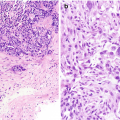
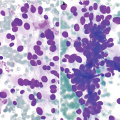
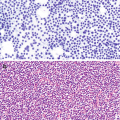
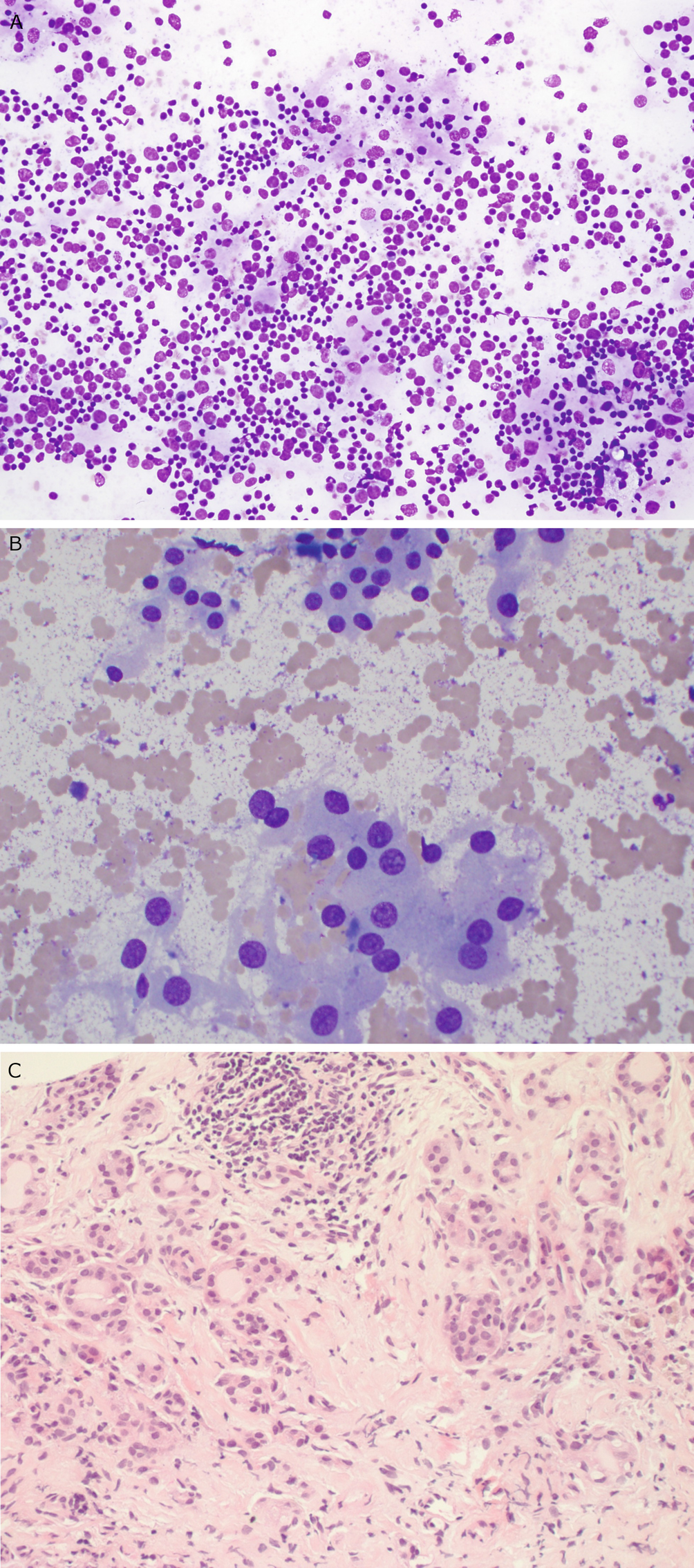
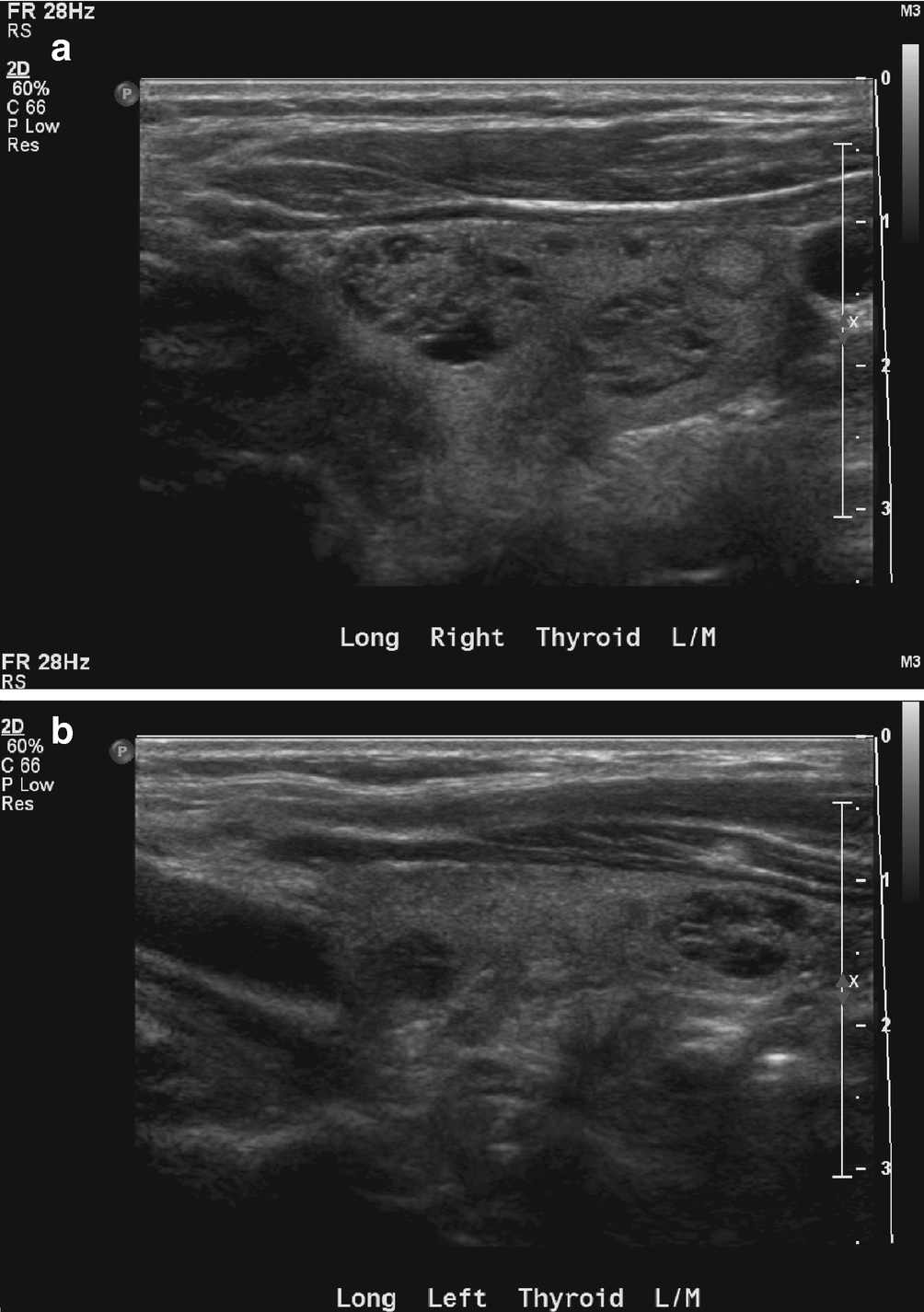
Chronic lymphocytic thyroiditis . (a) The right thyroid lobe is enlarged, diffusely heterogeneous, and hypoechoic. There is a micronodular pattern throughout the right lobe. (b) Color Doppler imaging demonstrates diffuse hypervascularity
Gross
Firm and diffusely enlarged thyroid.
Pale tan-yellow and nodular.
FNA
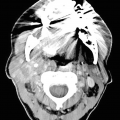
Usually hypercellular with a mixture of Hurthle cells, abundant polymorphous population of lymphoid cells, tingible body macrophages, and histiocytic aggregates (Fig. 8.2a, b) [8].
Variable numbers of plasma cells, lymphoid tangles, lymphoglandular bodies, germinal center fragments, skeletal muscle infiltrated by lymphoid cells, epithelioid histiocytes; rarely, granulomas may be seen in the background.
Colloid is often scant.
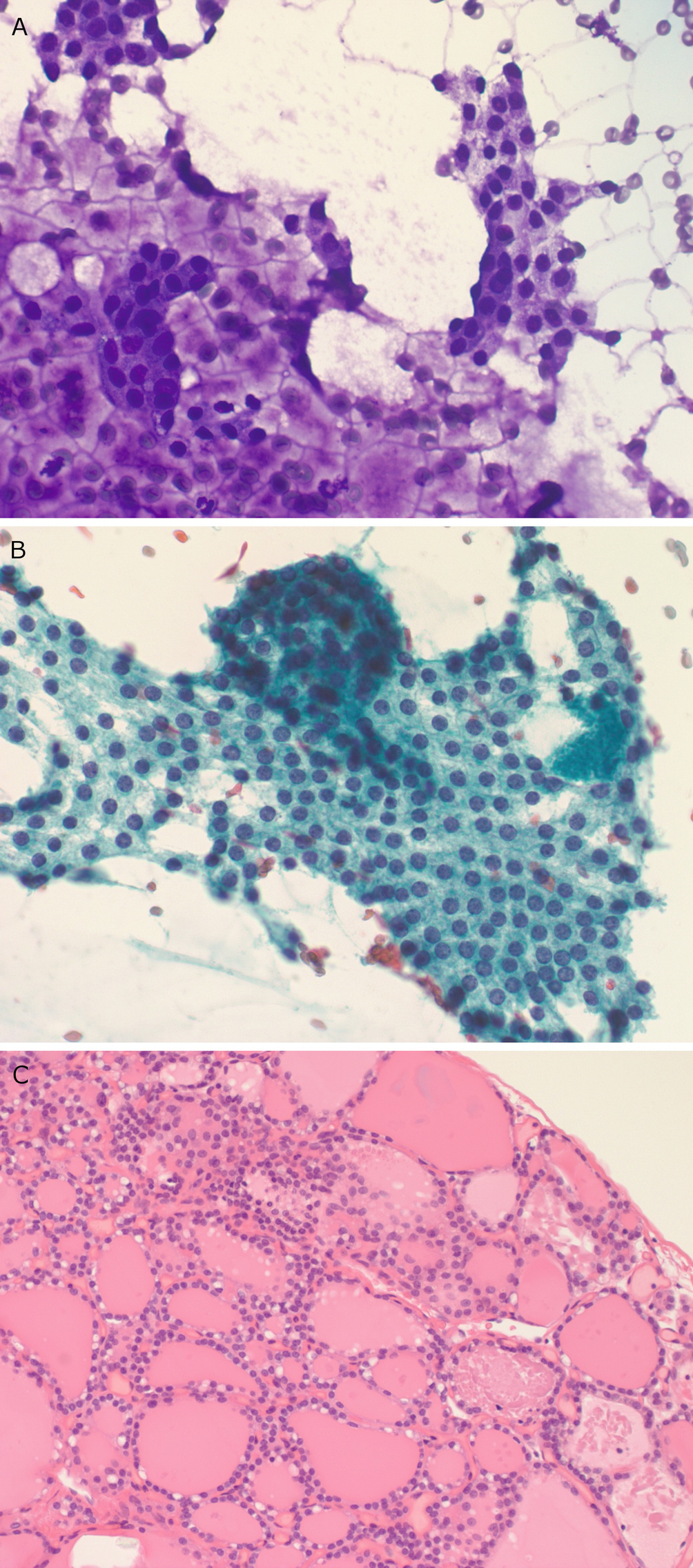
Chronic lymphocytic thyroiditis . (a) A Diff-Quik (DQ) stained aspirate slide demonstrates a polymorphous population of lymphoid cells with admixed histiocytes. (b) The follicular cells demonstrate Hürthle cell change with abundant granular cytoplasm and enlarged, round nuclei with prominent nucleoli. (c) A core biopsy demonstrates interstitial fibrosis, lymphoid aggregates, Hürthle cell change, and numerous small follicles (microfollicles)
Core Biopsy
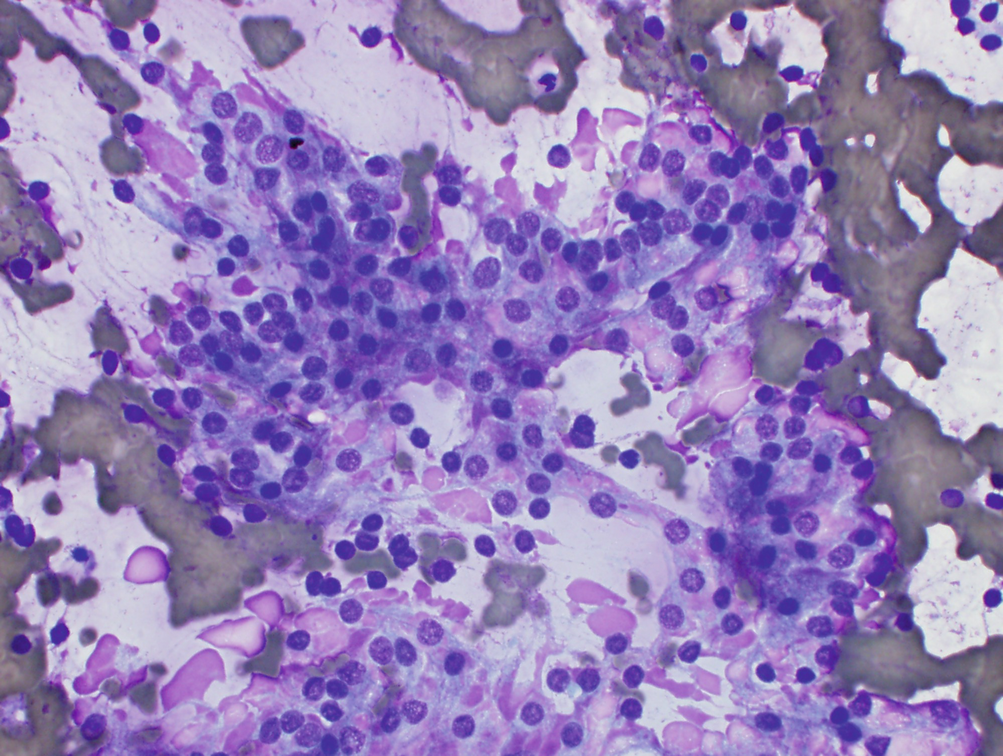
Needle core biopsy shows small follicles with focal lymphoid infiltrate in a background of fibrosis. The follicular cells often have Hurthle cell change with abundant eosinophilic granular cytoplasm and prominent nucleoli (Fig. 8.2c).
Comment
A microfollicular pattern is often seen in aspirates from cases in that colloid is often sparse and three-dimensional cellular clusters as well as microfollicles are frequently seen. If a lymphoid component is present in the aspirate, architectural atypia should be disregarded unless it is extremely striking.
Similarly, in cases of chronic lymphocytic thyroiditis, nuclear enlargement, elongation, and pallor may be seen. Unless the nuclear features of papillary thyroid carcinoma are incontrovertible, this cytologic atypia should be ignored. Of note: intranuclear pseudoinclusions, if strictly defined, are not seen in cases of chronic lymphocytic thyroiditis.
Graves Disease
Clinical
Autoimmune thyroid disease found most commonly in young women.
Clinically presents with ophthalmopathy (exophthalmos) and dermopathy (pretibial edema).
Increased T3 and T4, decreased TSH, and IgG antibodies to TSH receptor.
Ultrasound
In Graves disease there is mild to moderate diffuse, symmetric enlargement of the thyroid gland, including the isthmus (Fig. 8.3).
On US, there is a hypoechoic, heterogeneous, “spotty” parenchymal echo pattern [6].
There is a marked increase in parenchymal vascularity on color Doppler, which is sometimes referred to as “thyroid inferno” [9].
The hypervascularity tends to decrease in response to treatment [10].
Graves disease . (a) The right thyroid lobe is enlarged, heterogeneous, and hypoechoic. (b) Color Doppler demonstrates marked parenchymal hypervascularity or “thyroid inferno.” (c) A 24-hour I-123 uptake scan demonstrates a markedly elevated uptake of 74% in a diffusely enlarged thyroid gland. There is diffusely prominent uptake in both lobes
Nuclear Medicine
Technetium-99 m pertechnetate or iodine-123 scans show homogeneously increased uptake with high target to background levels. There is increased radioactive iodine uptake (normal is 8–25%), with an uptake exceeding 50–80%; this confirms the diagnosis of Graves disease.
Gross
Diffusely, symmetrically enlarged thyroid with a beefy red cut surface.
FNA
Marked cellularity
Flame cells (fire flare): follicular cells with blebs and marginal vacuoles containing colloid (Fig. 8.4).
Lymphocytes, Hurthle cells, and giant cells may be present. Colloid is typically scant [11].
Three-dimensional cell groups and papillary clusters can be seen but lack the nuclear features of papillary thyroid carcinoma.
Graves disease . The follicular cells in this aspirate slide are enlarged with large round nuclei. Marginal vacuoles containing colloid can be seen at the periphery of the cells, and magenta colloid is present in the background
Core Biopsy
Scant colloid with hyperplastic columnar follicular cells with papillary formations and lymphocytic infiltrate. Scalloping of colloid may be seen at the luminal surface of the follicular cells.
Comment
While the follicular cells in Graves disease are enlarged and contain large nuclei, the nuclear: cytoplasmic ratios are low in contrast to those of papillary thyroid carcinoma.
Papillary hyperplasia may be seen in aspirates from cases of Graves disease. As such, papillary fronds in the absence of the nuclear features of papillary thyroid carcinoma should not be considered atypical or diagnostic for malignancy.
Hyperplasia/Multinodular Goiter
Clinical
A hyperplastic multinodular goiter in some cases may reflect impaired synthesis of thyroid hormone.
Hyperplastic nodules can be solitary or present in a background of a multinodular thyroid gland.
If extensive, multinodular hyperplasia may present with airway obstruction, dysphagia, or dyspnea.
Radiography
On chest radiograph, an enlarged thyroid gland may result in tracheal deviation.
Ultrasound
Hyperplasia/multinodular goiter . (a) There is enlargement of the right thyroid lobe with several well-defined solid and mixed solid and cystic nodules of varying echogenicity. (b) There is enlargement of the left thyroid lobe with several well-defined solid and mixed solid and cystic nodules of varying echogenicity
Computed Tomography (CT)/Magnetic Resonance Imaging (MRI)
There is diffuse, heterogeneous enhancement on postcontrast imaging with multiple solid and cystic masses. CT or MRI can be used to better evaluate the mediastinal extent of the goiter and the severity of airway compression.
Gross
Enlarged thyroid with solid and cystic nodules of various sizes; may be asymmetric.
Fine Needle Aspiration
Benign follicular epithelial cells in a background of abundant colloid (Fig. 8.6a).
Bland and uniform follicular epithelial cells in a flat monolayer sheet with delicate cytoplasm, round nuclei, and granular chromatin (Fig. 8.6b).
Variable cellularity. May present with abundant colloid and rare follicular cells, or colloid may be moderate with high epithelial cellularity.
Other cellular patterns seen include intact follicles (spherules), tissue fragments and dissociated single follicular cells, and largely cystic aspirates (colloid cysts). A minor microfollicular component may be seen.
Hurthle cells are a variant of follicular epithelial cells with more abundant granular cytoplasm, mildly enlarged round nuclei, and prominent nucleoli.
Involuting/degenerating nodules can show a variety of changes, some of which can cause concern radiologically or cytomorphologically. These include pigment-laden foamy macrophages and multinucleated giant cells, cyst-lining cells, mesenchymal repair, dystrophic calcifications, and usually birefringent calcium oxalate and/or cholesterol crystals and perifollicular fibrosis.
Hyperplasia/multinodular goiter . (a) This DQ-stained aspirate slide contains abundant colloid in the background as well as flat sheets of thyroid follicular cells with round small nuclei. (b) In this Papanicolaou-stained aspirate slide, the follicular cells form a flat sheet. The granular chromatin pattern and the lack of nuclear crowding can be appreciated. (c) A core biopsy demonstrates numerous follicles of varying sizes
Comment
A predominant microfollicular component with scant colloid, three-dimensional follicular cell clusters, and microfollicles (follicles less than 12–15 cells in circumference) may be seen in aspirates from hyperplastic thyroid nodules, thereby raising concern for the presence of a follicular neoplasm. If a significant macrofollicular component composed of abundant colloid and flat sheets of follicular cells is seen, the microfollicular component may be safely disregarded.
If using smears, we recommend that the FNA pass numbers are noted on the slides so that one can get a sense of the extent of sampling and cytologic-histologic correlation
Malignant Neoplasms: Primary
Papillary Thyroid Carcinoma (PTC)
Clinical
The most common thyroid malignancy (85% or greater); it can be accurately diagnosed by FNA (96–100% confirmed as PTC on histologic examination).
Usually an asymptomatic thyroid nodule; locally advanced cases may present with lymphadenopathy.
More common in women (in Japan 9:1).
Associated with radiation exposure, Hashimoto thyroiditis, familial adenomatous polyposis coli (FAP), Cowden syndrome, and possibly Carney complex [13].
Overall excellent prognosis; 10-year survival rate in excess of 95%.
Poor prognostic features include male sex, older age, gross extrathyroidal extension, and large size.
Ultrasound
Common sonographic features are a hypoechoic texture, microcalcifications (correspond to psammomatous calcifications), well-defined margins, and intrinsic hypervascularity (Fig. 8.7) [14].
Punctate microcalcifications are highly specific for papillary carcinoma and are seen as small echogenic foci that may or may not demonstrate posterior shadowing.
Involved lymph nodes are usually hyperechoic to the adjacent muscle and contain microcalcifications or show cystic change [15].
Large tumors may show signs of invasion into adjacent muscle or the esophagus, trachea, or vessels.
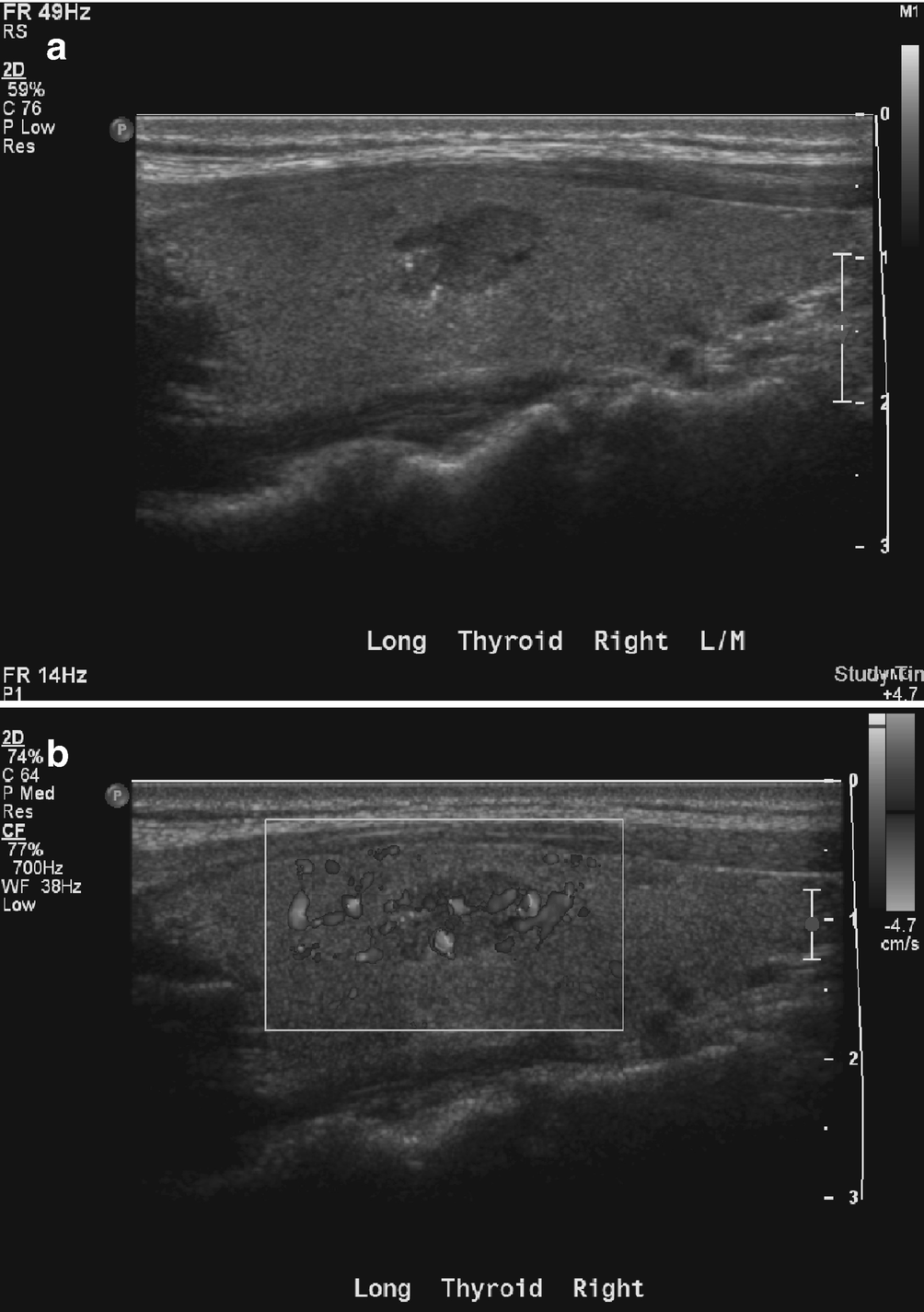
Papillary thyroid carcinoma . (a) There is a well-defined hypoechoic right thyroid lobe nodule with several echogenic microcalcifications. (b) On color Doppler imaging, the nodule demonstrates intrinsic hypervascularity
Computed Tomography/Magnetic Resonance Imaging
CT or MRI may be used to stage large tumors. Although the findings are nonspecific, the primary tumor may demonstrate heterogeneous enhancement on postcontrast imaging with invasive margins, which is the key to diagnosis.
Nuclear Medicine
Diagnostic Iodine-131 scan is performed after thyroidectomy to detect local recurrence or metastatic disease. If the scan is positive, therapeutic ablation with I-131 may be performed.
Gross
Papillary thyroid carcinoma is grossly often a poorly circumscribed firm white scar-like lesion.
May be well-circumscribed, diffuse, or multifocal.
Fine Needle Aspiration
Typical aspirate is cellular with irregular sheets of follicular epithelium (Fig. 8.8a). In most cases, papillary fragments are absent or rare.
Flat, crowded sheet of neoplastic epithelial cells with nuclear enlargement and ovoid nuclei with dense cytoplasm and conspicuous cell borders. The background frequently contains macrophages (Fig. 8.8b).
Sheet of neoplastic cells with ovoid nuclei, fine, pale chromatin, and inconspicuous nucleoli; occasional nuclear grooves and intranuclear pseudoinclusions are typical (Fig. 8.8c) [16].
Papillary carcinoma can have a wide variety of architectural patterns: classic papillary, follicular variant, and cystic. The most commonly seen architectural pattern is crowded monolayer sheets of cells.
The major diagnostic features are nuclear and include:
Fusiform/oval nuclei with fine powdery chromatin
Intranuclear cytoplasmic pseudoinclusions
Linear chromatin ridges (i.e., nuclear grooves)
Single small nucleolus, often located near the nuclear membrane
Minor diagnostic criteria include:
Architecture: syncytial sheets, papillary tissue fragments with fibrovascular cores, and swirl-like pattern of cell clusters [17]
Dense squamoid cytoplasm and septate cytoplasmic vacuoles
Bubble gum colloid; dense magenta colloid on modified Giemsa stain
Multinucleated foreign body-type giant cells
Psammomatous calcifications
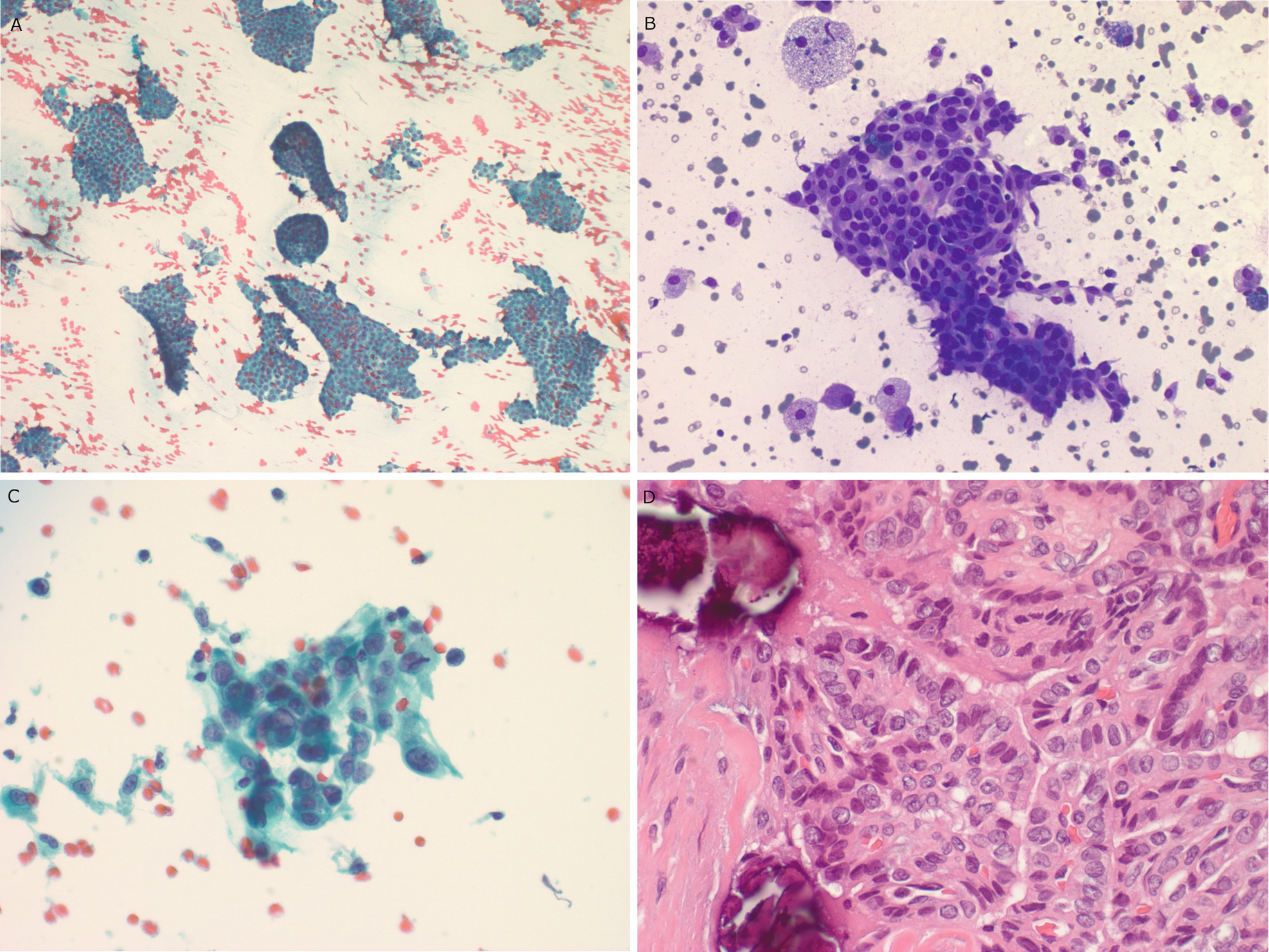
Papillary thyroid . (a) Sheets of follicular cells with crowded elongated nuclei are seen in this Papanicolaou-stained aspirate slide. (b) Crowded sheets of follicular cells with ovoid nuclei are seen in this DQ-stained aspirate slide. Macrophages and cystic debris are seen in the background. (c) Scattered intranuclear pseudoinclusions can usually be identified in aspirate smears. (d) In this histologic section, compressed papillary fronds can be seen with crowded ovoid nuclei, nuclear pallor, and grooves. Lamellated psammomatous calcifications are visible in the background
Core Biopsy
Papillary fronds lined by neoplastic cells with ovoid to elongated nuclei with nuclear membrane irregularities, fine chromatin with clearing, indistinct nucleoli, and occasional nuclear grooves. Psammomatous calcifications are present (Fig. 8.8d)
Comment
The newly described entity, noninvasive follicular tumor with papillary thyroid carcinoma-like nuclei (NIFTP) , is morphologically extremely similar to papillary thyroid carcinoma in cytologic preparations [18]. In resected specimens, this tumor is characterized by a follicular growth pattern, circumscription, and a lack of capsular or vascular invasion. The nuclei seen in these tumors are extremely similar to those seen in cases of papillary thyroid carcinoma. Based on currently available data, this tumor can be expected to behave in an indolent fashion when strict diagnostic criteria are used. These tumors cannot be reliably distinguished from conventional papillary thyroid carcinoma in aspirate specimens or core biopsies. This diagnosis may be considered when the nuclear features of papillary thyroid carcinoma are seen but intranuclear pseudoinclusions, true papillae, and psammoma bodies are absent [19].
Ancillary studies such as thyroglobulin on the FNA specimen from the thyroid bed or from a lymph node to exclude metastatic disease can be useful when cytology is less than definitive.
Medullary Thyroid Carcinoma
Clinical
Accounts for 5–10% of cases of thyroid carcinoma.
Neuroendocrine tumor derived from either the parafollicular cells or C cells of the thyroid gland, which produce calcitonin.
Eighty percent are sporadic; 20% are associated with MEN syndrome IIA or IIB or are familial forms of medullary thyroid carcinoma [13]. Multicentricity is seen in familial cases.
Generally present at a higher stage than well-differentiated thyroid carcinomas. Seventy-five percent of patients with a palpable thyroid mass have metastases to the cervical lymph nodes.
While good outcomes are seen in patients with disease confined to the thyroid (95% 10-year survival in adults), patients with regionally advanced disease have a 70% 10-year survival rate.
Ultrasound
Medullary carcinoma is usually hypoechoic and solid, with well-defined borders (Fig. 8.9).
Echogenic foci may be seen in 80–90% of lesions and are usually dense and coarse.
Metastatic lymph nodes are usually hypoechoic with coarse calcifications that demonstrate posterior shadowing.
Color Doppler imaging shows chaotic intratumoral and intranodal vascularity.
On US, medullary carcinoma is difficult to differentiate from papillary carcinoma. The best clues for differentiation are the presence of shadowing tumoral calcification (punctuate in papillary) and hypoechoic nodes (hyperechoic in papillary) with coarse shadowing [20].
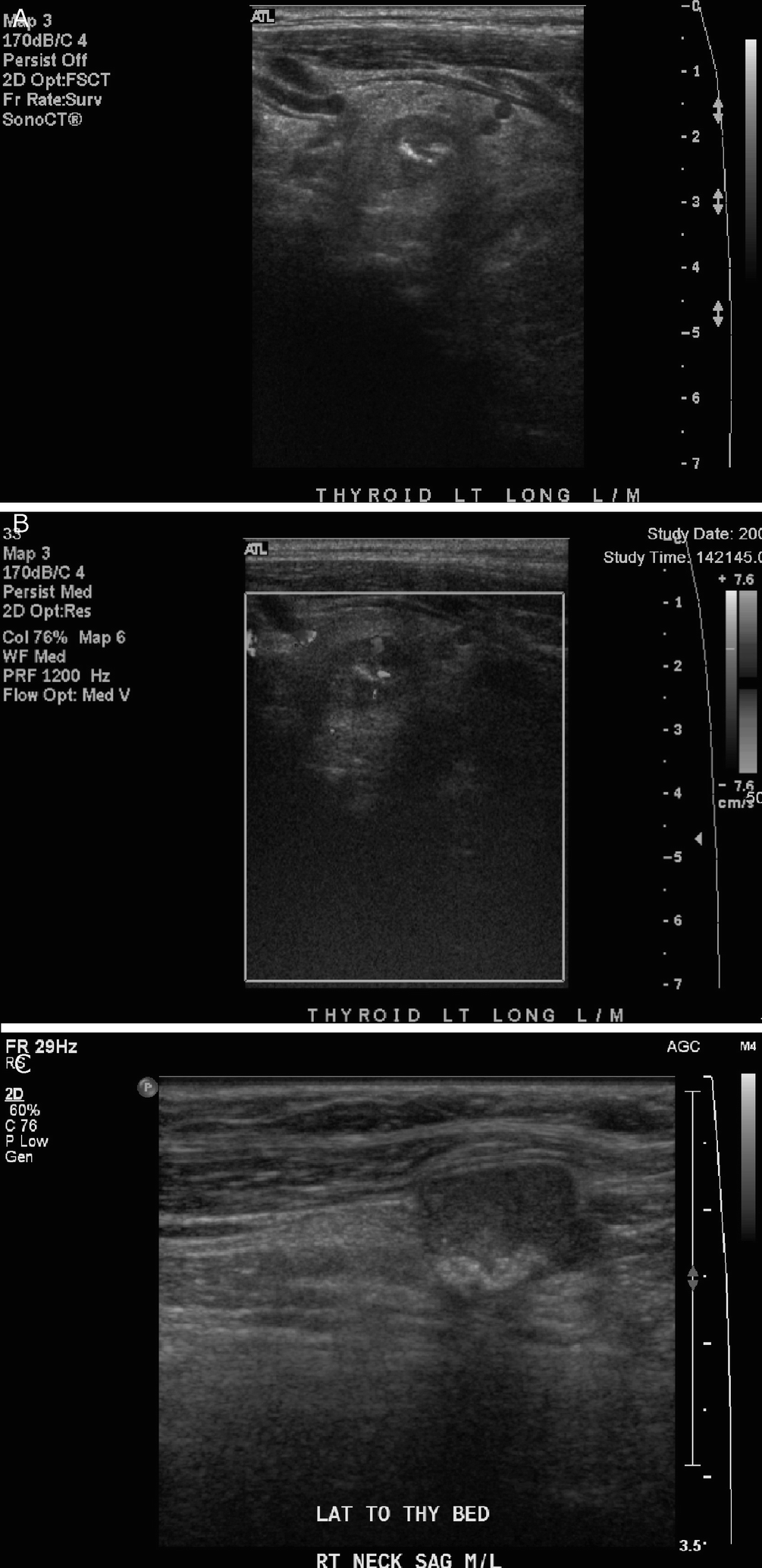
Medullary thyroid carcinoma . (a) There is a well-defined hypoechoic left thyroid lobe nodule with several large coarse echogenic calcifications that demonstrate posterior acoustic shadowing. (b) On color Doppler imaging, there is some intratumoral vascularity. (c) In a different patient with metastatic medullary thyroid carcinoma, there is a hypoechoic right cervical lymph node with several coarse echogenic calcifications at the posterior aspect that demonstrate posterior acoustic shadowing
Nuclear Medicine
Positron emission tomography (PET) scan using fludeoxyglucose (FDG) may be used when there are elevated tumor markers but no gross disease on cross-sectional imaging.
Octreotide scintigraphy (In-111-pentreotide) or I-131-MIBG may be taken up by the tumor.
Gross
Circumscribed nodule with a tan-yellow cut surface.
Commonly arises in the upper and middle third of the thyroid lobe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree