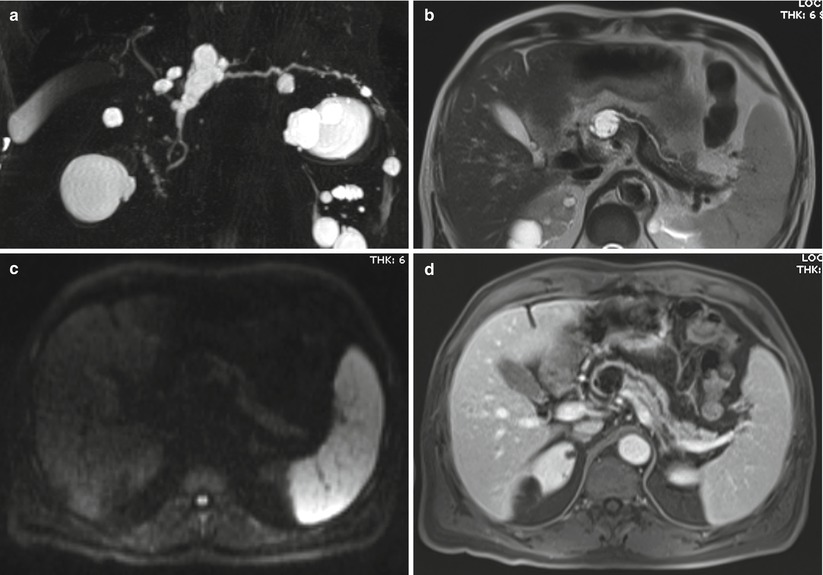
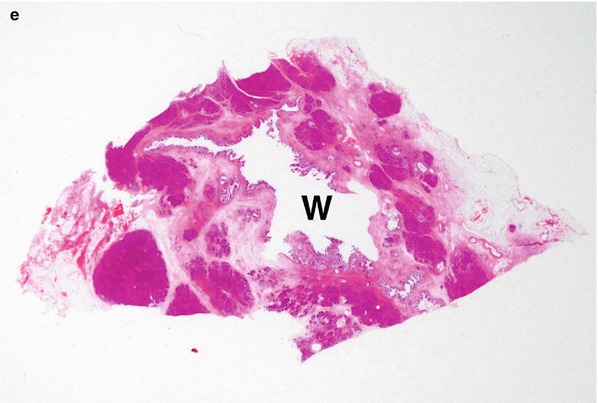
Fig. 43
IPMN mixed type. (a–d) MRI study: at MRCP (a) significant segmental dilation of the main pancreatic duct is visible in the neck of the pancreas. A BD-IPMN is recognizable in the body of the pancreas. With T2 HASTE (b), thin septa can be appreciated in the main lesion due to the involvement of branch duct. With DWI at b 800 (c), no solid nodules are highlighted. After Gd injection (d), slight enhancement of the septa can be appreciated. No solid nodules enhancement. (e) Macrosection: the Wirsung duct (W) appears enlarged with small endoluminal papillary proliferations
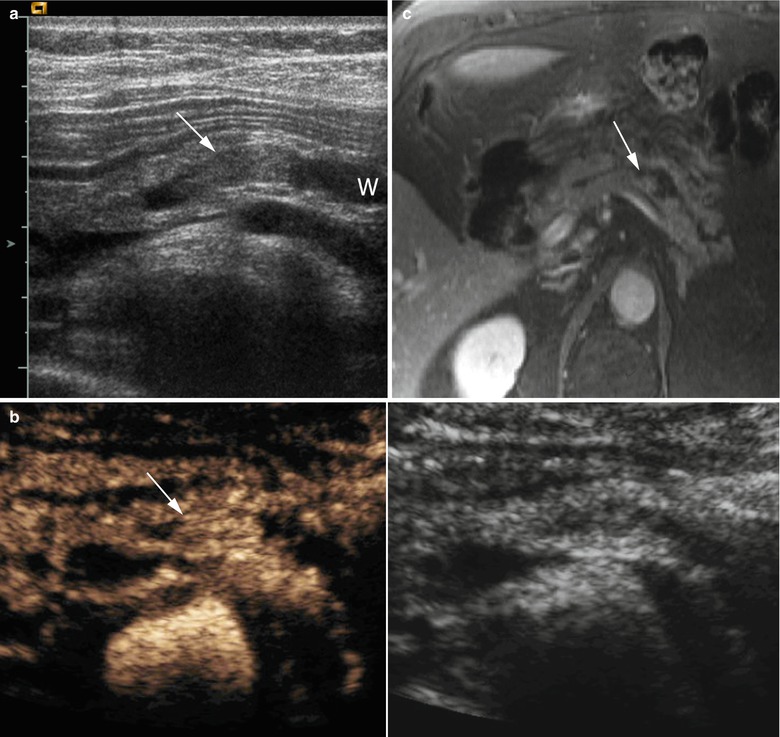
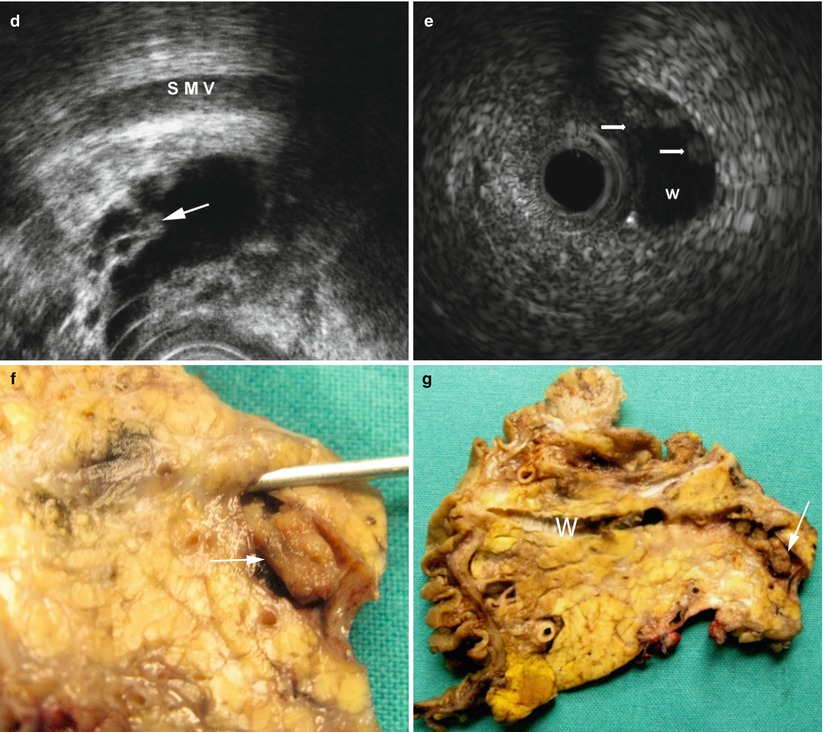
Fig. 44




IPMN main-duct type: nodular/papillary vegetations. (a, b) Ultrasound study: at conventional ultrasound (a) a dilated Wirsung duct (W in a) is visible with echoic vegetation (arrow in a) in the lumen. After contrast medium administration (b), the enhancement of the endoductal nodule becoming hyperechoic (arrow in b) is appreciable. (c) Dynamic MRI: enhancement of small nodule (arrow) completely inside a focally dilated Wirsung duct. (d) EUS: small papillary vegetations (arrow) inside a cystic pancreatic lesion. Superior mesenteric vein (SMV). (e) Intraductal ultrasound: endoscopic ultrasound inside the Wirsung duct (W) shows some very small nodules (arrows) on the duct wall. (f, g) Surgical specimen (pancreaticoduodenectomy): in the Wirsung duct (probe in f) a nodule (arrow in f, particular) is visible. After longitudinal cut of the Wirsung duct (W in g), the nodule (arrow in g) is confirmed to originate from the ductal wall of the main pancreatic duct. Moreover, other small irregularities of the Wirsung wall are present
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree