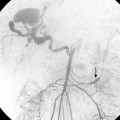
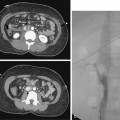
CHAPTER 2 Patient evaluation and care
In 1967, Dr. Alexander Margulis proposed a new subspecialty within the family of imaging sciences which he called “interventional radiology.”1 For some time thereafter, interventional radiologists (IRs) were consultants who performed minimally invasive angiographic procedures (at the request of clinicians) with little or no responsibility for patient care before or after. This practice model has been transformed over the past 40 years. Interventionalists (including the many subspecialists from other fields who also do this work) now assume full clinical responsibility for their patients—they are true “clinicians.” As such, IRs are obligated to conduct the initial patient assessment, determine the best course of therapy, and provide long-term care and management after the procedure is completed. Experienced interventionalists will agree that a successful and safe technical outcome depends as much on preprocedure and postprocedure care as it does on performing the case itself. The concepts set down in this chapter form the cornerstone of modern interventional radiology practice. The details and nuances may vary among institutions, but the principles are universal.
Preprocedure care
Patient referral and contact
The interventionalist should review the medical history and all pertinent diagnostic tests and imaging studies before seeing the patient. With this approach, one can avoid raising the specter of an intervention that is ultimately not indicated. The initial conversation between patient and physician is vitally important and should occur as far away (in time and space) from the interventional suite as possible. The goals of the interview and examination are to establish rapport, review the history firsthand, explain the procedure in detail (and thus obtain informed consent), and reduce anxiety. Family and significant others are encouraged to participate in the discussion. Ideally, inpatients are assessed the day before the case is scheduled. Outpatients are evaluated in a clinic or office dedicated to this work, where support staff (trainees, nurses, physician extenders, and administrative assistants) are fully engaged in IR.2
History and physical examination
The clinical evaluation includes several components (Box 2-1). The physician must be confident that there are appropriate indications for the proposed intervention based on “best practice” criteria established in the medical literature or endorsement by the Society of Interventional Radiology (SIR) and the Cardiovascular and Interventional Radiology Society of Europe (CIRSE).3 Risk factors that may require a delay or modification of the proposed procedure or an alternative therapy are sought (Boxes 2-2 and 2-3). A focused physical examination is performed, but it is prudent to assess the airway, lungs, heart, and abdomen in almost all patients. For angiographic procedures, the interventionalist should evaluate and document the following parameters:
Thrombophilic (hypercoagulable) states are an important risk factor for vascular thrombosis and can be associated with significant complications from diagnostic and therapeutic vascular procedures.4–6 The major hereditary and acquired disorders are listed in Chapter 1 (see Boxes 1-4and 1-5). These conditions should be suspected when thrombosis occurs in young patients, at atypical sites, in the absence of underlying vascular disease, with familial tendency, or with apparent resistance to anticoagulants.
Sedation and analgesia requirements
Most procedures on adults are performed with moderate sedation under the supervision of the operating physician. It is wise (and often hospital policy) to have an anesthesiologist or nurse anesthetist handle sedation and analgesia for sicker patients (e.g., American Society of Anesthesiology physical status classification system categories 3 or above7 [Box 2-4]). In certain circumstances, regional, monitored, or general anesthesia is preferable (Box 2-5).
Informed consent
It is the obligation of the physician or physician extender performing any medical procedure to explain the proposed intervention to the patient, to the parent of a minor patient, or to the legal representative or the closest relative if the patient is not competent to give consent.8,9 If the patient is not fluent in the native language of the health care team, a trained medical translator (not a relative or friend) should assist with consent. If telephone consent from a family member or legal representative is necessary, a witness must document the conversation. In the United States, the “implied consent” doctrine is considered to be in force with any medical procedure in which a delay could lead to severe disability, severe pain, or death. In this rare situation, consent is unnecessary if the patient cannot give his or her own approval and no legal representative is immediately available.9
The particular risks for specific diagnostic and interventional procedures are discussed in later chapters. As a rough guide, the overall incidence of major complications (Box 2-6) should be no more than 1% to 2% for the more common interventions (e.g., vascular access placement, inferior vena cava filter placement, percutaneous biopsy and drainage procedures, dialysis access interventions).10–12 However, older patients and those with established risk factors are more likely to suffer a bad outcome such as bleeding, infection, thrombosis, renal dysfunction, or allergic reactions to administered drugs.13
Laboratory testing
The purpose of preprocedure laboratory testing is to minimize risk by detecting (and when feasible correcting) relevant abnormalities, altering the technique as needed, or canceling the case and choosing a safer treatment. Preprocedure studies may be routine (screening) or selective (directed).14 Indiscriminate testing has proved to be of little value in virtually every medical and surgical study ever published.15–17 However, selective testing is warranted before vascular and interventional procedures. Screening is generally unnecessary in otherwise healthy patients younger than 40 years of age. Testing is certainly advisable in older adults and those with predisposing risk factors. The acceptable interval between test result and procedure varies among hospitals and clinical situations and cannot be generalized.
Renal function
Serum creatinine is still widely used as a proxy for kidney function, but it is an imprecise measure of such. Estimated glomerular filtration rate (eGFR) is a more accurate indicator of renal insufficiency. Contrast-induced nephropathy (CIN) is marked by a significant rise in serum creatinine level (0.5 mg/dL or 25% of baseline) 1 to 3 days after intravascular administration and by resolution at 7 to 10 days. This (usually) transient dysfunction is related to direct toxic effects on the kidney by oxygen free radicals or ischemia of the renal medulla.18 In the general population and in patients with eGFR greater than 60 mL/min (stage 1 or 2 chronic kidney disease), the overall risk of CIN after diagnostic angiography is low (<2%). The risk increases to about 5% in patients with preexisting mild renal dysfunction and 33% or greater in patients with diabetes and severe renal insufficiency (eGFR <30 mL/min, stage 4 or 5 chronic kidney disease).19 Only a small fraction of patients who suffer this complication require long-term hemodialysis. However, some experts believe concerns about CIN are exaggerated and that use of iodinated contrast should not be avoided in patients with moderate renal dysfunction.19
Several pharmacologic regimens may reduce the likelihood of CIN (see discussion below).
Until recently, gadolinium-based contrast agents were favored as a safe alternative to iodinated materials during intravascular interventions in patients with baseline renal insufficiency. Some of these agents pose a risk (albeit very small) for causing nephrogenic systemic fibrosis in individuals with preexisting severe chronic or acute renal insufficiency (eGFR <30 mL/min.). This rare disorder is characterized by widespread and often debilitating dermal (and sometimes visceral organ) sclerosis.20,21 As such, gadolinium-based agents are no longer used during vascular procedures unless renal function is essentially normal.
Coagulation parameters
Significant bleeding from interventional procedures is uncommon. It is an axiom in interventional radiology (IR) that the individual risk is largely a function of the coagulation status of the patient (Box 2-7), the likelihood of traversing a major artery or vein, and the ability to detect and then manually control bleeding when it occurs. In fact, there are equivocal data regarding the value of coagulation screening tests in predicting the likelihood of bleeding from invasive procedures.22,23 Nonetheless, routine screening for coagulopathy is the practice in many institutions based on tradition and sometimes stated policy. A more judicious approach is favored by some practitioners:
Commonly performed coagulation tests are outlined in Box 2-8. Thresholds for defining a coagulopathy and measures for correcting them22–25 are outlined in Tables 2-1 and 2-2. Based on limited but promising experience using more relaxed parameters for tunneled central venous catheter placement, some practitioners insert such devices when the INR is less than 2.0 or the platelet count is greater than 25,000/dL.26
Box 2-8 COAGULATION SCREENING BEFORE INTERVENTIONAL RADIOLOGY PROCEDURES
Routine
Table 2-1 Safety Thresholds for Coagulation Parameters
| Parameter | Threshold |
|---|---|
| International normalized ratio (INR) | 1.6-1.8 |
| Prothrombin time (PT) | <3 sec from control |
| Partial thromboplastin time (PTT) | <6 sec from control |
| Platelet count (normal INR/PTT) | >50,000/mm3 |
| Platelet count (abnormal INR/PTT) | >50-100,000/mm3 |
| Bleeding time | <8 min |
Table 2-2 Correction of Coagulation Abnormalities
| Parameter | Response |
|---|---|
| International normalized ratio | Withhold warfarin, bridge with heparin or low molecular weight heparin (see Box 2-9) |
| Fresh-frozen plasma (FFP), 2-4 bags or 10-15 mL/kg | |
| Vitamin K, 1-3 mg IV; may be repeated after 6-8 hr | |
| Partial thromboplastin time | Withhold heparin 2-6 hr before procedure |
| FFP, 2-4 bags or 10-15 mL/kg | |
| Platelet count | Platelet transfusion (10 units to increase count by 50,000-100,000/mm3) |
| Bleeding time | Cryoprecipitate (0.2 bag/kg) |
| Desmopressin (DDAVP), 0.4 μg/kg over 30 min | |
| Platelet transfusion | |
| Fibrinogen | FFP, 10-15 mL/kg |
IV, intravenously.
Patient preparation
Diet and hydration
When moderate sedation is planned, oral intake or gastrostomy feeding restrictions must comply with institutional guidelines. Typically, patients are limited to clear liquids within 6 hours and are given nothing by mouth (NPO) within 2 hours of the expected start time to prevent aspiration from vomiting caused by contrast agents, sedatives, or individual patient factors.27 For inpatients who will receive significant volumes of intravascular contrast, overnight IV hydration should be considered when feasible. Outpatients are encouraged to drink plenty of fluids. IV fluids should be ordered in consultation with the referring physician for patients with cardiac or renal disease.
Medications