Pediatric abdominal masses are commonly encountered in the pediatric population, with a broad differential diagnosis that encompasses benign and malignant entities. The primary role of abdominal imaging in the setting of a suspected pediatric abdominal mass is to establish its presence, as nonneoplastic entities can mimic an abdominal mass, and to identify characteristic imaging features that narrow the differential diagnosis. In the setting of a neoplasm, various imaging modalities play an important role to characterize the mass, stage extent of disease, and assist in presurgical planning. The purpose of this article is to discuss a practical imaging algorithm for suspected pediatric abdominal masses and to describe typical radiological findings of the commonly encountered abdominal masses in neonates and children with emphasis on imaging guidelines and recommendations.
Key points
- •
The primary role of abdominal imaging in the setting of a suspected pediatric abdominal mass is to establish its presence and to identify salient imaging features that can narrow the differential diagnosis.
- •
Abdominal radiography may help assess the mass effect on bowel loops and detect calcifications associated with abnormal masses.
- •
Abdominal ultrasound is the preferred initial imaging modality to determine the origin of the mass and evaluate its vascular supply with Doppler imaging.
- •
If a neoplasm is suspected on ultrasound, cross-sectional imaging, such as computed tomography (CT) or magnetic resonance imaging (MRI), is indicated to further characterize the mass, stage extent of disease, and assist in presurgical planning.
Introduction
Pediatric abdominal masses are commonly encountered in the pediatric population with a broad differential diagnosis that encompasses benign and malignant entities. Although there are American College of Radiology (ACR) appropriateness imaging guidelines for abdominal masses in adults, the ACR appropriateness criteria do not specifically address pediatric abdominal masses. Therefore, the purpose of this article is to discuss an evidence-based practical imaging algorithm for pediatric abdominal masses. In addition, the characteristic imaging appearances of the most commonly encountered abdominal masses in infants and children are reviewed with an emphasis on reaching an accurate diagnosis which, in turn, can lead to optimal pediatric patient management.
Evidence-based imaging algorithm
The primary role of abdominal imaging in the setting of a suspected pediatric abdominal mass is to establish its presence, as nonneoplastic entities can mimic an abdominal mass, and to further characterize the mass location as well as other salient imaging features that might help narrow the differential diagnosis. Abdominal radiographs may help with determining the mass effect on bowel loops and with the detection of tumoral calcifications. Abdominal ultrasound is the preferred initial imaging modality to determine the origin of the mass and to evaluate the vascular supply with Doppler imaging. If a tumor is confirmed on ultrasound, then cross-sectional imaging [computed tomography (CT) and/or magnetic resonance imaging (MRI)] is indicated to further characterize the mass, stage extent of disease, and assist in presurgical planning.
The 3 most common abdominal malignancies in children are neuroblastoma, Wilms tumor, and hepatoblastoma, and therefore, we discuss the specific evidence-based imaging recommendations for these entities; clinical features and imaging characteristics for these 3 malignancies are further discussed in the subsequent section entitled, “Spectrum of Neonatal and Pediatric Abdominal Masses.” Additionally, currently recommended imaging protocols that exist to screen pediatric patients with certain syndromes and conditions such as Beckwith–Wiedemann and Wilms–aniridia–genitourinary–mental retardation (WAGR)syndromes who have an increased risk of abdominal malignancy are summarized in Table 1 .
| Genetic Syndrome | Associated Abdominal Masses | Surveillance Imaging Recommendations |
|---|---|---|
| Beckwith–Wiedemann Syndrome | Wilms tumor, nephroblastomatosis, hepatoblastoma, neuroblastoma, rhabdomyosarcoma, adrenal carcinoma | Abdominal US every 3–4 mo |
| Wilms–Aniridia–Genitourinary–mental Retardation (WAGR) | Wilms tumor | Abdominal US every 3–4 mo |
| Denys–Drash syndrome (DDS) and Frasier Syndrome | Wilms tumor, gonadoblastoma | Abdominal US every 3–4 mo |
| DICER1 Syndrome | Renal lesions (cystic nephroma and Wilms tumor), ovarian sex cord-stromal tumors, genitourinary rhabdomyosarcomas | Abdominal US every 6 mo until age 8 y and then every year until age 12 y. For women: abdominal and pelvic US every 6–12 mo from age 8–10 y to age 40 y. |
| Von Hippel–Lindau Syndrome | Clear cell renal cell carcinoma, pheochromocytoma, pancreatic neuroendocrine tumors, epididymal and broad ligament cystadenoma, and renal and pancreatic cysts | Variable recommendations based on expert consensus, with annual screening with either abdominal US or MRI starting as early as 10 y of age. |
| Tuberous Sclerosis Complex | Renal angiomyolipomas, renal cell carcinoma | Surveillance for renal lesions (interval not specified). |
| Multiple Endocrine Neoplasia Syndrome 1 , | Pancreatic neuroendocrine tumors, adrenal lesions | Annual imaging surveillance for pancreatic neuroendocrine tumors and adrenal lesions with CT, MRI, or US before the age of 10 y. |
| Multiple Endocrine Neoplasia Syndrome 2 | Pheochromocytoma | Variable screening guidelines, with some favoring imaging in the setting of biochemical abnormalities, and others suggesting abdominal imaging for pheochromocytoma every 3 y. |
| Li-Fraumeni Syndrome | Rhabdomyosarcoma, adrenal cortical tumors | Abdominal US every 4 mo |
Neuroblastoma
Although neuroblastoma may initially be detected by abdominal radiographs or ultrasound, further evaluation with CT or MRI is necessary for tumor staging and clinical management. Both CT and MRI have various advantages and disadvantages, without a clear consensus favoring one modality over the other for initial staging. CT is more sensitive than MRI for detecting calcifications. MRI is superior to CT for the characterization of intraspinal extension, bone marrow disease, and liver metastasis. Nuclear medicine metaiodobenzylguanidine (MIBG) imaging is also indicated at initial staging, because 90% of neuroblastoma tumors demonstrate MIBG avidity. For MIBG-avid neuroblastoma tumors, MIBG imaging complements CT or MRI for treatment planning and monitoring tumor response. Data are still emerging regarding the use of fluorodeoxyglucose/positron emission tomography (FDG/PET) for the 10% of neuroblastoma lacking MIBG avidity.
Wilms Tumor
Similar to neuroblastoma, Wilms tumor is typically initially diagnosed by ultrasound but requires CT or MRI for staging. CT and MRI have similar accuracy with respect to staging, and therefore, the institutional preference often dictates the choice of modality. Contrast-enhanced chest CT is indicated for diagnosing pulmonary metastatic disease, including nodal involvement of the mediastinum and hilum, as well as potential tumor thrombus within the inferior vena cava and heart.
Hepatoblastoma
As with neuroblastoma and Wilms tumor, the diagnosis of hepatoblastoma is often suspected by ultrasound findings. MRI is preferred over CT for staging due to its superior contrast resolution, use of hepatobiliary-specific contrast agents, and potential for multiphase, dynamic contrast-enhanced imaging in the absence of ionizing radiation that is inherent to CT. Given the propensity for hepatoblastoma to involve the hepatic vessels, a thorough evaluation for vessel involvement with tumor is mandatory for staging. In the absence of MRI, CT may also be used for staging hepatoblastoma.
Practical imaging approach to pediatric abdominal masses
Various imaging modalities are currently available for evaluating pediatric abdominal masses. A clear understanding of their advantages and disadvantages as well as specific situations for certain imaging modalities is essential for the practical imaging approach to pediatric abdominal masses.
Radiography
Conventional radiographs of the abdomen are often requested by clinicians as the initial imaging modality for the evaluation of a possible abdominal mass in children based on the clinical history and physical examination. The results of radiography are often nonspecific, but can be helpful, especially if an abdominal mass is large enough to cause local mass effect on adjacent organs such as the bowel. For example, a large hepatic mass can displace bowel loops inferiorly to the lower abdomen or pelvis, whereas a pelvic mass such as an ovarian tumor tends to displace the bowel loops superiorly. A large kidney or adrenal mass can displace the bowel loops to the contralateral side of the abdomen. Additionally, the presence of abnormal calcifications can provide clues to diagnosis. When a pediatric patient presents with abdominal pain, at least 2 radiographs of the abdomen are needed. These should include a frontal supine view, and either a decubitus, upright, or cross-table lateral view to assess for bowel obstruction and pneumoperitoneum.
Ultrasound
Abdominal ultrasound is the preferred initial imaging modality in children with a suspected abdominal mass mainly due to its accessibility, flexibility, lack of ionizing radiation, lack of sedation, and relatively low cost compared to CT and MRI. Ultrasound has the potential to determine the anatomic origin and borders of the mass, as well as to characterize internal characteristics such as solid, cystic, and vascular components. Specifically, the vascularity and vascular supply of the mass can be evaluated by Doppler imaging. Local and distant abdominal metastasis in the abdomen can be assessed as well, but may lack sensitivity compared with CT and MRI. A potential pitfall of ultrasound is operator dependency; an inexperienced sonographer may completely fail to image a mass. Furthermore, the acoustic windows for visualizing structures in the abdomen may be limited by bowel gas. When successful, however, ultrasound characteristics of the abdominal mass narrow the differential diagnosis and thus help to guide management.
Computed Tomography
CT of the abdomen and pelvis with contrast is recommended if abdominal ultrasound is inconclusive or if further characterization of a mass or extent of disease is needed. The clinical setting and suspected diagnosis dictate the exact CT protocol. For instance, hepatic tumors such as hepatoblastoma, require identifying which hepatic segments are involved as well as the relationship of the mass to the hepatic vessels for surgical planning. Therefore, a dual-phase CT with both arterial and venous phases may be needed. Additionally, given the propensity for hepatoblastoma to metastasize to the lungs, a chest CT is typically also simultaneously performed. Advantages of CT include its wide availability and rapid acquisition, particularly with the newer generation CT machines, which may avoid the need for sedation in many pediatric patients. CT also excels in characterizing abdominal masses and provides a more global view of anatomy than ultrasound, and therefore, can assist in problem-solving and staging. An important disadvantage of CT is the inherent use of radiation, and therefore, it is important to routinely apply the as low as reasonably achievable (ALARA) principle with respect to radiation dose and technique.
Magnetic Resonance Imaging
MRI has been steadily gaining popularity in pediatric imaging due to advances in technology. Faster imaging acquisition, specialized pediatric coils, and dynamic contrast imaging allow superior contrast resolution than other imaging modalities to aid in accurately diagnosing an abdominal mass. Newer techniques, such as excretory MRI, can provide functional and quantitative information for obstructive processes involving the kidney. Although neonates can be imaged using the “wrap-and-feed” technique, most infants and young children may still require sedation for diagnostic quality MRI. Thus, the risks of anesthesia should be carefully considered when deciding MRI as an imaging modality for the evaluation of abdominal masses in infants and children.
Nuclear Medicine
Nuclear medicine is a helpful adjunct imaging modality in select clinical scenarios in the pediatric population. For instance, MIBG scans can help to differentiate neuroblastoma from other adrenal lesions, including adrenal tumors and adrenal hemorrhage. MAG3 (mercaptoacetyltriglycine) diuretic renal scans can help to differentiate ureteropelvic junction (UPJ) obstruction from other congenital kidney conditions. It is important to note, however, that there is both inherent risks of radiation and limited spatial resolution with nuclear medicine.
Interventional Radiology
Interventional radiology (IR) is an integral component of radiology that facilitates the diagnosis and treatment of abdominal masses. Minimally invasive image-guided procedures performed by interventional radiologists may provide both diagnosis and treatment in lieu of open surgery. For example, some lesions may be amenable to image-guided percutaneous biopsy. Additionally, therapeutic procedures such as fluid drainage, embolization of tumors, and sclerotherapy may also be provided by IR. Unfortunately, pediatric IR service is currently not universally available and is often limited to tertiary children’s hospitals.
The spectrum of neonatal and pediatric abdominal masses
The differential diagnosis of pediatric abdominal masses is broad and includes congenital and acquired lesions that may be benign or malignant ( Tables 2 and 3 ). The most common entities that comprise the differential diagnosis, with a focus on the characteristic imaging appearance and optimal imaging strategies for each condition, are reviewed in the following section.
| Congenital | Infectious/Inflammatory | Neoplasm | Miscellaneous |
|---|---|---|---|
| Enteric duplication cysts | Abscess | See Table 3 | Adrenal hemorrhage |
| Subdiaphragmatic extralobar pulmonary sequestration | Pancreatic pseudocyst | Constipation | |
| Congenital kidney disorders Abdominal lymphatic malformation | Meconium pseudocyst |
| Origin | Neoplasm |
|---|---|
| Liver | Infantile hepatic hemangioma, hepatoblastoma |
| Pancreas | Pancreatoblastoma, solid pseudopapillary epithelial neoplasm (SPEN) |
| Kidney | Wilms tumor |
| Adrenal Gland | Neuroblastoma |
| Blood Cells | Lymphoma |
Congenital Disorders
Enteric duplication cysts
Enteric duplication cysts (EDCs) are a subtype of foregut duplication cysts that result from the defective development of the ventral foregut. EDCs can occur anywhere along the gastrointestinal tract from the mouth to the rectum. Clinical presentation depends on the location and histologic type of the EDCs. Gastric and intestinal duplication cysts can cause abdominal pain, nausea, and bloating due to the local mass effect on the adjacent bowel. Intussusception and bowel obstruction with EDCs acting as lead points can also occur. The most serious complications typically present when EDCs are lined with gastric mucosa, which may result in mucosal ulceration and even bowel perforation.
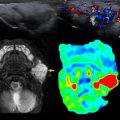
With technological advances in prenatal ultrasound, there has been increased detection of EDCs in prenatal imaging. Ultrasound is the imaging modality of choice for the diagnosis of EDCs. On ultrasound imaging, EDCs are known for a characteristic “gut signature” or a “double wall” sign corresponding to alternating bands of the bowel wall layers. When complications of EDCs occur, CT and MRI can further aid in surgical planning ( Fig. 1 ). With symptomatic EDCs, surgical resection is needed for treatment.

Subdiaphragmatic extralobar pulmonary sequestration
Subdiaphragmatic extralobar pulmonary sequestration (EPS) consists of malformed pulmonary parenchyma that lacks a normal connection to the bronchial tree and receives direct arterial supply from the aorta or its branches. EPS is believed to develop from the abnormal budding of the foregut, often associated with bronchial atresia. Subdiaphragmatic EPS is uncommon and accounts for approximately 10% of EPS; 90% of EPS occur within the chest, between the lower lobe and diaphragm. EPS is associated with other congenital anomalies such as diaphragmatic hernia, vertebral body anomalies, and congenital heart disease. Affected pediatric patients with EPS often present in infancy with a respiratory illness.
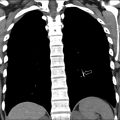
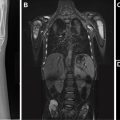
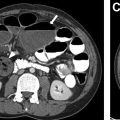
EPS is typically first evaluated with abdominal ultrasound and is characterized by a homogenous round or triangular-shaped mass that is inferior to the diaphragm ( Fig. 2 A, B ). Occasionally, arterial supply arising from the aorta can be appreciated on Doppler ultrasound. Multidetector CT (MDCT) can accurately detect the arterial supply from the aorta as well as determine venous drainage, and therefore, MDCT is often chosen as the next imaging step ( Fig. 2 C, D). The management of EPS is surgical resection if the patient is symptomatic with recurrent infections. Some cases of EPS may regress spontaneously within 4 years without treatment, particularly when the affected pediatric patient is asymptomatic and systemic arterial supply is diminutive on initial imaging,.

Congenital kidney disorders
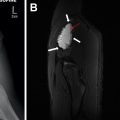
A variety of congenital kidney disorders such as hydronephrosis, UPJ obstruction, ureterovesical junction (UVJ) obstruction, multicystic dysplastic kidney (MCDK), and autosomal recessive polycystic kidney disease (ARPCKD) can present in children as an abdominal mass by the virtue of either the degree of dilation of the collecting system or the extent of nephromegaly related to the pathologic parenchymal process. Although a detailed description of these various entities is beyond the scope of this article, many of these entities are either suspected or diagnosed in utero on screening ultrasound, and are further evaluated with postnatal ultrasound ( Fig. 3 A). In the case of suspected UPJ or UVJ obstruction, functional imaging can be further accomplished by either nuclear medicine scintigraphy or MR urography ( Fig. 3 B).