Tropical diseases affecting the central nervous system include infections, infestations, and nutritional deficiency disorders. This article discusses the commonly encountered diseases. The infections include bacterial, mycobacterial, fungal, parasitic, and viral infections with varied clinical manifestations. Imaging sensitivity and specificity for the prediction of the cause of infections has improved with application of advanced techniques. Microbial demonstration and histology remain the gold standard for diagnosis. Understanding the basis of imaging changes is mandatory for better evaluation of images. Nutritional disorders present with generalized and nonspecific imaging manifestations. The pathology of commonly encountered vitamin deficiencies is also discussed.
Tropical diseases affecting the central nervous system (CNS) include a wide array of infections and infestations as well as nutritional deficiency disorders. It is a vast subject, so in this article the description is limited to the commonly encountered diseases. The infections include bacterial, mycobacterial, fungal, parasitic, and viral infections with varied clinical manifestations. Imaging sensitivity and specificity for the prediction of the cause of the infection has improved with application of advanced techniques. Microbial demonstration and histology remain gold standard for diagnosis. Understanding the pathology basis of imaging changes is mandatory for better evaluation of images. Nutritional disorders present with generalized and nonspecific imaging manifestations. The pathology of commonly encountered vitamin deficiencies, like thiamine and B 12 deficiency, are also discussed.
Bacterial infections
Bacterial infections of the CNS include suppurative acute, subacute, and chronic infections. Mycobacterial CNS disease forms a large proportion of cases and is discussed separately. Bacterial infections may manifest as meningoencephalitis, brain abscess, and subdural empyemas.
Brain Abscess
Brain abscess is a capsulated focal suppurative lesion within the brain parenchyma that begins as a localized area of cerebritis. The incidence of brain abscess in the developed world is as low as 1% to 2%, whereas in the developing countries it is up to 8% of all intracranial space-occupying lesions.
Infection may be direct from the contiguous site, such as otitis media or sinusitis, after trauma or neurosurgical procedures, or may be caused by a bacteremia from a distant primary source such as bacterial endocarditis. Patients with pulmonary arteriovenous shunts or hereditary hemorrhagic telangiectasias are also prone to develop cerebral abscesses. Pyogenic abscesses of hematogenous origin are solitary in more than 50% of cases and are usually located at the gray-white matter junction in the anterior or middle cerebral artery distribution.
Most abscesses are produced by pyogenic bacteria. In one-third of patients, more than 1 type of organism is found. Frequently isolated microbes include streptococci (both aerobic and anaerobic) and staphylococci. Both aerobic and facultative anaerobes may be present.
Pathogenesis
When bacteria attach to the parenchyma vessel wall and pass into the parenchyma, they cause disruption of the blood-brain barrier and facilitate brain invasion.
Brain abscess evolves through 4 stages initiating with early cerebritis, and progressing to late cerebritis, early capsule formation, and late capsule formation. Early cerebritis stage is the initial stage of cerebritis occurring during the first 4 to 5 days of infection. During this stage, the brain reacts by developing an area of local inflammation with vascular congestion, petechial hemorrhage, and edema. By the end of first week during the late cerebritis stage, 1 or more microabscesses with acute inflammation and surrounding edema are evident. Granulation tissue with proliferating new vessels and fibroblasts starts developing around the abscess in the early capsule stage by the end of the second week and is characterized by the formation of a collagenous capsule surrounding the liquefied necrotic core. During the late capsular stage, a thick capsule consisting of 3 layers develops: an inner layer of granulation tissue infiltrated by lymphocytes and macrophages, a middle collagenous layer, and an outer gliotic layer with reactive astrocytes and proliferating blood vessels. It is not always possible to separate these layers on histopathology. The wall is lined by necrotic material with an inner polymorphonuclear infiltrate ( Fig. 1 ). Organisms may be detected by special staining techniques. Identification of the causative organism requires a battery of special stains in histology including Gram stain; Gomori methenamine silver (GMS) for fungus, actinomycosis, and nocardia; Ziehl Neilson stain for acid-fast bacilli (AFB); and Warthin-Starry for spirochetes. The sensitivity of tissue staining methods in the identification of organisms is low. On magnetic resonance (MR) images, mature pyogenic abscesses show an isointense to slightly hyperintense rim on T1-weighted images that appears hypointense on T2-weighted images, a feature that could relate to coagulative necrosis; increased accumulation of hemorrhagic products and paramagnetic materials such as iron, magnesium, and manganese; and production of free radicals, secondary to bacterial metabolism.
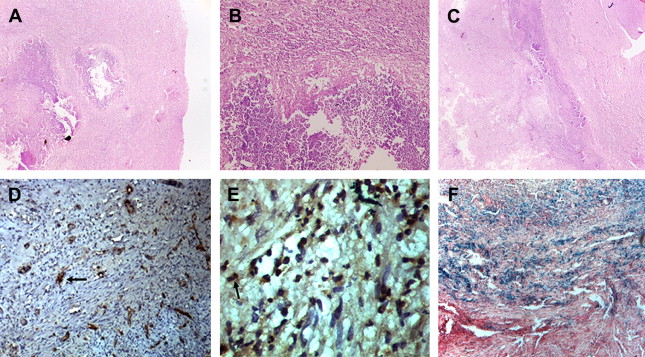
As the abscess heals, the cavity gradually shrinks. Complications of cerebral abscesses include ventriculitis, choroid plexitis, subdural empyema, and purulent leptomeningitis. Remnant fibrogliotic scars may result in epileptic foci.
Meningitis
Bacteria may cause an acute meningitis with neutrophilic pleocytosis or a subacute or chronic meningitis with a predominantly lymphocytic pleocytosis. Frequent causative pathogens are Streptococcus pneumoniae and Neisseria meningitidis in children and young adults, group B streptococci in newborns, and Listeria monocytogenes in newborns and the elderly. Haemophilus influenzae has shown a vaccine-related decline. The cornerstone in the diagnosis of bacterial meningitis is cerebrospinal fluid (CSF) examination. The CSF in meningitis shows hundreds, even thousands, of neutrophils and is teeming with organisms. CSF protein is increased and glucose is low. Staining and culture on appropriate media and other tests, including antigen detection in CSF and bacterial polymerase chain reaction (PCR), help clinch the diagnosis.
The exudates cover the cerebral hemispheres and settle along the base of the brain, around cranial nerves and the openings of the fourth ventricle. MR imaging shows enhancement and high fluid-attenuated inversion-recovery (FLAIR) signal intensity in the meninges, corresponding with the pathology. Brain damage in meningitis is caused not only by bacteria but probably more by host responses. These responses have a protective purpose (to eliminate bacteria) but are excessive and indiscriminate and set in motion destructive cascades that damage mostly host tissues. The results of inflammation are tissue and vascular injury (vasculitis) and increased intracranial pressure. Increased intracranial pressure is caused by increased vascular permeability and leakage of proteins in the interstitial space (cerebral edema) and CSF. Vasculitis causes infarcts, and increased intracranial pressure aggravates hypoxic-ischemic insult. The late complications of meningitis include cranial nerve deficits and ischemic infarction. The thick fibrinopurulent exudate in the subarachnoid space organizes into fibrous tissue that blocks the exits of the fourth ventricle and impairs CSF circulation around the cerebral convexities, causing hydrocephalus.
The infection is limited by a thick, tight mesh of astrocytic processes, joined by dense junctions and covered by basement membrane that resists penetration by bacteria and neutrophils. It provides an effective barrier that prevents the infection from spreading into brain tissue.
The commonest bacterial cause of chronic meningitis in the tropics is tuberculosis. Other pathogenic conditions and bacteria resulting in chronic meningitis include brucellosis, spirochetes (increasingly being encountered in human immunodeficiency virus [HIV] infection), borreliosis or relapsing fever ( Borrelia recurrentis , louse-borne; Borrelia duttonii , tick borne), and Lyme disease ( Borrelia burgdorferi , tick borne). The neural manifestations include meningitis, encephalitis, focal cranial neuropathies, radiculitis neuropathy, and encephalopathy. The pathology of chronic meningitis is discussed in detail later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree