- •
CMD can be diagnosed invasively or through multiple noninvasive methods, including PET and CMR MPI and Doppler echocardiography.
- •
CMD is highly prevalent in patients with concomitant CAD and in those with no obstructive CAD without (INOCA) and with myocardial infarction (MINOCA).
- •
The functional and economic impact of CMD are substantial, with high symptom burden, reduced activity capacity, and missed work.
- •
A diagnosis of CMD increases risk for all-cause mortality and MACES.
- •
In patients with concomitant CAD, a reduced CFR guides need for revascularization.
- •
Treatment in patients with CMD and INOCA or MINOCA is two-pronged: risk factor modification and symptom reduction.
- •
CFR reclassified risk beyond typical parameters in disease states with high morbidity, such as DM and CKD.
- •
Emerging applications of CMD assessment include in patients with coronary allograft vasculopathy and with systolic heart failure.
- •
Future research is needed to optimize diagnostic and treatment algorithms for CMD and manage risk appropriately.
Introduction
Until recently, the focus of ischemic heart disease evaluation has been entirely on the identification of obstructive atherosclerosis in the epicardial coronary arteries; however, advanced noninvasive imaging and invasive assessment of the microvasculature in the new millennium have identified complex pathophysiologic processes leading to coronary microvascular dysfunction (CMD). CMD can be present across multiple clinical scenarios as shown in Table 13.1 and raises unique diagnostic and prognostic considerations. In the setting of obstructive coronary artery disease (CAD), CMD has been associated with a worse prognosis and may affect treatment benefit. ,
| Clinical Scenario | Context |
|---|---|
| CMD with epicardial CAD | CFR can be reduced in the setting of obstructive epicardial CAD but it is also possible to have concomitant epicardial CAD and coronary microvascular dysfunction, which may portend a worse prognosis. CMD in this setting can be more difficult to diagnose, particularly in patients with multivessel obstructive CAD. |
| CMD without epicardial CAD (INOCA) | CMD in patients with signs/symptoms of ischemia and no obstructive CAD (INOCA) has substantial functional and economic impact and is associated with a substantial increase in risk for downstream cardiac events. |
| CMD with MI but no epicardial CAD (MINOCA) | MINOCA is associated with a high risk for future cardiac events, and CMD is an important cause of this condition. Prognosis has not been specifically studied in this subpopulation. |
| CMD in special populations | The diagnostic and prognostic role of CMD may be highest in special populations, such as patients with diabetes mellitus and chronic kidney disease. |
| Secondary CMD | CMD can be present in the setting of other diseases of the myocardium, including dilated, hypertrophic, and restrictive cardiomyopathy or severe hypertensive or valvular disease. |
In addition, recent studies have shown a high rate of negative invasive coronary angiography irrespective of referral indication. , These patients have typically received false reassurance that their symptoms are because of alternative etiologies and their prognosis is good. These patients with symptoms of ischemia and normal coronary arteries (“ischemia with no obstructive coronary artery disease,” or INOCA) have substantial morbidity with ongoing chest pain and reduced functional capacity that lead to a high economic burden from missed workdays and reduced productivity. Moreover, this population has a substantially higher rate of major adverse cardiovascular events (MACEs) compared to patients with similar comorbidities without CMD. , This problem extends to patients with myocardial infarction (MI) and normal epicardial coronary arteries (“myocardial infarction with no obstructive coronary artery disease,” or MINOCA) and is particularly prevalent and morbid in special populations, including patients with diabetes mellitus (DM) and chronic kidney disease (CKD). ,
In this chapter, we will review the evaluation of suspected CMD and compare the multiple diagnostic tools available to assess this morbid condition. We will then present four representative cases and associated epidemiology, prognostic information, and treatment approaches for patients with CMD and obstructive CAD, INOCA, MINOCA, and high-risk comorbidities. Finally, we will briefly discuss emerging applications in CMD.
Evaluation of suspected coronary microvascular dysfunction
For patients who present with signs and symptoms of CMD, secondary CMD causes should be excluded, including myocardial disorders such as hypertrophic, restrictive, and dilated cardiomyopathies; severe valvular heart disease; and high-output states such as anemia and hyperthyroidism. In patients without a secondary cause, empiric therapy can be considered, particularly in the absence of persistent symptoms. In patients in whom diagnosis is pursued, there are both invasive and noninvasive approaches. Noninvasive techniques such as exercise electrocardiography (ECG) and stress imaging without coronary blood flow assessment have limited sensitivity and specificity for the diagnosis of CMD. Likewise, the coronary microcirculation cannot be directly visualized using invasive or noninvasive coronary angiography. Myocardial blood flow (MBF) can be directly measured or estimated using both invasive and noninvasive techniques at both rest and with maximal coronary vasodilation induced with a stress agent such as adenosine. The ratio of stress MBF to rest MBF is defined as the coronary flow reserve (CFR). A reduced CFR identifies an inadequate augmentation of MBF from rest to maximal coronary vasodilation and is the diagnostic hallmark of CMD. Prognostic benefit has been identified using varying thresholds for CMD from 1.5 to 2.6. Although CFR may be less helpful in diagnosing CMD in patients with severe left ventricular (LV) dysfunction or advanced severe renal dysfunction, it has established prognostic utility in these groups. The noninvasive approaches currently used to evaluate CMD include vasodilator stress PET myocardial perfusion imaging (MPI), vasodilator stress CMR MPI, and vasodilator stress Doppler echocardiography, all with CFR assessment. Each diagnostic modality has specific advantages and disadvantages that should be considered when assessing CMD, as shown in Table 13.2 .
| Modality | Advantages | Disadvantages |
|---|---|---|
| Echocardiography |
|
|
| Positron emission tomography |
|
|
| Cardiovascular magnetic resonance |
|
|
| Invasive angiography |
|
|
Noninvasive approaches
Echocardiography
Echocardiography can assess coronary blood flow through direct visualization of the distal left anterior descending (LAD) coronary artery and measurement of the coronary blood flow velocity (CBFV) using Doppler. CFR is calculated as the ratio of the peak diastolic CBFV during maximal vasodilation to the CBFV at basal state. Although this was one of the first noninvasive techniques employed, it suffers from multiple limitations. The distal LAD must be well visualized and is subject to the challenges of poor echocardiographic windows, particularly in obese patients or in those with pericardial fluid. Extracardiac thoracic arteries can be mistaken for the LAD. Finally, the measurement is dependent on the angle of incidence. These limitations have led to high variability. Nevertheless, technical advances in ultrasound equipment, including high-frequency transducers, digital imaging, and low Nyquist limit Doppler, have led to improved success. CFR measured by Doppler echocardiography has been shown to correlate closely to positron emission tomography (PET) CFR in healthy patients (r = 0.942, p < 0.001) with high reproducibility. , In the iPOWER study of women with angina and no obstructive CAD, 97% of patients were able to have CFR measured. An alternative echocardiographic technique, myocardial contrast echocardiography, uses microsphere destruction and ultrasound contrast refill time to measure absolute MBF. CFR obtained by this technique has been shown to correlate closely to that obtained with Doppler echocardiography (r 2 = 0.73). Nevertheless, myocardial contrast echocardiography is highly operator dependent and thus is only performed in a few centers.
Positron emission tomography
PET MPI with absolute flow assessment has become the most used noninvasive method for assessing CMD in clinical practice because of a variety of factors, including expansion in the number of PET scanners, increased availability of PET tracers, and streamlined efficiency from automated MBF quantification in commonly used software programs. In contrast to echocardiographic approaches, PET allows for quantification individually in each coronary distribution. In comparison to single photon emission computed tomography (SPECT), PET has higher temporal resolution and tracers with more accurate correlation of radionuclide uptake to changes in MBF (see details in Chapters 2 and 4 ). Methodologic considerations for MBF quantification are discussed in detail in Chapter 3 . When multiple PET absolute flow quantification techniques have been compared, the estimation of MBF varied considerably (r = 0.42 to 0.62), complicating diagnostic and prognostic cutoffs. In contrast, because it is a ratio, CFR correlates more closely across PET quantification techniques (r >0.90). CFR assesses blood flow in both the macro- and microcirculations. It correlates well with fractional flow reserve (FFR) for single-vessle obstructive CAD ; however, differentiating between multivessel CAD, diffuse atherosclerosis, and CMD is more challenging. A normal CFR rules out both multivessel CAD and CMD. A low CFR could represent either condition, but the magnitude of CFR reduction along with the presence of focal perfusion defects may help differentiate, with low global CFR values especially when associated with regional perfusion defects more likely to represent multivessel epicardial CAD.
Cardiac magnetic resonance
Stress cardiac magnetic resonance (CMR) imaging can quantitate regional myocardial perfusion with high spatial resolution and has advantages as detailed in Table 13.2 , including its noninvasive nature, wide scanner availability, and absence of radiation. Several contraindications, the need for careful manual processing of images, and a lack of necessary expertise in most centers currently limit its use. The risk of nephrogenic systemic fibrosis from gadolinium exposure has been almost eliminated with the use of cyclic agents.
The test is performed by injecting a gadolinium-containing contrast agent at peak vasodilation and at rest and measuring the change in myocardial signal intensity within the heart. Myocardial perfusion can be assessed semiquantitatively through the upslope integral technique with high accuracy but is dependent on the pharmacodynamics of the contrast agent. Quantitative analysis can be performed through measurment of the arterial input (blood) and tissue functions and then performance of compartment kinetic modeling or Fermi-function deconvolution. This approach identifies a reduced CFR in patients at risk for CMD versus healthy controls. CMR absolute MBF has been validated against microspheres and has compared favorably with PET in healthy volunteers. In a population with known or suspected CAD, absolute MBF correlated, but only weakly, at both rest (r = 0.32, p < 0.0001) and stress (r = 0.37, p < 0.0001). Nevertheless, given that CMR underestimated the MBF compared with PET equally at both rest and stress, the CFR correlated more strongly (r = 0.79, p < 0.0001); PET and CMR CFR had similar accuracy for detecting significant CAD.
Invasive approaches
Invasive absolute MBF assessment involves placement of a catheter in a single coronary artery (typically the LAD) and measurement through thermodilution or intracoronary Doppler ultrasonography. Both endothelial-dependent and endothelial-independent pathways are assessed with graded doses of intracoronary acetylcholine (endothelial dependent) and adenosine (endothelial independent). This process can be time consuming. Dilution techniques calculate flow using the temperature gradient between the coronary arteries and the coronary sinus. For Doppler techniques, flow velocity is measured and MBF is calculated as the product of the LAD arterial cross-sectional area and peak flow velocity divided by 2. MBF is measured at peak stress and at rest, and the ratio of these constitutes the CFR. Both thermodilution and intracoronary Doppler measures of CFR correlate well with microspheres in a porcine model (r = 0.85 and r = 0.72, respectively, both p < 0.001). An additional approach includes the assessment of the index of microvascular resistance (IMR). In patients with stable nonobstructive atherosclerosis, abnormal IMR was associated with worse CVD outcomes. A major drawback to catheter-based approaches in the diagnosis of CMD is their invasive nature; however, invasive CMD diagnosis has an excellent safety record when studied in over 1500 patients, with less than 1% procedure-related adverse events, similar to levels with invasive coronary angiography.
Diagnostic challenges
Although the diagnosis of CMD by invasive and multiple noninvasive modalities has improved with increased focus over the past several years, diagnostic challenges remain. These include the importance of ruling out secondary causes of CMD, variations in CMD diagnostic threshold and MBF estimates by modality, and optimization of empiric therapy as a diagnostic approach.
The first step in evaluating patients with signs and symptoms of INOCA is to exclude secondary causes of CMD. Alternative causes of low CFR include myocardial disorders such as hypertrophic, restrictive, and dilated cardiomyopathies; valvular heart disease, including severe aortic stenosis; and high-output states (e.g., hyperthyroidism and anemia). These conditions have different prognosis and treatment strategies and must be ruled out before making a diagnosis of primary CMD.
Cutpoint variation is an additional diagnostic challenge when evaluating for CMD, with thresholds from 1.5 to 2.6 having been used in studies evaluating the prognostic significance of CMD ( Fig. 13.1 ). The optimal threshold of CFR for the identification of CMD has not been determined, partially because of the known variation in CFR by age and sex among healthy individuals. Additional complexity stems from differences in MBF values by imaging modality (such as PET vs. CMR) and by calculation method. A comparison of multiple methods of 82 rubidium (Rb) PET MBF quantification found a variable relationship between stress MBF and cardiac mortality by input method and extraction model. Fortunately, the relationship between CFR methods and cardiac death was more robust across methods because the differences are generally present at both rest and stress and the ratio cancels out these differences. Given differences in cutpoint chosen and variations by demographic, ranges have been suggested, such as those by Loffler et al., in which values of CFR less than 1.5 are labeled as definite CMD, greater than 2.6 as no CMD, and between 1.5 and 2.6 as borderline CMD.

An alternative diagnostic approach is to provide a trial of empiric therapy. If the patient has improved symptoms, this supports a diagnosis of CMD. This approach has been challenging because there are no clear data supporting use of aspirin and statins in this cohort. Empiric therapy has thus been advocated primarily in patients with elevated atherosclerotic cardiovascular risk scores, who would benefit more from these treatments even if they do not have CMD.
Patient-centered clinical applications
Case vignette 1: CMD with concomitant obstructive epicardial CAD
A 65-year-old female with a history of hypertension, dyslipidemia, and insulin-dependent DM with poor control developed pelvic pain from necrotizing fasciitis and Fournier’s gangrene and underwent a right radical vulvectomy and thigh revision. In the postoperative setting, she developed substernal chest pressure and was found to have a non-ST elevation myocardial infarction (NSTEMI) with peak troponin of 0.15, for which she was managed medically with aspirin, a high-intensity statin, and a beta-blocker. She had a complicated and prolonged recovery with persistent anemia that required multiple transfusions and Candida difficile colitis. She was seen in cardiology clinic in follow-up and underwent a vasodilator rest/stress 13 N-ammonia PET MPI for risk stratification. The team hoped she would be manageable medically given her multiple recent issues.
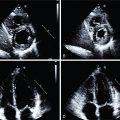
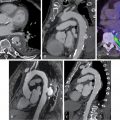
She received 0.4 mg of IV regadenoson with an associated increase in heart rate from 77 to 88 beats per minute (bpm). She had flushing shortly after administration but no chest pain. Her ECG revealed nonspecific ST-T changes throughout the test. Her relative perfusion images ( Fig. 13.2 ) revealed an apical to basal, mild, predominately reversible perfusion defect in the inferolateral wall with an associated mild wall-motion abnormality that was concerning for focal ischemia and infarction. The most likely involved coronary artery based on these images was the left circumflex or one of its branches. Her stress left ventricular ejection fraction (LVEF) was 45%. Based on these images alone, the scan would have been considered lower risk and medical therapy would have been pursued for the presumed one-vessel CAD.

Absolute blood flow quantification, however, revealed a diffuse severe reduction in stress flow and coronary flow reserve (see Fig. 13.2 ). Invasive coronary angiography revealed a proximal severe obstruction of a large obtuse marginal branch of the left circumflex coronary artery that was opened and a drug-eluting stent placed. Her remaining coronaries had nonsignificant obstruction. The final diagnosis was severe one-vessel obstructive epicardial CAD with concomitant CMD. Unfortunately, the patient suffered a fatal ST-elevation MI 30 months later.
Epidemiology and diagnosis
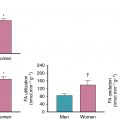
CFR assesses the entire coronary arterial system, including the large epicardial arteries and microvasculature; thus, an abnormal CFR can represent either obstructive epicardial CAD or CMD. Because of this overlap, establishing the prevalence of concomitant obstructive CAD and CMD is challenging without coronary angiography as illustrated in this case, particularly in patients with multivessel disease. Likewise, establishing the diagnosis of CMD in the setting of obstructive CAD can be difficult. A normal CFR excludes high-risk obstructive CAD with high accuracy. Naya et al. showed that a CFR greater than 1.93 excludes high-risk CAD with a sensitivity of 86% and a negative predictive value of 97%. In a cohort of 329 intermediate- to high-risk patients sent to intermediate-care areas after PET MPI, there was a significant but modest negative correlation (r = −0.26, p < 0.0001) between CFR and extent and severity of CAD (as measured by the CAD prognostic index). There was a wide range of CFR values across all values of the CAD prognostic index, including those with no obstructive CAD but also those with single-vessel or multivessel disease. In this cohort, those with an impaired CFR of less than 1.6 had obstructive CAD appreciated in 83% of patients, and 35% of these had one-vessel disease, indicating likely concomitant CMD. There was a gender difference, with the majority of women in this group having no obstructive CAD and men having multivessel CAD. Incorporation of relative perfusion pattern; high-risk findings, such as transient ischemic dilation; and gated function in concert with MBF and CFR can often help differentiate between obstructive and CMD phenotypes but is not always conclusive. Although CFR cannot definitively differentiate between CMD and multivessel obstructive CAD, the likelihood of the latter increases as the CFR decreases, with a substantial increase in specificity for multivessel CAD below CFR values of 1.5.
Prognosis
The prognostic value of CMD in patients with obstructive CAD has predominately been established in studies that included both patients with and without obstructive CAD. Murthy et al. showed that the lowest tertile of CFR (<1.5) in a population referred for PET MPI had a 5.6-fold increase in risk for cardiac death over a median 1.4-year follow-up. The addition of CFR correctly reclassified 51% of intermediate-risk patients. Fukushima et al. similarly found that abnormal CFR (cutoff <2.11) was a significant predictor of MACE events (hazard ratio [HR] 2.93, 95% confidence interval [CI] 1.3 to 6.65, p = 0.009). Furthermore, the adverse prognostic impact of CFR persists in patients with abnormal myocardial perfusion, suggesting the value of this marker in patients with obstructive CAD. Ziadi et al. showed that patients with a summed stress score (SSS) greater than four have an increased rate for cardiac death/nonfatal MI that increases from 1.1% with normal CFR to 11.4% with a CFR of less than 2. CFR had incremental prognostic value over SSS for MACE events ( p = 0.0002). The increased risk with abnormal CFR in patients with abnormal perfusion has been shown to persist over a 10-year time horizon. Application of these studies to the obstructive CAD population is limited by lack of invasive coronary angiographic data. Tio et al. examined a cohort of 344 patients with confirmed obstructive CAD and no revascularization. In this group, each standard deviation decrease in CFR had an age- and sex-adjusted HR of 4.11 (95% CI 2.98 to 5.67) for cardiac death; this marker had higher risk than a decreased LVEF.
Impact on treatment
Given their indication for aspirin and statin from their CAD diagnosis, identification of CMD does not have as great an impact on therapy as in those without concomitant CAD. Although it has not been studied, the worse prognosis in patients with a reduced CFR suggests that more aggressive pharmacologic treatment thresholds should be considered. The relationship between CFR and revascularization has been studied, however. Using the same cohort of 329 patients previously described, Taqueti et al. assessed rates of cardiovascular death and heart failure admission by CFR and receipt of percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or no revascularization. There was no difference in events in those with a high CFR. In contrast, the event rate increased significantly from 0.9% for those with low CFR receiving CABG to 11.5% in those with low CFR treated medically ( Fig. 13.3 ). Of note, this analysis included all patients with obstructive CAD and did not assess specifically those with concomitant CMD.