Fig. 1
IgA nephropathy. (a) Kidneys present normal morphological appearance on grayscale ultrasound, and (b) normal renal parenchymal vascularization is revealed by power Doppler ultrasound. (c, d) On duplex Doppler, normal resistive index values (0.62) are revealed
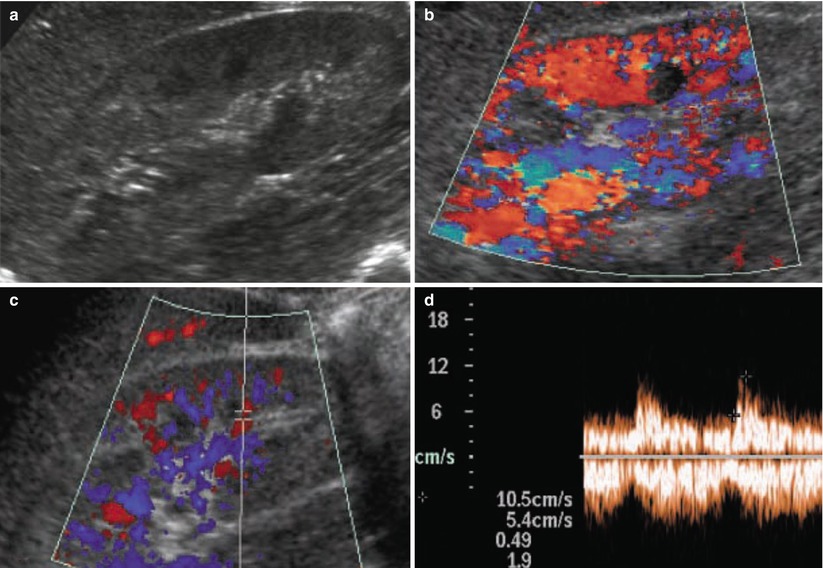
Fig. 2
Membranous glomerulonephritis. (a) Kidneys reveal a slight increase in cortical echogenicity with an increased corticomedullary differentiation on grayscale ultrasound. (b) Normal renal parenchymal vascularization is revealed by color Doppler ultrasound, whereas on (c, d) duplex Doppler, normal resistive index values (0.49) are revealed
1.2 Renal Vasculitides
Since vascular and interstitial components are the most represented in renal parenchyma and since they are both involved in renal vasculitides, US may reveal early involvement. Renal vasculitides, such as Wegener granulomatosis (Fig. 3) and polyarteritis nodosa (Fig. 4), manifest as an increased cortical echogenicity with reduced corticomedullary differentiation. US may also reveal multiple cortical hypoechoic areas of variable size and shapes with cortical distortion, expressing regions of parenchymal edema. The RI values are significantly correlated with creatinine level and presence of interstitial disease.
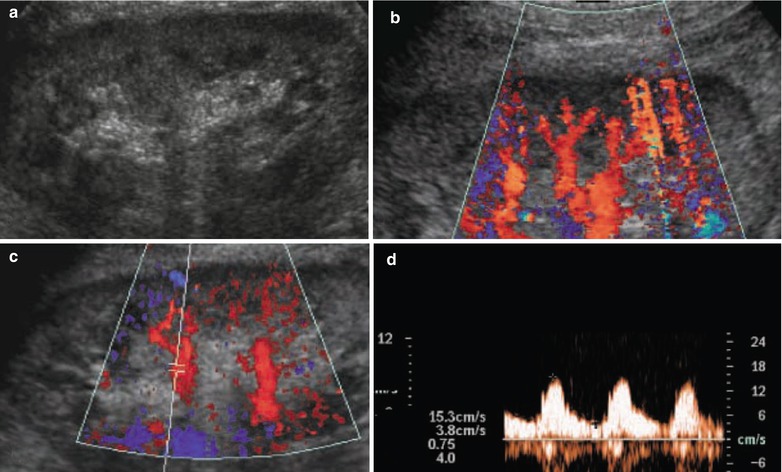
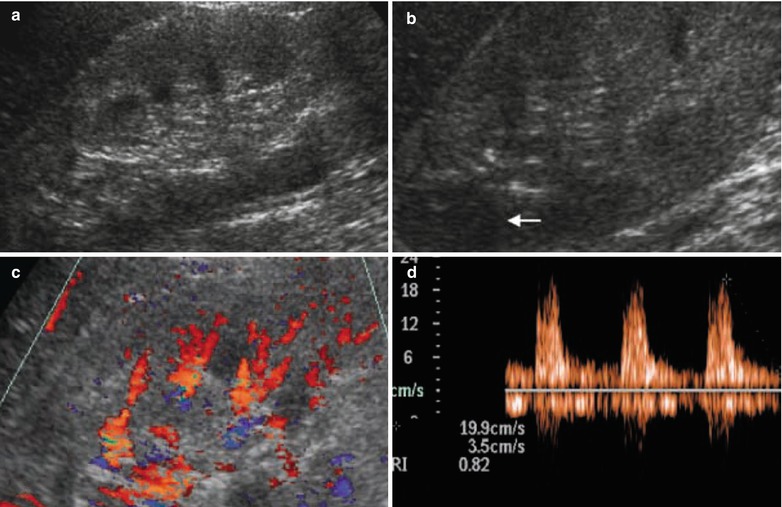

Fig. 3
Wegener granulomatosis. (a) On grayscale ultrasound, kidneys reveal a clear increase in renal parenchymal echogenicity with a low corticomedullary differentiation, whereas on color Doppler ultrasound, (b) a clear reduction of renal parenchymal perfusion is documented. (c, d) Duplex Doppler reveals diffusely increased resistive index values (0.75)

Fig. 4
Polyarteritis nodosa. (a) On grayscale ultrasound, kidneys reveal a clear increase in renal parenchymal echogenicity, with a poor corticomedullary differentiation and (b) some hypoechoic cortical areas (arrow) which can be an expression of localized cortical edema. (c) Reduced renal parenchymal vascularization is documented on color Doppler ultrasound, whereas (d) a clear increase in resistive index values (0.82) is revealed by pulsed Doppler
Polyarteritis nodosa is a systemic necrotizing vasculitis of medium and small arteries which consists of foci of fibrinoid necrosis that begin in the media. Renal involvement occurs in 90 % of patients with polyarteritis nodosa. Inflammation then spreads to involve the intima and adventitia and results in small aneurysms and vascular thrombosis. These small aneurysms occur at the bifurcation of interlobular or arcuate arteries and are best seen at digital subtraction angiography (Fig. 5), even though they can be effectively identified by contrast-enhanced CT during the corticomedullary phase (Fig. 6). Subsequent renal ischemia occurs, and renin-mediated hypertension is common. The small aneurysms seen in polyarteritis nodosa may occasionally rupture with resultant intraparenchymal or perinephric hematoma. Bilateral multiple small wedge-shaped hypovascular area (Figs. 6 and 7) is identified in the renal parenchyma on contrast-enhanced CT (Pope et al. 1981; Ozaki et al. 2009) corresponding to multiple renal cortical infarctions from vasculitis of the interlobar and arcuate arteries. Perirenal hematoma by aneurysmal rupture represents another typical imaging finding (Wilms et al. 1986; Ozaki et al. 2009).
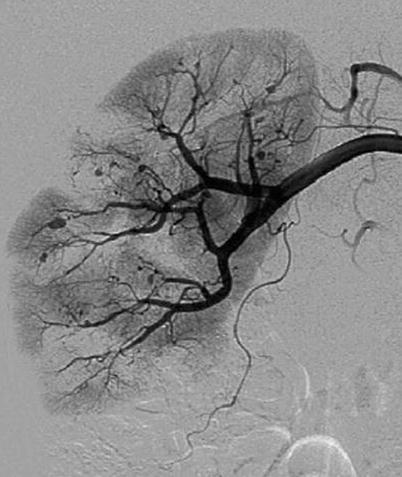
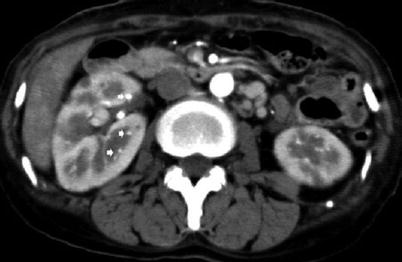
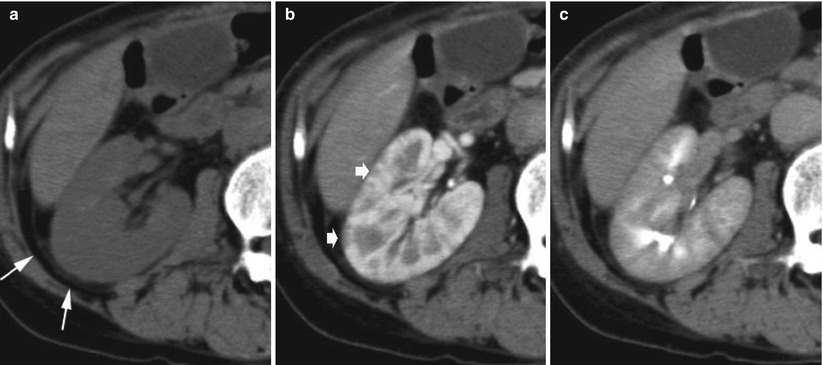

Fig. 5
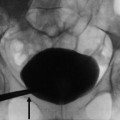
Polyarteritis nodosa. A 51-year-old man with fever of unknown origin. Digital subtraction angiography of the right renal artery. Multiple microaneurysms located at the interlobular and arcuate arteries. Irregularities, narrowing, ectasia, and occlusion in small arteries are also identified. Multiple wedge-shaped hypoperfusion areas are demonstrated (Courtesy of Dr. Kumi Ozaki, Kanazawa, Japan)

Fig. 6
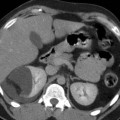
Polyarteritis nodosa. A 48-year-old woman. Contrast-enhanced CT during corticomedullary phase shows multiple microaneurysms (arrows) located at the interlobular and arcuate arteries and multiple wedge-shaped hypoperfusion areas in both kidneys (Courtesy of Dr. Kumi Ozaki, Kanazawa, Japan)

Fig. 7
Polyarteritis nodosa. A seventy-one-year-old man with fever of unknown origin. (a) Unenhanced CT shows diffuse enlargement and slight thickening of Gerota’s fascia (arrows). (b) Contrast-enhanced CT during corticomedullary phase shows multiple small wedge-shaped less-enhanced areas (arrows). The margin between the cortex and medulla is indistinct during the excretory phase (c) (Courtesy of Dr. Kumi Ozaki, Kanazawa, Japan)
2 Systemic Diseases Involving the Kidney
2.1 Diabetic Nephropathy
Renal disease is an important cause of morbidity in patients with diabetes mellitus, and kidney is involved in 30–50 % of patients with long-standing type I insulin-dependent diabetes mellitus (juvenile-onset diabetes mellitus) and in 1–2.5 % of patients with non-insulin-dependent diabetes mellitus (Soldo et al. 1997). Diabetes mellitus determines the development of end-stage renal disease in approximately 25–40 % of patients entering chronic dialysis (Myers 1992). Although dialysis and transplantation prevent death from uremia, the 5-year survival in diabetic patients with end-stage renal disease is much worse than that of nondiabetic patients. Therefore, it is important to recognize the very early stage of diabetic nephropathy and to institute appropriate therapy, especially when urinary albumin excretion is >300 mg/day.
Diabetic glomerulopathy, a complex disorder associated with a diffuse expansion of collagenous component of the glomerulus with accumulation of extracellular matrix resulting in an expansion of mesangium and thickening of glomerular basement membrane (diffuse intercapillary glomerulosclerosis), is the predominant cause of renal failure (Myers 1992). The diabetic patient is also prone to other renal disease, including pyelonephritis, papillary necrosis, and obstructive nephropathy which lead to renal failure, and diabetic patients with glomerulopathy are more susceptible to these associated renal diseases (Myers 1992). Several factors have been implicated in the development of diabetic nephropathy including poor glycemic control (fasting plasma glucose >140–160 mg/dL), genetic factors, hemodynamic abnormalities (increased GFR), systemic hypertension, altered vascular permeability, metabolic disturbance, and hyperlipidemia. According to the Diabetes Control and Complications Trial Research Group (DCCT) (1993) and the United Kingdom Prospective Diabetes Study (UKPDS) (2005), the tight glycemic control has been shown to be the primary prevention from all microvascular complications in the diabetic patient including nephropathy, retinopathy, and neuropathy.
Early abnormalities of glomerular function appear almost in all patients with type I insulin-dependent diabetes mellitus, but only part of them will develop a progressive proteinuric form of diabetic glomerulopathy. The stage 1 of diabetic glomerulopathy is devoid of clinical symptoms and signs of glomerulopathy, and it manifests with a 20–40 % elevation of the GFR above that found in age-matched normal control subjects due to a generalized hypertrophy of the glomeruli (Myers 1992). Stage 2 of diabetic glomerulopathy is characterized by increasing proteinuria (with a microalbuminuria >250 μg/min), reduced GFR, and development of hypertension and edema. Diffuse thickening of the glomerular basement membrane is noted along with increased mesangial volume. The stage 3 of diabetic glomerulopathy represents the last 2–3-year advanced stage of what is a 20–25-year process, and it is characterized by the development of azotemia and progressive worsening of hypertension and edema. At this stage, the mesangium further expands and occupies a greater portion of the glomerular volume, while the thickness of the glomerular basement membrane is not necessarily increased. Frequently, characteristic nodular hyaline-like deposits situated at the periphery of the glomerulus, named nodular glomerulosclerosis or intercapillary glomerulosclerosis or Kimmelstiel–Wilson lesions, are evident in the center of the peripheral glomeruli (Olefsky 1992).
Diabetic patients, with initial renal involvement, present enlarged kidneys, increased parenchymal thickness, and increase of the renal parenchymal echogenicity with increased visibility of renal pyramids and corticomedullary differentiation on grayscale US (Fig. 8). This appearance is related to the increased GFR in the initial stages of renal disease, whereas in diabetic patients with mild renal involvement, renal length and parenchymal thickness are usually normal. In diabetic patients undergoing hemodialysis renal length is reduced. In advanced diabetic nephropathy, renal parenchymal echogenicity may appear increased or normal according to vascular and interstitial compartment involvement, whereas renal margins are usually diffusely irregular. Multiple irregularities of the renal cortex profiles with indentation between renal calyces may simulate fetal lobations even though in diabetic nephropathy the overall parenchyma thickness is reduced by atrophy (<12 mm). The RIs are typically elevated in advanced diabetic nephropathy, whereas RIs are often normal in the early stage of disease (Platt et al. 1994). The RIs are highly correlated with serum creatinine concentration and creatinine clearance rate, whereas an elevated RI (≥0.70) is associated with impaired renal function, increased proteinuria, and poor prognosis (Platt et al. 1994).
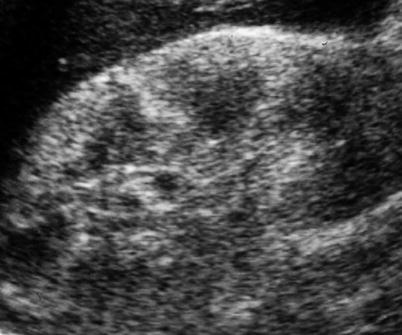

Fig. 8
Diabetic nephropathy. Grayscale US. Longitudinal scan. Enlarged kidneys and increased parenchymal thickness with diffuse increase of the renal parenchymal echogenicity with increased visibility of renal pyramids and corticomedullary differentiation
2.2 Hypertension
Coexistence of hypertension and decreased renal function may be due to nephrosclerosis secondary to hypertension, or primary renal disease with secondary hypertension. Nephrosclerosis represents the second most common diseases that result in referral of patients for transplantation (Curtis 1992). Even though hypertension is better treated than in the past, yet the incidence of end-stage renal failure due to hypertension has not decreased. Kidney transplantation in this group of patients often restores normal renal function and normal blood pressure control.
Benign nephrosclerosis is the term used for the renal pathology associated with sclerosis of the renal arterioles and small arteries due to medial and intimal thickening as a response to hemodynamic changes, aging, genetic defects, or some combination of these and to hyaline deposition in the arterioles (Alpers 2005). The resultant effect is focal ischemia of the renal parenchyma supplied by the vessels with thickened walls and consequent narrowed lumen (Alpers 2005). Some degree of nephrosclerosis is present at autopsy with increasing age preceding or in the absence of hypertension. Hypertension and diabetes mellitus increase the incidence and severity of the lesions. Malignant nephrosclerosis is the form of renal disease associated with the malignant or accelerated phase of hypertension (Alpers 2005). This pattern of hypertension may occasionally develop in previously normotensive individuals but often is superimposed on preexisting essential benign hypertension, secondary forms of hypertension (renal, endocrine, vascular, or neurogenic), or an underlying chronic renal disease, particularly glomerulonephritis or reflux nephropathy (Alpers 2005). Kidneys present a size which is related to the duration and severity of the hypertensive disease. Small petechial hemorrhages may appear on the renal cortical surface from rupture of the arterioles or glomerular capillaries. The two histological landmarks of malignant nephrosclerosis are the fibrinoid necrosis of the arterioles and the hyperplastic arteriolitis with the typical onion-skinning appearance.
The renal vascular alterations of hypertension depend on the severity of the blood pressure elevation and whether the process accelerates to malignant hypertension. Arteriolosclerosis of the small cortical renal arteries, interlobar, arcuate, and interlobular, is a common feature of the kidney in patients with systemic arterial hypertension (Quaia and Bertolotto 2002). In nephroangiosclerosis both kidneys are usually symmetrically reduced in their diameters with reduction of the renal parenchymal thickness, cortical scars, or renal contour irregularities and frequently present an increased cortical echogenicity with increased corticomedullary differentiation (Fig. 9a). Color and power Doppler US reveal nonspecific reduction of vascularization. If compared to normal patient population, renal RIs are typically increased (>0.7 and frequently around 0.8) (Fig. 9b). Renal perforating arteries and veins (Bertolotto et al. 2000) are much more visible in kidneys of nephroangiosclerotic patients in comparison with normal subjects, since they enlarge in nephroangiosclerosis and present normally directed flows from the kidney toward the renal capsule. Renal RIs are increased with values ranging from 0.8 to 0.98.
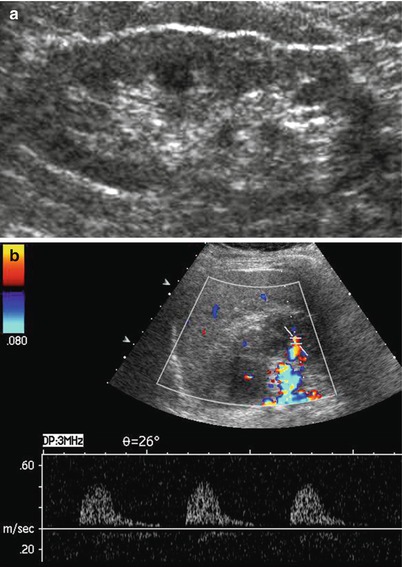

Fig. 9
Nephroangiosclerosis. (a, b) A seventy-year-old patient with slightly increased creatinine levels (1.5 mg/dL) and hypertension (170/110 mmHg). (a) Longitudinal US scan of the left kidney. Reduction of the renal parenchymal thickness, renal contour irregularities, and increased corticomedullary differentiation. (b) Increased value of the resistive index (0.9) measured on a segmental artery
2.3 Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease involving multiple organs. The kidneys are the most commonly affected organs, with significant associated morbidity and mortality and renal involvement in 15–85 % of cases (Kim and Kim 1990). Renal lesions in systemic lupus erythematosus are classified into five types: type 1, normal or minimal-change disease; type 2, mesangial glomerulonephritis; type 3, focal proliferative glomerulonephritis; type 4, diffuse proliferative glomerulonephritis; and type 5, membranous glomerulonephritis (Kim and Kim 1990).
Grayscale US has been reported to have a sensitivity of 95 % in lupus nephritis detection (Longmaid et al. 1987). In lupus nephritis, kidneys may present reduced or increased dimensions and an increased cortical echogenicity with reduced corticomedullary differentiation. US may also reveal multiple cortical hypoechoic areas of variable size and shapes with cortical distortion, expressing regions of parenchymal edema. The RI values are significantly correlated with creatinine level and presence of interstitial disease, whereas normal RI values are considered as a good prognostic factor (Platt et al. 1997).
2.4 Metabolic and Hematological Disease-Related Nephropathies
In hyperoxaluria, and particularly in enteric hyperoxaluria due to enhanced absorption of dietary oxalate in patients with ileal disease, kidneys present normal or reduced dimensions with smooth margins and a hyperechoic renal cortex and medulla. In gout nephropathy hyperechoic papillary spots or a diffuse hyperechoic medulla may be observed. Renal stones are usually present, while kidneys present normal or reduced dimensions with smooth margins. In nephrocalcinosis, hyperechoic calcium depositions are observed mainly in renal cortex but also in renal pyramids.
The kidneys are frequently involved in all forms of systemic amyloidosis and represent major complications of the disease (Amendola 1990) both in the AL (amyloid light chain) and AA (amyloid-associated) types (Kim and Kim 1990




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree