CHAPTER 1 ![]()
Patient Education, Assessment, and Diagnostic Options
Summary of Important Points
PATIENT EDUCATION AND ASSESSMENT
Reasons for Patient Communication
• Helps to relax the patient
• Helps to identify patients with unusually sensitive breast
• Helps to educate the patient and combat misconception or fear of mammography
• Helps to identify concerns and answer questions before the patient leaves the facility
• Helps to ensure compliance with request for follow-ups
Factors that can Influence the Patient Compliance or Noncompliance
Influencing Compliance
• Pleasant experience
• Understanding mammographer
Influencing Noncompliance
• Painful experience
• Unsympathetic mammographer
The Role of the Mammographer
Link between patient and radiologist
• Provide appropriate clinical and didactic education
• Obtain patient’s medical information
• Conducts physical assessment of the patient’s breast
• Performs visual inspection
• Performs manual palpation
• Documents assessment findings
The Informed Consent
• Consent given by the patient before any invasive procedure or a procedure of significant risk
• Patient must be of legal age and mentally competent.
• Patient must offer consent voluntarily.
• Patient must be adequately informed about the medical care being given.
Types of Consent
• Written consent—consent given in writing. It should include the name of the health-care professional who discussed the proposed treatment with the patient, the name of the health-care provider who is to perform the procedure, and the date, time, and location where the consent was signed.
• Oral consent—consent given verbally.
• General consent or authorization for treatment—consent signed at the time of admission to a hospital or health-care setting. It authorizes general treatment and excludes invasive procedures.
• Expressed consent—assumed by nonverbal cues such as nodding or lifting the arm when told that an injection is required or oral.
• Implied consent—assumed in an emergency situation where the patient is unable to make a decision. Generally, additional documentation is required.
Objective and Subjective Data
• Objective data defines information collected from the patient that can be seen, heard, or felt, e.g., a lump in the breast.
• Subjective data is perceived by the affected individual only (e.g., pain in the breast). Subjective data should never be disregarded. However, it should be clearly defined.
Method of Questioning
• Listening (rather than asking questions) will allow the patients to tell their stories unasked. Listening is used to assess the patient’s life experience and educational background and can help in determining whether the patient will understand medical or technical terms for future questions.
• Open-ended questions use nondirected or nonleading methods to avoid leading the patient and to allow an unbiased input.
• Facilitation uses gestures such as a nod or saying “yes,” “okay,” or “go on” to encourage elaboration.
• Silence is used to give the patient time to remember and ensure accuracy.
• Probing questions are used to get more detail of the problem.
• Repetition will reword the question to clarify information and can be used to verify that the patient has not changed his/her mind.
• Summarization is used to verify the accuracy of the information.
Medical or Clinical History Documentation
• Used to document information on symptoms or any abnormalities of the breast
• Serves as a guide for the radiologist
Documentation Using the Medical or Clinical History
• Imaging history and patient limitations
• Previous mammogram—when and where it was obtained.
• The previous mammograms are vital for comparison.
• The mammographer should document any limitation (mental or physical) that will affect the imaging, e.g., the placement of a pacemaker could limit the imaging of the pectoral muscle on the mediolateral oblique (MLO).
• Signs or symptoms—palpable or visible abnormalities of the breast
• Changes in nipple or areola
• Nipple discharge
• Inverted nipple
• Dimpling or puckering of skin
• Skin thickening
• Accessory breast tissue or nipples can develop along the milk line. Examples include congenital abnormalities such as asymmetry in breast size, shape, or position.
• Accessory tissue must be documented.
• Imaging will depend on the policy of the facility.
• Persistent skin conditions such as swelling, eczema, itchiness, or redness.
• Lump/s in breast or axilla.
• Trauma to the breast especially recent trauma should be documented.
• Skin lesions or scars.
• Skin lesions or palpable lumps should be identified.
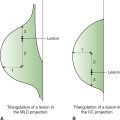
• Schematic diagram can be used to localize scars.
• Personal and family history of breast cancer
• Documentation of first-degree relatives with breast cancer and include the age cancer was detected.
• Major and/or minor risks factors.
• Surgical history related to the breast
• History of augmentation or reduction including type and date of surgery
• Type of breast biopsies with results and date of surgeries
• History of lumpectomy or mastectomy including dates of surgeries
• Autologous reconstruction—surgical flap procedures
• Childbearing or gynecological history
• Patient’s age at birth of first child
• Patient’s age at menarche
• Date of patient’s last menstrual period
• Patient’s age at menopause, hysterectomy, or oophorectomy
• Hormonal history including status of menstrual cycle
• Types of hormone taken including start date and duration
The Breast Self-Examination
The breast self-examination (BSE) can serve as an important tool in the detection of breast cancer. Often the woman herself is the first to notice abnormal changes in the breasts. The American Cancer Society suggests that women aged 20 and older should perform a BSE every month.
• The best time to conduct a BSE is a few days after the menstrual period ends when the breasts are least tender or swollen or 5-10 days after the start of the period.
• For women not having regular menstruation the BSE should be done on the same day every month.
The Clinical Breast Examination
The clinical breast examination (CBE) is a check of the breast by a qualified health-care professional. The examination is performed to locate any lumps or suspicious areas and to examine the texture, size, and shape of the breast.
• The CBE takes the same format as the BSE—looking and feeling for changes in the breast.
• In a CBE special attention is paid to lymph nodes in the axilla and around the clavicle because breast cancer leaves the breast via the lymph nodes.
• Both breasts should be examined in the upright and the supine positions because a cancer in the upper half of the breast is easier felt when the woman is upright whereas a cancer in the lower breast is best felt when the woman is supine.
Two Main Criteria in any Breast Examination
• Looking for changes
• The woman should check for indentations, retracted nipples, dimpling, or prolonged skin conditions such as eczema.
• Feeling for changes
• While feeling for changes the woman should examine her breast in both the upright and the supine positions.
Breast Screening Guidelines Approved by the ACR
• Women aged 20-39
• CBE every 3 years
• Monthly BSE
• Women older than 40 years
• Annual screening mammogram
• Magnetic resonance imaging (MRI) or ultrasound screening only for women with high-risk factors or very dense breast
• Annual CBE
• Monthly BSE
Advantages and Disadvantages of Mammography Screening
Advantages
• The mammogram is the single most effective tool in early breast cancer detection.
Disadvantages
• The sensitivity of the mammogram will be dependent on breast density, plus the age and hormone status of the patient.
• Cancer is generally visualized as a white area within the background density of the breast. If the background density is black (as in fatty tissue), the cancer will be easily seen. If the background density is “white” (as in dense breast tissue), the cancer will be harder to detect.
• The mammogram is not 100% effective.
• Mammography screening is generally acknowledged to have a 10%-15% miss rate. As the patient ages the density of the breast generally decreases, thus the sensitivity of the mammogram will increase.
• Mammography tends to understate the multi-focality of a lesion.
• The positioning skills of the mammographer can affect the outcome.
• Cancer can be missed due to inadequate compression or missed breast tissue due to poor positioning.
• The interpretation skills of the radiologist can affect patient outcome.
Dose Calculations in Mammography
• Most patient dose reports in radiology is a fact check of the ESE (entrance-skin-dose) only. However, the biological effect of a mammogram is assumed to be more closely associated with the total energy absorbed by the glandular tissue of the breast.
• The glandular dose is therefore the dose of choice when calculating radiation doses associated with mammography. In mammography screening, when compared to the skin dose, the glandular dose is very low because the dose falls off rapidly as the low photon energy beam penetrates the breast.
• The average glandular dose for a typical mammogram: The American College of Radiology (ACR) recommends that the average glandular dose on a single mammogram projection should not be greater than 3 mGy (0.3 rad or 300 mrad) with a grid or 1 mGy (0.1 rad or 100 mrad) without a grid.
Risk Factors for Breast Cancer
Risk factors are anything that increases a person’s chance of getting a disease. The risk for breast cancer is not the same for all women at a given age. Some women have an increased chance of developing breast cancer.
Increased Breast Cancer Risks
Major risk factors are often those outside of a woman’s control. These are factors that cannot be changed. Minor risks are often those within a woman’s control or do not significantly increase a woman’s chance of developing the disease. They may be cancer-causing factors in the environment or may be related to personal choices.
Major Risk Factors for Breast Cancer
• Gender: Although men can develop breast cancer this disease is much more common among women than men. The biggest risk factor for breast cancer is therefore gender, i.e., being female.
• Age: Age is an important risk factor because as a woman ages, the incidence of cancer increases.
• Genetic risk factors: About 5%-10% of breast cancer cases are hereditary. A woman with a hereditary risk for breast cancer may carry a harmful mutation of the BRCA1 or BRCA2 genes which are the most common. These are mutated genes that can be inherited from either parent. The genes help to control the rate of cell growth and division and are regarded as tumor suppressors. Women with a normal gene have a protective effect because the genes make proteins that keep cells from growing abnormally. Therefore with the mutated gene there is an increase susceptibility to breast cancer. It is estimated that women with the BRCA1 mutation have a 55%-65% risk of developing breast cancer before age 70, and often at a younger age. Women with a BRCA2 mutation have a 45% risk. Another gene, PALB2 (partner and localizer of BRCA2) provides instructions for making a protein that works with the BRCA2 gene to repair strands of DNA and control the rate of cell growth and division. Individuals with a defective PALB2 have a 33% chance of developing breast cancer by the age of 70. Molecular tests are available to identify the BRCA and PALB2 mutations in individuals.
• Family history of breast cancer: Breast cancer risk is higher among women whose close blood relatives (mother, sister, and/or daughter) have this disease. A blood relative can be from either the mother’s or father’s side of the family, but the closer the relative, the higher the risk factor. Women are also at a higher risk if the breast cancer occurs in a relative before age 50.
• Personal history of cancer or breast cancer: A woman with cancer in one breast has a greater risk of developing a new cancer in the other breast.
• Abnormal breast biopsy: A biopsy of atypical hyperplasia, lobular carcinoma in situ (LCIS) or ductal carcinoma in situ (DCIS), are associated with increased risks. Because some cases of DCIS will eventually become cancer, this type of breast change is actively treated. Women with atypical hyperplasia or LCIS are usually monitored carefully and not actively treated.
• Radiation therapy: Radiation therapy to the chest and breast before age 30 carry an increased risk. The younger a woman was when she received treatment, the higher is her risk of developing breast cancer later in life. Also, women who have had the chest area radiated because of a previous cancer treatment such as for Hodgkin disease (or non-Hodgkin lymphoma) are at a slightly increased risk for breast cancer.
Other Risk Factors for Breast Cancer
• Menstrual history: Starting menstruation at an early age (before age 12) or menopause at a later age (after age 55) are both considered increased risks for breast cancer. The greater the amount of menstrual cycles in the woman’s lifetime, the greater then would be the risk of breast cancer.
• Reproductive history: Pregnancy is thought to have a protective effect against having breast cancer because during the pregnancy the woman is not menstruating. It therefore follows that the longer a woman goes without getting pregnant the greater are her risks for breast cancer. Also, women who had their first full-term pregnancy after age 30 or who have never had a full-term pregnancy are at increased risk of breast cancer. Breast-feeding at any age has a protective effect.
• Hormone: The use is thought to influence breast cancer risk, but does not actually cause breast cancer. Women who used combined estrogen and progestin menopausal hormone therapy for more than 5 years have an increased chance of developing breast cancer.
• All factors that affect the reproductive hormones in a woman’s body increase risks for breast cancer.
• Diethylstilbestrol (DES): It was used by pregnant women in the United States during 1940-1971. It was used to prevent miscarriage and studies show that women who took DES during pregnancy have a slightly increased risk of breast cancer. Also, women who were exposed to DES in utero, whose mothers took DES while they were pregnant, may also have a slightly increased risk of breast cancer after age 40.
Purpose of Hormone Replacement Therapy
• Relieves symptoms of perimenopause:
• Hot flushes
• Sleep disturbance
• Insomnia
• Side effects can include fatigue, slower involution process, reduces osteoporosis.
Dangers of Hormone Replacement Therapy
• The combination estrogen and progesterone in hormone replacement therapy (HRT) may increase the risk of developing cancer in the breast and uterus.
• The administration of hormones generally increases the proliferation of glandular tissue, fibroadenomas, and breast cysts.
• Use of HRT increases the incidence of asthma, dementia, heart attacks, strokes, and blood clots.
• Cancers can be harder to detect in dense breast tissue, therefore the increase in glandular tissue caused by the HRT can reduce the effectiveness of the mammogram. However, other modalities can be very effective when imaging dense breast tissue.
BREAST IMAGING MODALITIES
Analog Imaging
• Analog imaging produces the images in a purely mechanical way with a final hard copy that cannot be modified.
• In analog imaging a film is used to acquire the diagnostic information, to display the information, and to store the information.
Disadvantages of Films
• Films can be costly.
• Storage costs are high and a large storage area is needed.
• Films are easily lost or stolen.
• Rigid techniques are required to produce optimum exposures and there is no adjusting for over or under exposure.
• The final image has a narrow dynamic range and the fixed gradient or contrast can be a challenge for the mammographer to optimize and for the radiologist to interpret.
Full-Field Digital Mammography and Computed Mammography
• Full-field digital mammography (FFDM) utilizes a totally cassetteless flat-panel detector system whereas computed mammography (CM) combines an analog imaging x-ray unit with digital technology using photostimulable phosphor (PSP) technology.
• Both are considered digital technologies and have the capability of providing high-quality mammography images.
Monitors—AWS versus RWS
Most modern mammography imaging uses a liquid crystal display (LCD) monitor.
• Acquisition workstation monitors (AWS) are the technologist monitors.
• Low-resolution monitor
• Allows rapid viewing of the image
• The mammographer can determine whether to repeat the examination immediately after the exposure.
• Allows postprocessing of an image while viewing the changes being made
• Images can be magnified, rotated, flipped, or inverted as needed.
• Patient’s demographic information can be modified or corrected and retransmitted.
• Images can be sent to multiple destinations.
• Review workstation monitors (RWS) are the radiologist monitors.
• The only monitor approved for interpreting the mammogram
• High-resolution monitor
Advantages of Digital Mammography
The biggest advantage of digital and computed systems is associated with the wide latitude of the digital signals.
• The higher sensitivity of the detectors demonstrates a linear response (input to output) to the intensity of x-ray exposure over a broad range. This allows better penetration of denser breast tissue, plus, because the response to exposure is linear, optimum contrast can be obtained for both fibroglandular and fatty regions of the breast.
• Improves an institution’s workflow by reducing the number of repeated examinations due to improper exposures.
• Reduces repeats, therefore reduces the cost associated with repeats.
• Offers an efficient imaging system, reducing the overall time needed to complete the examination.
• Reduces the possibility of lost images.
• Offers an easier and more efficient means of managing images or finding previous records.
• Eliminates poor copies because every copy is as exact as the original.
• Reduces the medicolegal risks and expenses associated with lost films.
• Offers multiple archival systems allowing a variety of storage and retrieval options.
• Allows picture archiving and communication system (PACS).
• Images from one modality can be linked with images from other modalities.
• Images from multiple modalities can be viewed quickly and easily from any monitor within the facility.
• Allows teleradiography.
• Images can be transmitted to workstations outside the facility.
Disadvantages of Digital Imaging
• High start-up cost
• Reduced awareness of manipulating individual technical factors
• Lack of awareness of radiation safety and patient protection
• Outsourcing of reporting with possible elimination of on-site radiologist
• Possibility of backup system failure, corruption, or loss of digital records
Digital and Patient Positioning
• Patient position in digital imaging is the same as with analog. However, because of the fixed size of the digital detector, modifications are sometimes needed especially when imaging the small breast.
Image Storage Devices
• Redundant array of independent (inexpensive) disk (RAID) can store 6-12 months of patient data and can transfer image files to the monitor in 2 minutes.
• Digital linear tape (DLT) is not recommended for high capacity storage. They can wear out with heavy use.
• Jukebox storage is used for long-term storage. They hold image data in digital tapes, disks, or other media and typically have a retrieval time of 2-5 minutes.
• Optical disk or magneto-optical disk (MOD) is similar to compact disk (CD) or digital versatile disk (DVD). They are more reliable and robust and are faster than jukebox storage.
• Ultra density optical (UDO) is the newest type of high capacity optical disk system that utilizes blue violet laser technology.
Digital Imaging and Communications in Medicine
• DICOM is a set of computer software standards that permit a wide range of digital imaging programs to understand each other.
Picture Archiving Communications System
• PACS is a computer network that allows images from multiple modalities to be viewed at a single monitor, transmitted or stored.
• The images can be accessed over the Internet, over the telephone, via satellite communication, or from a cable modem. This allows images to be sent from multiple modalities to remote locations across the nation or even across the world.
• All equipment used must be Digital Imaging and Communications in Medicine (DICOM)-compliant.
Teleradiography
• Teleradiography is the remote transmission and viewing of images. Transmission can be Internet, cable, or telephone based. The interface format of transmission is called DICOM.
Computer-Aided Detection
• The use of computers to preread the mammograms, by scanning every part of the radiograph and reporting any suspicious area.
• The computer will display or analyze suspicious areas to make the interpretation consistent from patient to patient.
• The computer system must be programmed to act as a second reader.
DIGITAL BREAST TOMOSYNTHESIS
Digital breast tomosynthesis (DBT) uses a high-resolution cross-section 3-dimensional imaging to eliminate tissue overlap.
Advantages and Potential Use of Tomosynthesis
• Detection and screening especially for women with dense breasts.
• Diagnostic workup or problem solving.
• Help to reduce recall rate.
The Hologic tomosynthesis unit does not use a grid and the number of reconstructed images is based on the breast thickness in millimeters. Reconstruction is always in 1-mm-thick slices with the first slice starting at the detector. The x-ray tube rotates in an arc around the breast taking 15 exposures in 4 seconds, and sweeping from –7 to 0 to +7 degrees with no movement of the patient. A typical tomosynthesis unit can image in 2D, 3D, or a combination of 2D and 3D (COMBO).
Hologic also offers synthesized 2D imaging
• This technique offers a reconstructed 2D image of the breast without the additional radiation. After taking a 3D projection, the computer will synthesize and reconstruct the tomosynthesis slices of the breast and create a 2D image without added radiation to the patient. The technology is called the C-view imaging by Hologic.
Disadvantages of Hologic Tomosynthesis
• Greater risks of motion due to the longer acquisition time.
• Long total exposure time of 12 seconds for COMBO.
• Motion artifacts hard to detect on the reconstruction images at the radiologist workstation.
• Large number of images.
• Degraded imaging of calcifications.
• Thin slicing may mask calcification. However, slabbing of the slices help with calcification visualization.
• Tomosynthesis is not possible for the From-Below (FB) projection, magnification and if the breast tissue is more than 24.5 cm.
• Higher overall radiation dose.
• 2D imaging gives approximately 1.2 mGy of radiation per projection.
• 3D tomosynthesis gives approximately 1.45 mGy of radiation per projection.
• COMBO imaging (2D + 3D) will give approximately 2.65 mGy per projection.
• Imaging implants in 3D gives even more radiation to patient because lower peak kilovoltage (kVp) is used.
Motion Checks in the Hologic System
• In the Hologic system the mammographer must review the projection dataset at the AWS. Because motion can occur at any point of the imaging, motion is best appreciated in the cine mode and a motion check must be made before submitting the images. Motion can be caused by inadequate compression, movement, or heavy breathing during the exposure. The long exposure time leading to patient motion is also a contributing factor.
• Motion is best visualized on the projection dataset of images at the AWS.
• Motion is seen as anterior/posterior movement of the breast tissue or of structures in the breast such as calcification or lymph nodes.
• Medial to lateral movement of breast tissue or structures on the tomosynthesis projection images is normal and does not indicate motion.
The GE SenoClaire 3D Breast Tomosynthesis takes 9 exposures and utilizes only one 3D projection, the MLO. The craniocaudal (CC) projection is imaged in 2D. The tube makes a complete stop after each exposure in a technology called step-and-shoot tube motion. This system reduces image blur. The GE system uses a grid. However, radiation dose is kept low because only 1 projection is taken in 3D. The GE system offers reconstructed 2D images called V-Preview. The disadvantage of the system is the higher recall rates when compared when compared to other screening systems.
The MicroDose Photon-Counting Tomosynthesis System uses a slot-scanning design. The detector consists of an array of parallel crystalline silicon strips combined with photon-counting electronics, designed to absorb 90% of the incident x-rays. The x-ray tube, collimator and detector pivot about the breast taking up to 21 images. Imaging uses high kVp—up to 40 kVp with a tungsten target and aluminum filtration. However, the dose is 1/20 the dose of 2D mammography imaging. As the tube moves laterally across the breasts, the images produced are reconstructed and sent for viewing. The disadvantage of the system is the higher recall rates when compared to other screening systems.
CONTRAST DIGITAL MAMMOGRAPHY
• This involves injecting a contrast while the breast is under compression. Precontrast and postcontrast images are taken.
• Dual-energy subtraction can be performed to assess the contrast in tumors. Dual-energy subtraction is used to subtract high- and low-energy images to separate soft tissue from contrast or calcium deposits. The technology can also be used to enhance masses and eliminate obscuring structures.
ULTRASOUND IMAGING OF THE BREAST
• Ultrasound uses the piezoelectric principle to generate high-frequency sound waves that are used to create images of the organs and blood vessels in the body.
• The patient can rest comfortably on a bed during the ultrasound examination of the breast.
• Higher frequency transducers provide greater resolution but have less penetrating power. Mammography imaging can use frequencies up to 16 MHz.
Ultrasound Transducer
The diagnostic ultrasound transducers serve 2 functions:
• Converts electric energy to acoustic pulses that are sent into the patient.
• Receives the reflected echoes and converts their weak pressure changes into electrical signals so the computer can process them.
Breast Imaging with Ultrasound
• Ultrasound does not use ionizing radiation.
• Ultrasound cannot image microcalcifications.
• No documented risks or harmful bioeffects have been reported.
• Sound cannot travel through a vacuum and will not travel easily through air. To eliminate the air gap between the skin and the transducer a water-based gel is always applied. This gel acts as a conducting medium to transmit sound from the transducer the skin surface.
COLOR DOPPLER ULTRASOUND
This technology can be used to provide an anatomic display of blood flow and can therefore be used to outline the vascularity of a lesion. The technology is based on the fact that highly vascular lesions are often malignant.
MAGNETIC RESONANCE IMAGING OF THE BREAST
• MRI uses very complex magnetic properties to create detailed images of the breast. There is no ionizing radiation and no documented side effects from an MRI examination. The MRI system is able to switch magnetic fields and radio waves to take images in any plane and from any orientation.
• MRI is performed with the contrast material, Gadolinium diethylenetriamine penta-acetic acid (DTPA). Gadolinium (Gd) is a paramagnetic metal which by itself is toxic. A complex solution of Gd and DTPA is stable with very little toxicity. This can be injected into a vein of the arm before the MRI examination to enhance image contrast.
• MRI of the breast has been approved by the FDA since 1991 as a supplemental tool in addition to the mammogram.
Risks and Potential Complications of MRI of the Breast
• All the major risks of MRI are associated with the magnetic properties of the MRI machine.
• During an MRI, the patient is placed within a powerful magnetic field. Patients are required to provide the technologist with a detailed medical history before the MRI examination because any ferromagnetic metal object can potentially be extremely dangerous inside the magnet of the MRI unit.
• The MRI cannot be used on patients with some pacemakers, aneurysm clips in the brain, nerurostimulators, shrapnel, surgical clips or plates, prostheses, metal implants, cochlear implants or if the patient has been exposed to metallic flakes or slivers as a result of working around metal finishing, welding, or grinding equipment.
• Patients must remove all metals from their bodies or clothing including metal piercings, coins, watches, keys, bobby pins, credit cards, pocketknives, and clothing with metal zippers, buttons, belts, or fasteners.
Disadvantages of MRI of the Breast
• Poor visualization of the axillary nodes mainly because contrast enhancement occurs for both normal and pathological nodes.
• MRI is unable to image microcalcifications—which is often associated with early-stage DCIS.
• MRI is expensive and not always an available option.
• The MRI examination is longer than the mammogram and the patient needs to lie prone/very still during the examination for 30-60 minutes.
• MRI is extremely sensitive and has a higher false-positive rate than mammography and often results in unnecessary biopsies.
• Patients can experience claustrophobia when placed in the bore of the MRI unit.
• Any contrast injection has associated risks and breast MR imaging must be done with contrast.
NUCLEAR IMAGING
Nuclear imaging represents the emerging molecular imaging technologies where the function of the organ can be assessed and not just the anatomy. In nuclear imaging a radioactive substance, called a tracer or radiopharmaceutical is injected into the vein. Nuclear imaging technologies include:
• Positron emission tomography (PET) imaging
• Breast scintigraphy or scintimammography
• Lymphoscintigraphy—sentinel node mapping
Positron Emission Tomography
• PET imaging is usually performed using fluorodeoxyglucose (FDG), which is the most common radioactive tracer. This tracer—(fluorodeoxyglucose, methionine, tyrosine, fluoro-estradiol, nor-progesterone), 2-deoxy-2-fluoro-D-glucose labeled with fluorine—also has a half-life of 109.8 minutes. It is used because the breast cancer has a particular affinity to it.
• Typically, 10-20 mCi of the radioactive substance is injected into an arm vein then a special gamma scanner is used to detect the radiation emitted.
• The technology works on the principle that most cancerous tissue uses vast amounts of sugar. The FDG is metabolized in the body like sugar. It will therefore go to the tissues that are most active. PET imaging is a valuable tool in detecting the metastatic spread of breast cancer.
• PET imaging can be modified using a special breast gamma camera in a technology called positron emission mammography (PEM). In PEM imaging, the breast can be position similar to the CC and MLO mammographic projections for easier comparisons.
Breast Scintigraphy or Scintimammography or Breast-Specific Gamma Imaging
• With scintigraphy, the radiopharmaceutical is injected into the arm vein of the patient.
• The radiopharmaceutical used is technetium-99m (Tc-99m) sestamibi which is sometimes marketed under the trade name of Miraluma. The radiopharmaceutical will accumulate only in malignant lesions in the breast. A benign lesion does not take up the radioactive isotope.
• In BSGI the patient is imaged with a special gamma camera which images the breast in the same positions and projections as the mammography unit.
Lymphoscintigraphy or Sentinel Node Mapping
• In this technique, the radiopharmaceutical is injected into subareola lymphatic plexus of the affected breast. The radioactive material is then taken up by the lymph system and travels to the lymph nodes under the arm. At surgery, the surgeon uses a Geiger counter to locate the radioactive lymph nodes. Surgery should follow 1-9 hours after the radiopharmaceutical injection.
• Often a blue dye is also injected into the region of the tumor and the dye is also then carried to the sentinel node.
• The first nodes picked up by the radiopharmaceutical can be removed for biopsy. If the nodes test negative for cancer then axillary node dissection is not needed.
• The procedure can provide prognostic information based on the presence or absence of regional lymph node metastases. And because the cancerous node is identified, fewer lymph nodes are removed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree