This article reviews the characteristic imaging appearances of parasitic diseases of the central nervous system, including cysticercosis, toxoplasmosis, cystic echinococcosis, schistosomiasis, amebiasis, malariasis, sparganosis, paragonimiasis, and American and African trypanosomiases. Routine precontrast and postcontrast MR imaging helps in localization, characterization, delineation of extension, and follow-up of the parasitic lesions. Moreover, recently developed tools, such as diffusion, perfusion, and MR spectroscopy, help to differentiate parasitic diseases of the central nervous system from simulating lesions. Combining imaging findings with geographic prevalence, clinical history, and serologic tests is required for diagnosis of parasitic diseases of the central nervous system.
Parasitic diseases are distributed worldwide, with increased prevalence in areas of poor sanitation. Although these diseases occur much more frequently in developing countries, sporadic cases occur in nonendemic areas because of an increase in international travel and immunosuppression caused by posttransplantation therapy or HIV infection. Parasitic diseases can involve the central nervous system (CNS) with multiple clinical presentations. The most common parasitic infection of the CNS is cysticercosis; other less frequent infections are toxoplasmosis, echinococcosis, and schistosomiasis. Rare parasitic diseases are sparganosis, paragonimiasis, malariasis, amebiasis, toxocariasis, and American and African trypanosomiases. MR imaging is the most helpful tool because it identifies characteristic features of the illness studied. Some advanced techniques, such as diffusion MR, perfusion MR, and MR spectroscopy, provide more clues for differentiation of parasitic diseases of the CNS from simulating lesions. The radiologist plays a vital role in the diagnostic work-up of these infections. By interfacing with clinicians, the radiologist can help direct appropriate testing and treatment, ultimately decreasing the morbidity and mortality associated with these infections.
Cysticercosis
Cysticercosis is the most common parasitic infection of the CNS. It is found worldwide, particularly in Central and South America, India, Africa, East Asia, and Eastern Europe. Cysticercosis is caused by the encysted larvae of the tapeworm Taenia solium . It develops after ingestion of eggs from the feces of a tapeworm carrier, which is followed by hematogenous spread to neural, muscular, and ocular tissue. Intracranially, the oncospheres, the primary larval form of T solium , develop into a secondary larval form called “cysticerci.” There are many types of cerebral cysticercosis. The parenchymal type is the most common type, whereas the subarachnoid (cisternal), intraventricular, and mixed types are also observed. Cysticercosis affects males and females equally and manifests predominantly in young adults, with a peak occurrence at 25 to 35 years of age. The clinical presentation is nonspecific. Patients present with epilepsy (50%–70%), headache (43%), symptoms of cerebrospinal fluid (CSF) flow obstruction (33%), and signs of meningeal irritation (<2%). Seizures are a result of perilesional inflammation in degenerating cysts, although infarction, vasculitis, and even calcified granulomas may also act as predisposing factors. The cysticercus-specific IgG antibody level, in either serum or CSF, has a specificity and sensitivity of 100% and 98%, respectively. The common sites of cysticercosis are the CNS, the eye, and muscle tissue. It commonly involves the brain parenchyma; the ventricles (20%–25%); or the subarachnoid space (2%–3%). The corticomedullary junction is the primary location of the parenchymal form of this parasite. More than one anatomic site is often involved, and one patient can have lesions in different stages of the disease simultaneously.
Parenchymal Cysticercosis
Parenchymal cysticercosis is divided into four stages: (1) vesicular, (2) colloidal vesicular, (3) granular nodular, and (4) nodular calcified.
Vesicular stage (active)
This stage consists of a viable larva with a scolex. On imaging, the larva appears as a cyst (4–20 mm) with signal intensity similar to that of CSF on T1- and T2-weighted images. The cyst wall is thin, with little or no enhancement. The scolex appears as a mural nodule of low signal intensity on the T2-weighted image, high signal intensity on T1-weighted and FLAIR images (similar to a hole with a dot or a pea in a pod) that may show some enhancement ( Fig. 1 ).
Colloidal vesicular stage (active)
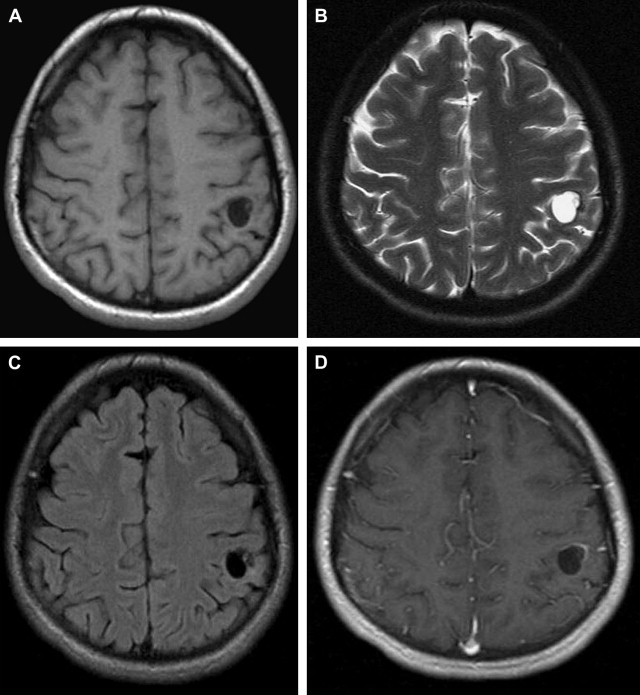
When the larva degenerates, the fluid becomes turbid, and its capsule thickens. Degenerating cysts may be hyperintense on both T1- and T2-weighted images because of their contents. Ringlike enhancement is seen in two-thirds of cases. Edema and contrast enhancement are also seen in this stage ( Fig. 2 ).
Granular nodular stage (active)
When the larva dies, the cyst begins to collapse, the capsule thickens, the scolex calcifies, and edema develops. It has an imaging appearance similar to the colloidal stage but with thicker, intensely enhanced walls and more surrounding edema ( Fig. 3 ).
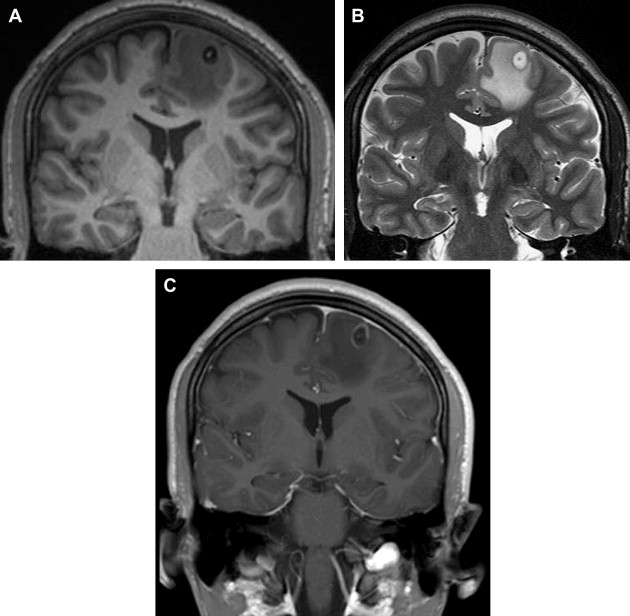
Nodular calcified stage (inactive)
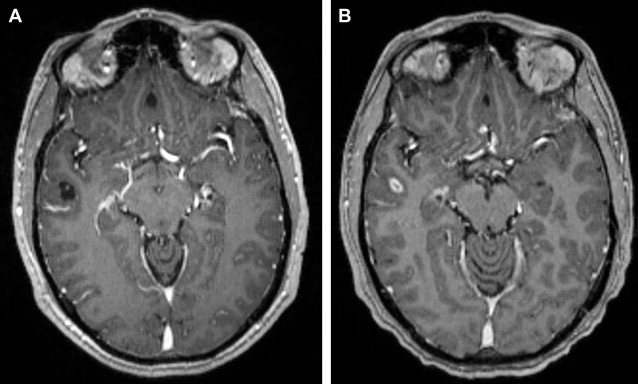
The lesion is completely mineralized with no active immune response from the host. A small, calcified lesion measuring 2 to 10 mm is typical. Usually, there are no mass effects or contrast enhancement. However, contrast enhancement or perilesional edema has been identified in some lesions and is likely related to recent seizure activity originating from chronic regions ( Fig. 4 ).
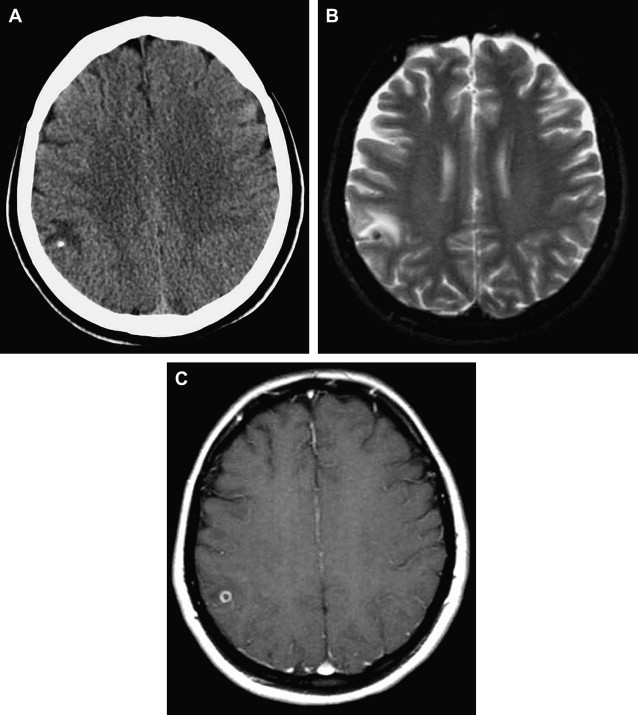
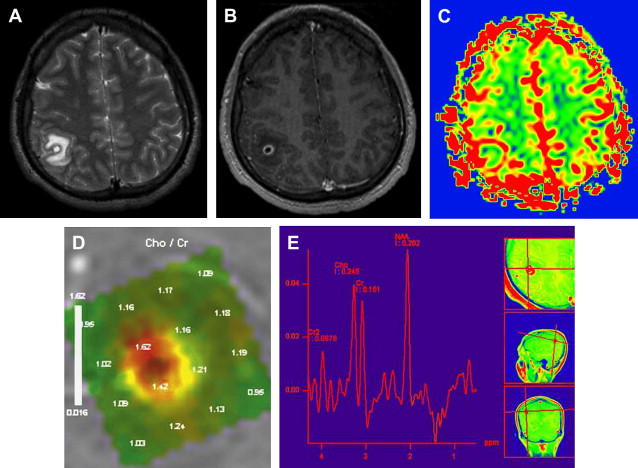
Delayed contrast MR imaging is a sensitive method for detection of lesions in the degenerative phase, which are prone to show enhancement related to the active inflammatory reaction of these stages. By diffusion-weighted imaging (DWI), the cysts have signal intensities similar to or slightly higher than that of CSF, and the scolex is detectable as a hyperintense nodule within the vesicle. MR spectroscopy helps to differentiate cysticercosis from abscesses and cystic metastasis. The presence of succinate alone or increased amounts of both succinate and acetate indicates the presence of degenerating cysticerci and differentiates them from anaerobic or tuberculous abscesses. The choline/creatine ratio may be increased, probably because of the presence of inflammatory cells. Perfusion (reduced regional cerebral blood volume [rCBV]) map shows low perfusion of a benign lesion (see Fig. 2 ).
When the cysticercosis grows larger than 1 to 2 cm in size, it may show surrounding edema, wall enhancement, or mural nodules. With these appearances, it can resemble tumor, so-called “pseudotumoral” form ( Fig. 5 ). This form is rarely seen. It may occur in the cerebral hemisphere but rarely in the cerebellar hemisphere. The main differential diagnosis includes glioma and echinococcus. Encephalitic cysticercosis occurs when multiple lesions are simultaneously in the granular stage and there is diffuse brain edema and overwhelming inflammation around the cyst. The disseminated miliary form represents a massive cysticercus infestation of the CNS and is characterized by multiple small cystic formations diffusely spread out in the brain parenchyma.
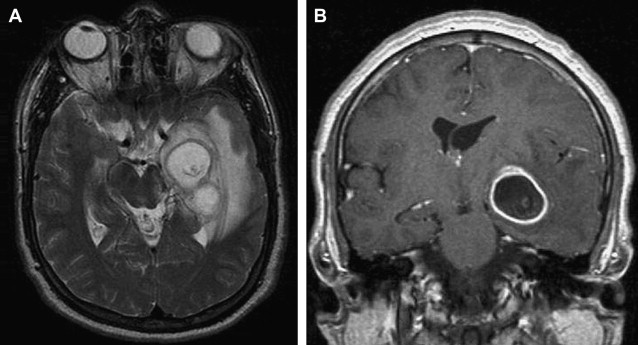
During the vesicular stage of cysticercosis, the lesions may be difficult to distinguish from echinococcosis (usually a large, solitary cyst in the region of distribution of the middle cerebral artery); sparganosis (seen in patients from Southeast Asia, multiple cystic lesions); cystic metastases; and multiple abscesses. During the colloidal vesicular and granular nodular stages of cysticercosis, both solitary and multiple cysts may be difficult to distinguish from metastases. DWI may be helpful in differentiating abscesses from cysticercosis. Pyogenic abscesses are nearly always bright by DWI, whereas cysticercal cysts are darker.
Treatment of cysticercosis depends on the form and the type of disease and the location and number of cysts. Treatment is a combination of cysticidal agents (ie, albendazole and praziquantel) and corticosteroids to control the host inflammatory response, because cysticidal therapy itself initiates a host inflammatory response that may result in more symptoms. Surgical intervention is rarely used because of early diagnosis. Imaging has been used to follow-up on the lesions after treatment ( Fig. 6 ).