Pediatric Applications
The role of point-of-care ultrasound in the emergent care of ill and injured pediatric patients continues to evolve and mature. Ultrasound technology is ideally suited for infants and children as it allows real-time visualization of anatomic structures without causing pain, requiring sedation, or exposing developing tissues to ionizing radiation.
There are numerous indications for emergency ultrasound common to both adult and pediatric emergency care. This has led to a greater understanding of the differences that exist when the same applications are used for both pediatric and adult patients. In addition, the functionality of emergency ultrasound has been further expanded by recent innovation and the development of several pediatric-specific applications.
 TRAUMA
TRAUMA
Trauma remains one of the leading causes of morbidity and mortality in children. In the United States, traumatic injuries result in hospital admission for approximately 600,000 children each year.1 In the pediatric age group, blunt trauma is more prevalent than penetrating trauma, and 20–30% of pediatric trauma cases involve the abdomen.2
The history and physical examination form the foundation of the patient evaluation; however, they may be difficult to obtain in children who have altered mental status, CNS trauma, or distracting injuries. In one study of children with blunt abdominal trauma, an initial physical examination was considered reliable in only 41% of cases.3 Furthermore, the exam may be misleading in up to 45% of injured children.4,5
The use of ultrasound in pediatric trauma with the focused assessment with sonography for trauma (FAST) examination has increased over the past decade, but it has not been as well accepted or widely used as it has for adult trauma care. In a survey of general emergency physicians, pediatric emergency physicians, and trauma surgeons, 91% of the respondents considered abdominal ultrasound to be “somewhat to extremely useful.”6 However, with regard to pediatric trauma patients, 73% of all respondents considered abdominal ultrasound to be useful, while only 57% of pediatric emergency physicians considered it so. Furthermore, only 14% of pediatric emergency physicians routinely use abdominal ultrasound for evaluation of their trauma patients.6
Numerous advantages exist for using ultrasound in pediatric trauma, mirroring its benefits in adult trauma (see Chapter 5, “Trauma”). In pediatrics, limiting the exposure of ionizing radiation is especially appealing.7 The results of a large prospective multicenter trial were recently reported, and showed that pediatric trauma patients with a low-to-moderate clinical suspicion for intra-abdominal injury were significantly less likely to undergo abdominal CT scanning if they underwent a FAST exam.8
CLINICAL CONSIDERATIONS
The 1980s saw a transition away from diagnostic peritoneal lavage toward the use of abdominal CT. CT is still the most commonly used modality in evaluating pediatric abdominal injuries.9–18 The primary advantage of CT is that it accurately and reliably identifies and characterizes most intraperitoneal and retroperitoneal injuries. A major disadvantage of CT is that it exposes patients to significant doses of ionizing radiation. Children are 10 times more sensitive to the induction of cancer than adults, and one study estimates that a single abdominal CT in a young girl results in a risk of fatal cancer later in life of approximately 1 in 1,000.19 Some physicians have questioned the widespread use of CT and advocate the use of ultrasound for screening pediatric blunt trauma patients.20–22
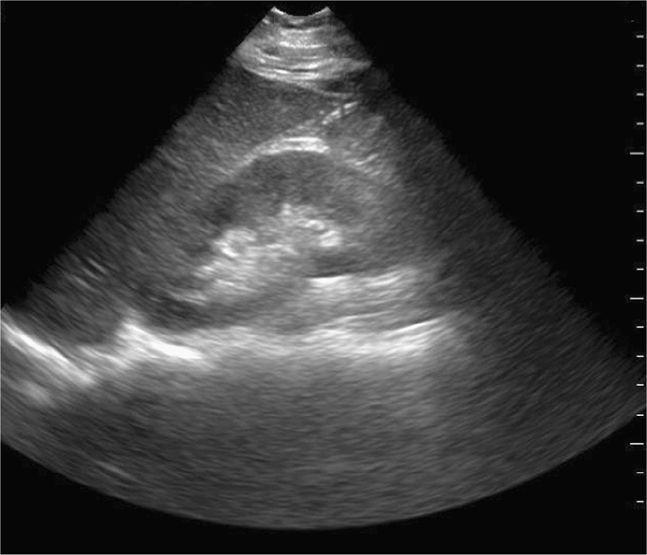
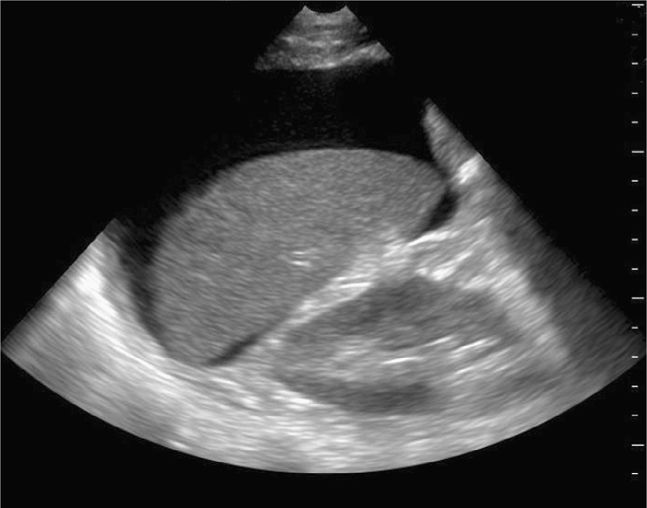
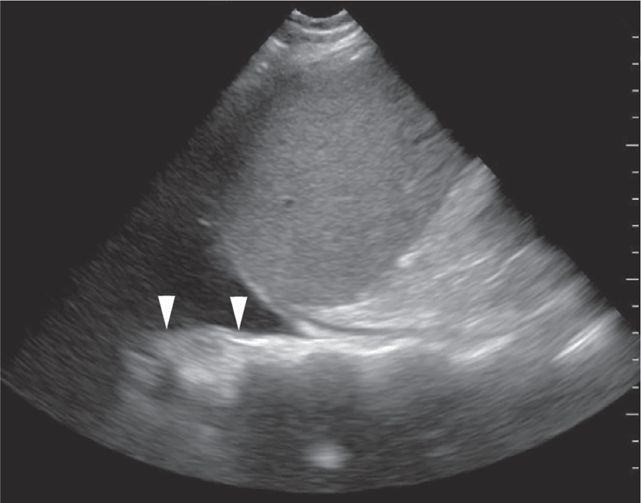
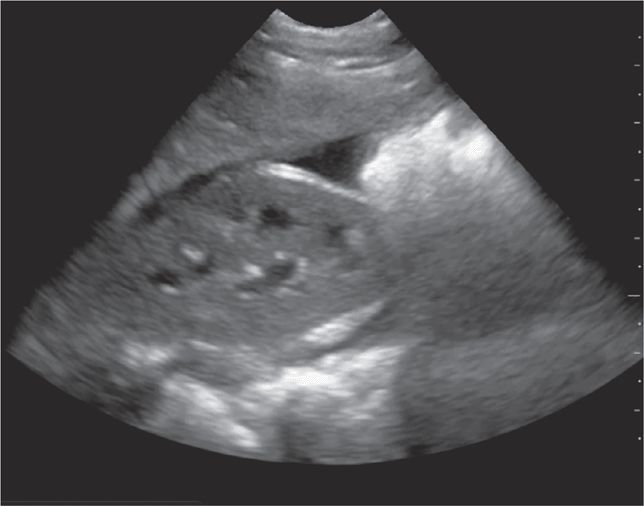
The FAST examination is a noninvasive diagnostic tool for detecting hemoperitoneum, hemopericardium, and hemothorax (Figure 20-1).6 A systematic review assessed 25 studies consisting of 3838 children undergoing abdominal ultrasound after sustaining trauma. All studies were observational and the methodology, ultrasound protocols, and outcome definitions were all highly variable. The meta-analysis showed that the FAST exam had a sensitivity of 80% for identifying hemoperitoneum.23
Figure 20-1. Hemoperitoneum. Ultrasound techniques and findings are outlined in the corresponding sections of this chapter.
The sensitivity fell to 66% when patients with intra-abdominal injuries, but without hemoperitoneum, were included.23 Over one-third of pediatric solid organ injuries are not associated with free intraperitoneal fluid,26,27 and failure to identify patients with intra-abdominal injury without hemoperitoneum is a known limitation of the abdominal ultrasound examination and is not considered a reasonable outcome measure for FAST by most providers.26,28
A prospective observational study evaluated the test characteristics of the FAST examination to detect any amount of fluid as well as clinically significant fluid in pediatric patients who suffered blunt abdominal trauma.24 Test characteristics for detection of significant intra-abdominal fluid show a sensitivity of 52% and a specificity of 96%. Clinically significant fluid was defined as moderate free fluid on abdominal CT scans or if patients went directly to the operating room for intra-abdominal injury. When evaluating the test characteristics of the FAST for detection of any intraperitoneal fluid the sensitivity dropped to 20%, while the specificity remained high at 98%. These data suggest that the FAST exam is useful when positive; however, when it is negative, it does not exclude the presence of intra-abdominal injury.
The clinical utility of the FAST exam as a screening tool that can potentially decrease the use of CT is not addressed in the pediatric trauma literature. But as in adults, the FAST exam is very sensitive for hemoperitoneum in pediatric patients who are hypotensive as the result of intraperitoneal blood loss.25
The E-FAST or “extended” FAST examination includes evaluation of the lungs for pneumothorax. Use of the E-FAST examination has been well established in adults (see Chapter 5, “Trauma”). Ultrasound evaluation for pneumothorax has been shown to be more sensitive (98%) than a supine chest radiograph (76%) in adult trauma patients, when compared to CT as the gold standard.29 A case report from a neonatal special care nursery had a similar finding.30 The E-FAST examination can be performed at the bedside in 3 minutes or less31,32 and is easily repeatable.
CLINICAL INDICATIONS
An extended point-of-care ultrasound examination is indicated in children with:
 Significant abdominal or thoracic trauma
Significant abdominal or thoracic trauma
 Patients with unexplained hypotension
Patients with unexplained hypotension
 Patients with altered mental status
Patients with altered mental status
Significant Abdominal or Thoracic Trauma
A complete E-FAST exam should be performed on all pediatric patients with significant blunt or penetrating thoracoabdominal trauma as part of the secondary survey. In hemodynamically unstable pediatric trauma patients, the E-FAST examination may rapidly identify an abdominal or thoracic source of hypotension and assist with decision making regarding timing of diagnostic testing versus operative intervention. The abdominal portion of the E-FAST exam has the best test performance in children who are hemodynamically unstable with significant hemoperitoneum.25 If free intraperitoneal fluid is identified, and the patient remains hypotensive after a bolus of IV fluid, the decision to perform exploratory laparotomy should be considered. If the patient stabilizes with a fluid bolus, abdominal CT scanning may be considered to guide selective laparotomy.
The E-FAST examination may also allow for prioritization of imaging studies in hemodynamically stable patients after initial evaluation and resuscitation. Patients with positive E-FAST examinations are typically triaged to abdominal CT with greater expediency than those patients with normal E-FAST examinations. E-FAST may also be useful in evaluating alert, hemodynamically stable pediatric trauma patients without abdominal tenderness, who would otherwise not routinely undergo abdominal CT, and who may occasionally have intraabdominal injuries.
In hemodynamically stable patients, the information provided by a negative E-FAST examination may be sufficient for the clinician to decide against abdominal CT.8,23 It may be prudent to admit such patients for observation and serial ultrasound and physical exams. Patients who are hemodynamically stable and have a negative E-FAST examination should undergo abdominal CT scanning if they demonstrate peritoneal signs, abdominal distention, seat belt abrasion, hematuria, or persistent tachycardia.
The E-FAST examination is also indicated in pediatric patients with penetrating trauma. The main benefit of using the E-FAST exam in penetrating thoracoabdominal trauma is not necessarily to rule out injury and avoid CT scanning, but to quickly identify which body cavity is injured to guide the sequence of surgical exploration or management.
ANATOMICAL CONSIDERATIONS
The FAST examination was designed to assess the three primary dependent areas of the peritoneal cavity (right upper quadrant, left upper quadrant, and pelvis) along with the thoracic cavity. The location of the fluid depends primarily on the source of the bleeding, but may be affected by the position of the patient. For the purpose of understanding the anatomy as it relates to the FAST examination, the abdomen is divided into quadrants by the mesentery of the transverse colon horizontally and by the spine vertically (see Figure 5-6A). Relevant anatomy needs to be identified in order to evaluate for surrounding intra-abdominal, thoracic, or pericardial fluid. There are some pediatric considerations, which are discussed below.
In the right upper quadrant, Morison’s pouch is the potential space between the liver and the right kidney and represents the most dependent supramesocolic area. Blood from a liver laceration will accumulate in this area; blood from a splenic injury may also spill over the lumbar spine into Morison’s pouch. Blood from an inframesocolic injury can spread over the sacral promontory into Morison’s pouch as well via the right paracolic gutter. Since most major blunt abdominal injuries involve the liver and spleen, the view of Morison’s pouch is regarded as the most important of the four views in the FAST examination in adults.33 Alone, it has been found to be 51–82% sensitive in detecting free fluid.34–36 In pediatric patients, however, free fluid tends to accumulate in the pelvis.37
Blood from a splenic injury will accumulate first in the left subphrenic space. There is no equivalent of Morison’s pouch as the splenorenal ligament attaches the spleen and kidney. Therefore, intraperitoneal fluid accumulates circumferentially around the spleen: commonly below the diaphragm, at the inferior pole of the spleen, and less so in the splenorenal fossa. Blood from this area can flow into Morison’s pouch, and will preferentially reach the pelvis by spilling down the right paracolic gutter because the left upper quadrant is separated from the left paracolic gutter by the phrenicocolic ligament.38
Blood from inframesocolic injuries will accumulate first in the rectovesicular pouch in boys and the retrouterine pouch of Douglas in girls. These areas are the most dependent portions of the peritoneal cavity.38 One study found an isolated pelvic view to be 68% sensitive in detecting free intraperitoneal fluid.34 In children, the pelvis view is the region most likely to be positive if a patient has intra-abdominal free fluid due to an isolated liver or spleen laceration.37
Ultrasound examination of pneumothorax is based on observation of sliding of the visceral pleura of the lung on the parietal pleura of the chest wall. In a supine trauma patient, thoracic air tends to accumulate between the anterior chest wall and the lung, separating the parietal and visceral pleurae.
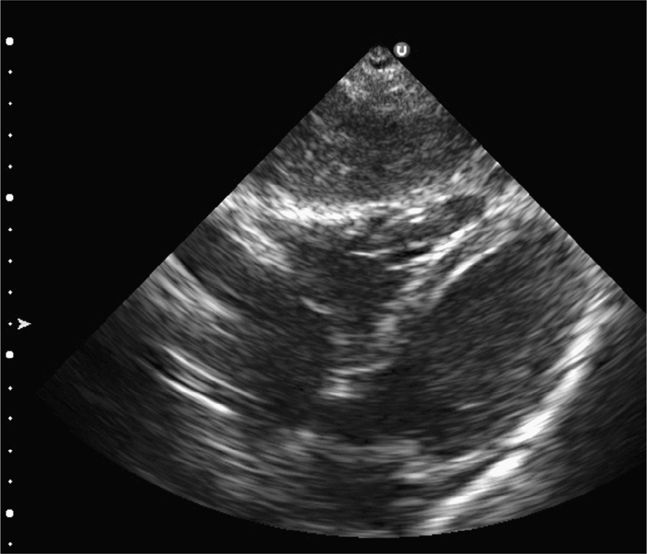
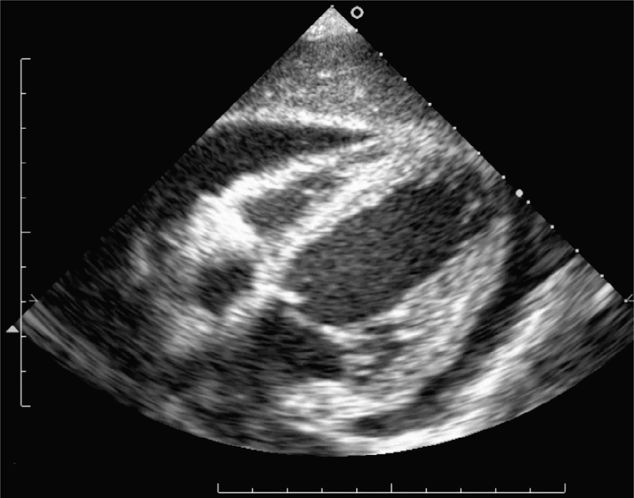
The classic FAST view of the heart is the subxiphoid view. In children, this view may be limited due to air in the stomach, from crying, or from bag mask ventilation. If the subxiphoid view is insufficient, consider the parasternal long-axis view of the heart. This view will visualize the right and left ventricles, left atrium, aortic and mitral valves, the pericardium, and the descending aorta. The descending aorta is the landmark used to differentiate pericardial from pleural effusions posterior to the heart.
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
The FAST examination of pediatric patients consists of subxiphoid, right and left upper quadrant, and suprapubic views, which are identical to those in adults (Figure 20-2; Video 20-1: Pediatric Considerations). The E-FAST examination adds bilateral lung views to assess for pneumothorax. Views of the hemidiaphragms and caudal thorax, to assess for hemothorax, should be obtained with the right and left upper quadrant views. Use a low-frequency transducer between 2 and 6 MHz (includes the phased-array transducer and curvilinear or convex transducers) in children. Phased-array transducers are preferred for younger children as the flat, small footprint allows for better imaging between the ribs. Some authors recommend using a 5.0 MHz (or even 7.5–10 MHz) transducer for finer resolution.21,39 The E-FAST examination should be performed with the patient supine, which is how the patient is transported on a backboard after blunt trauma. By convention, the index marker on the transducer should be directed cephalad or to the patient’s right. Move the transducer up or down one or more rib spaces to optimize the view.
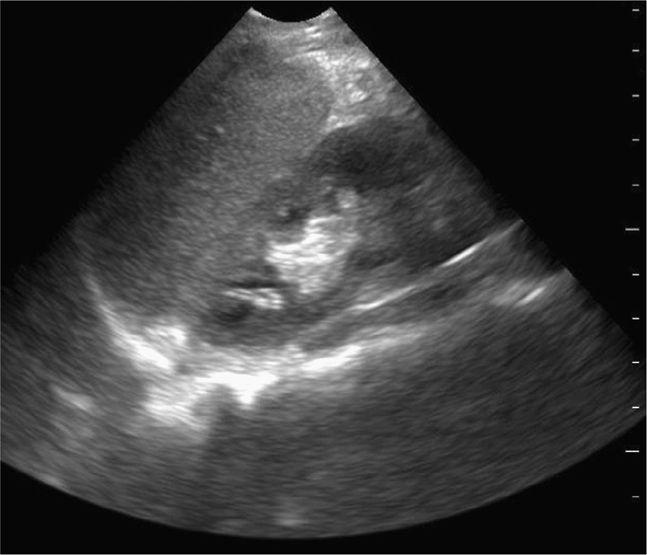
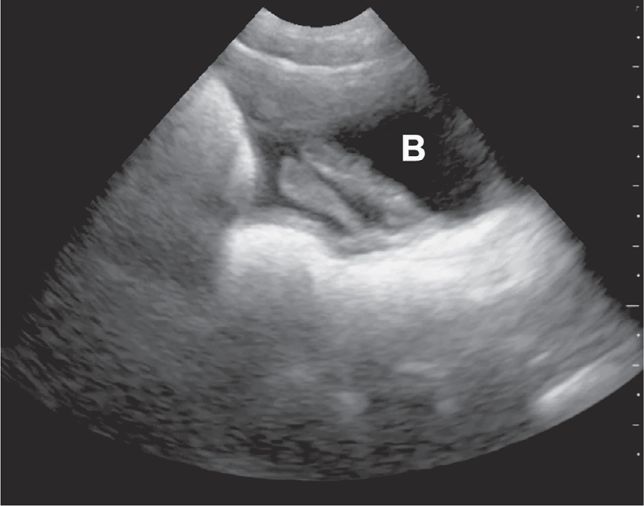
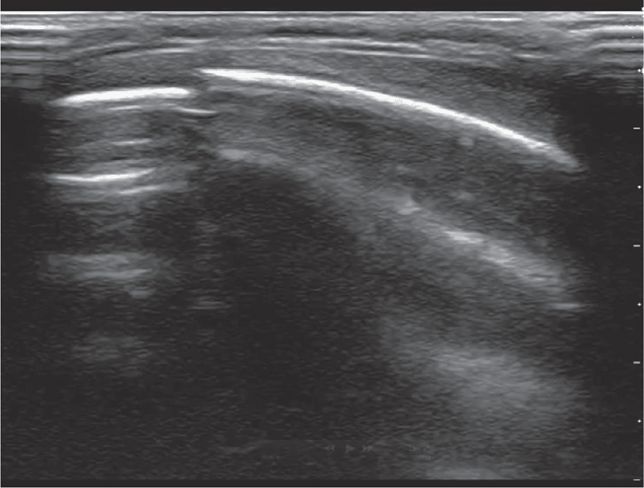
Splenic or hepatic injuries account for 74% of cases of hemoperitoneum in pediatric blunt trauma.40 With the index marker cephalad, place the transducer in a coronal plane in the mid to anterior axillary line at the 10th intercostal space or below (Figure 20-3). The kidney lies retroperitoneal, so direct the transducer dor-sally to maximize the view of the liver–kidney interface. Rotate the transducer counterclockwise and align it with the intercostal space to minimize shadowing. Sliding or tilting the transducer cephalad brings the lower thorax into view, displaying the mirror image artifact of the liver above the diaphragm (Figure 20-4).
Visualize the diaphragm superiorly and the tip of the liver inferiorly. Scan the inferior pole of the kidney to assess for free intraperitoneal fluid in the right paracolic gutter. Visualization of the inferior pole of the liver is necessary since free fluid may accumulate there before progressing to Morison’s pouch (Figure 20-5).
The goal of the left upper quadrant view is to examine the potential space around the spleen, between the spleen and the diaphragm, and around the inferior pole of the spleen. With the index marker cephalad, place the transducer in the posterior axillary line in the coronal plane at the 9th intercostal space or below (Figure 20-6). Rotate the transducer clockwise and align it with the intercostal space to minimize rib shadows. Inspect the lower left thorax for intrathoracic blood, as described above. Visualize the diaphragm superiorly and the lower pole of the spleen inferiorly (Figure 20-7). Scan the inferior pole of the left kidney to evaluate for intraperitoneal fluid in the paracolic gutter.
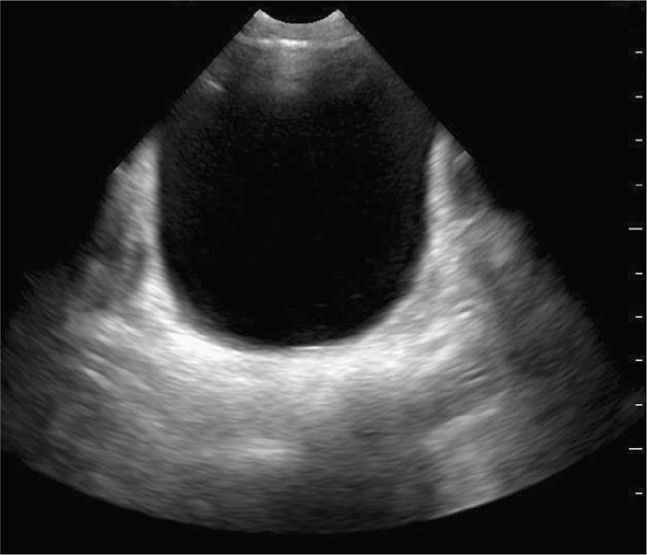
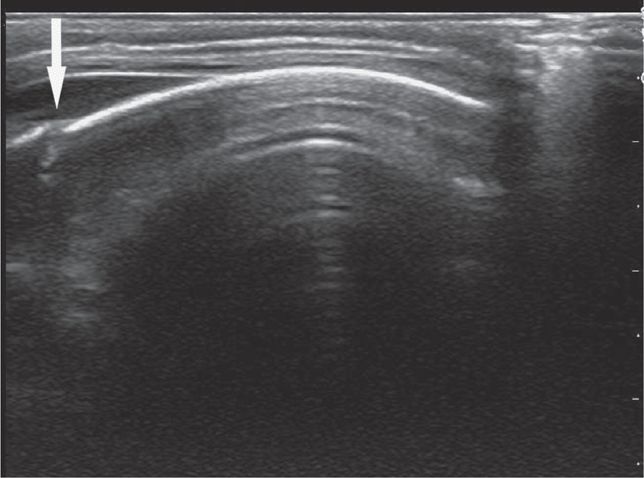
Scan the pelvis for free intraperitoneal fluid with both transverse and sagittal views. The bladder is used as a landmark to evaluate for hemoperitoneum. Intraperitoneal fluid accumulates cephalad and posterior to the bladder. Ideally, scan the suprapubic view prior to insertion of a Foley catheter. Urine in the bladder provides an important acoustic window. Place the transducer just cephalad to the symphysis pubis in the midline sagittal plane with the index marker cephalad and in the transverse plane with the index marker to the patient’s right (Figure 20-8). Urine appears hypoechoic or anechoic and is well circumscribed by the bladder wall (Figure 20-9). The uterus in girls and prostate in boys can be seen posterior to the bladder. The transition in tissue densities between urine in the bladder and the surrounding soft tissue can produce bright echoes, so reducing the far gain will enhance subtle soft tissue details and help avoid missing free fluid in this area.
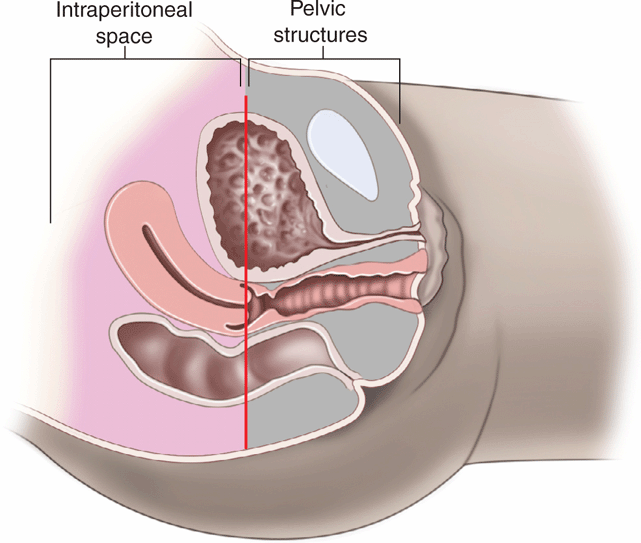
Obtain a midline sagittal view first to get an overview of the pelvic anatomy. In the midline sagittal view, the posterior angle of the bladder divides the intraperitoneal space containing the bowel (and the uterus in girls) from the pelvic structures (prostate and seminal vesicles in boys, vagina in girls) (Figure 20-10). Free intraperitoneal fluid only accumulates cephalad (to the left) to the posterior angle of the bladder, so it is an important anatomic landmark to recognize.
Figure 20-10. Midline sagittal view of the pelvis. This drawing demonstrates a simple technique for understanding where free intraperitoneal fluid may be seen in the pelvic views. An imaginary line (in red) drawn from the posterior angle of the bladder separates the intraperitoneal space (pink shading) from the pelvic structures (gray shading). In girls, the uterus projects into the peritoneal space (to the left) and may be surrounded by free fluid in trauma patients. In boys, free fluid will be adjacent to the bladder. Structures posterior and caudad to the bladder (to the right) include the vaginal stripe in girls, and prostate and seminal vesicles in boys, and free intraperitoneal fluid will not be seen in this region.
The amount of free intraperitoneal fluid that can be reliably detected in children has not been studied. In adults, 400 mL is a reasonable estimate.41,42 One study suggests that placing the patient in Trendelenburg or decubitus positions allows for the detection of intraperitoneal fluid with only two-thirds of the amount of fluid required in the supine position.43 The reverse Trendelenburg position may help with identification of pleural or pelvic free fluid.
Always begin the FAST examination with the subxiphoid view of the heart for penetrating trauma patients in whom cardiac injury is suspected. Place the transducer in the subxiphoid region in the coronal plane with the index marker to the patient’s right. Direct it toward the left shoulder at a steep angle (relative to the abdominal skin) using the liver as an acoustic window (Figure 20-2). This provides a four-chamber view of the heart surrounded by a hyperechoic pericardium (Figure 20-11). In the crying child, slide the transducer laterally, slightly to the right of the midline, to avoid the air in the stomach by using the liver as an acoustic window. If this view is insufficient, consider the parasternal long-axis view. Place the transducer along the axis of the heart from the right shoulder to the left hip. This approach will visualize the right and left ventricles, left atrium, aortic and mitral valves, as well as the pericardium surrounding the heart and the descending aorta. The descending aorta is the landmark used to differentiate pericardial from pleural effusions.
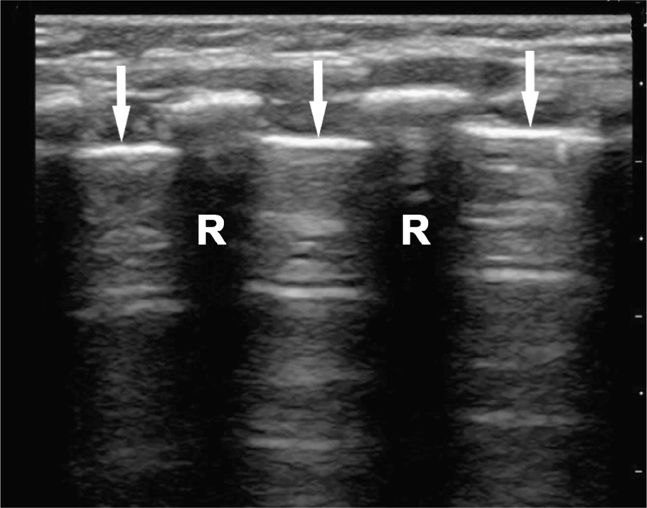
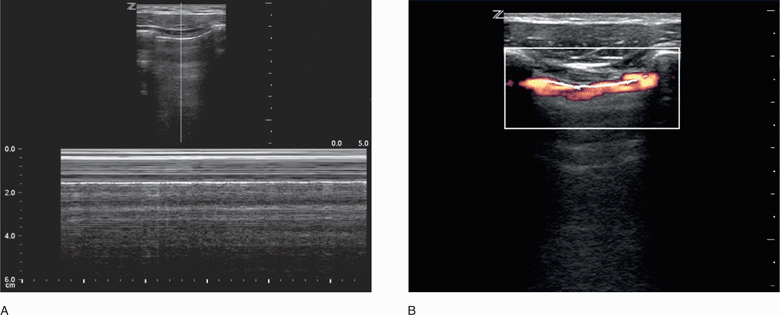
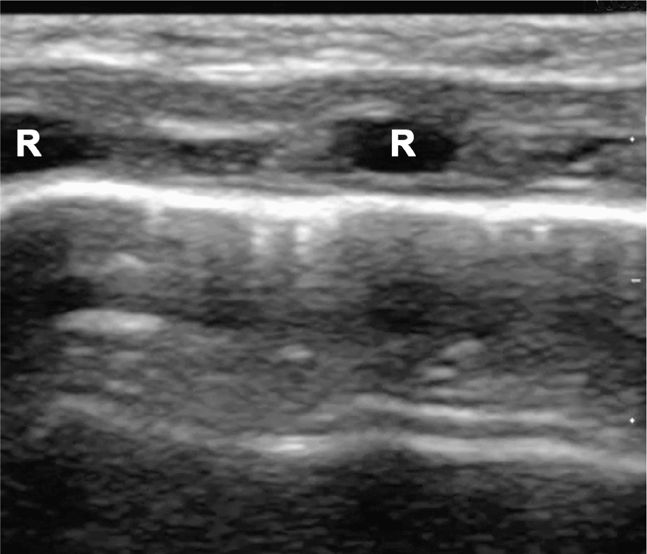
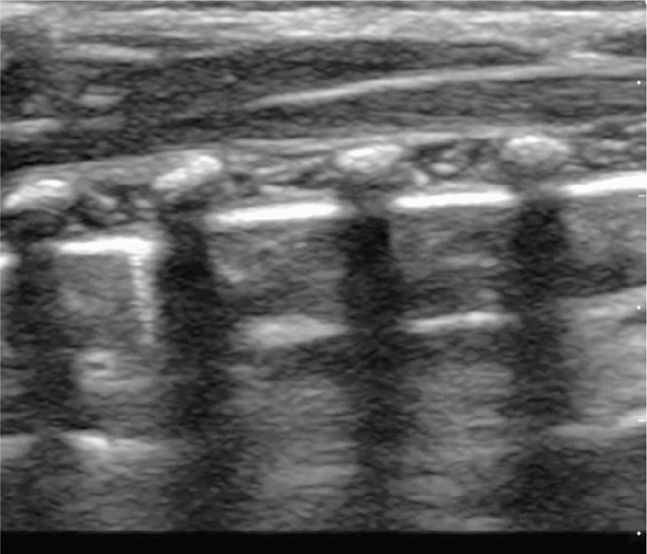
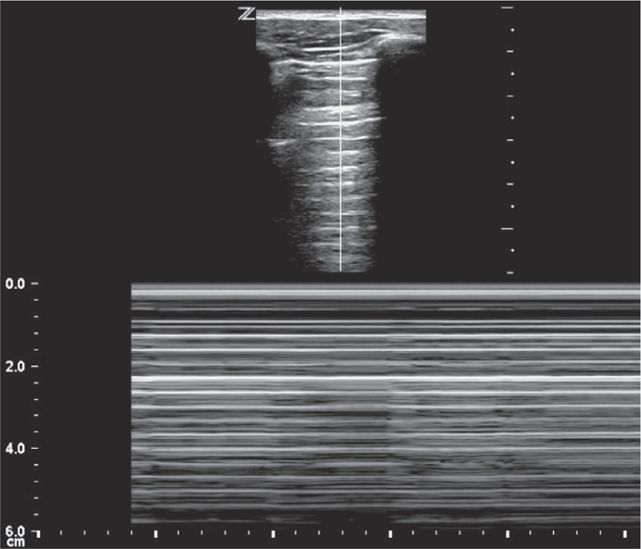
Perform the lung ultrasound examination with a linear transducer or any transducer with a higher frequency selected. Place the transducer in a longitudinal orientation with the hyperechoic ribs as landmarks on either side of the image. Decrease the depth to optimize the view of the pleural interface. Rapidly assess both sides of the chest for the presence or absence of lung sliding by scanning in the midclavicular line in the second or third intercostal space bilaterally. The interface of the visceral and parietal pleura is a thin line just deep to the ribs and appears bright and echogenic (Figure 20-12). Pleural sliding is seen in real time as scintillating, to-and-fro movement at the level of the pleural line. If pleural sliding is not obvious, observe the pleural line for at least three respiratory cycles and examine other sites on the chest wall. M-mode or color Doppler is commonly used to help assess and document pleural movement (Figure 20-13, and also see Chapter 5, “Trauma”). When evaluating the lungs in infants and toddlers, the pleura may be visible through the ribs (as a continuous hyperechoic line) because the ribs are primarily cartilage (Figure 20-14). In younger children, the ribs produce anechoic shadows but they are not as strong as the rib shadowing in adolescents and adults because the ribs are not yet completely ossified (Figure 20-15).
Figure 20-12. Ultrasound: Normal lung with rib shadowing (R), and location of the pleural line indicated (arrows).
Figure 20-13. Ultrasound: Lung. M-mode (A) and color Doppler (B) used as adjuncts. M-mode displays the “seashore sign.”
Figure 20-14. Ultrasound: Normal lung of a neonate. The ribs (R) are hypoechoic and have minimal shadowing artifacts.
Figure 20-15. Ultrasound: Normal lung of a young child. Ribs and associated shadows are more easily identified.
COMMON AND EMERGENT ABNORMALITIES
Hemoperitoneum
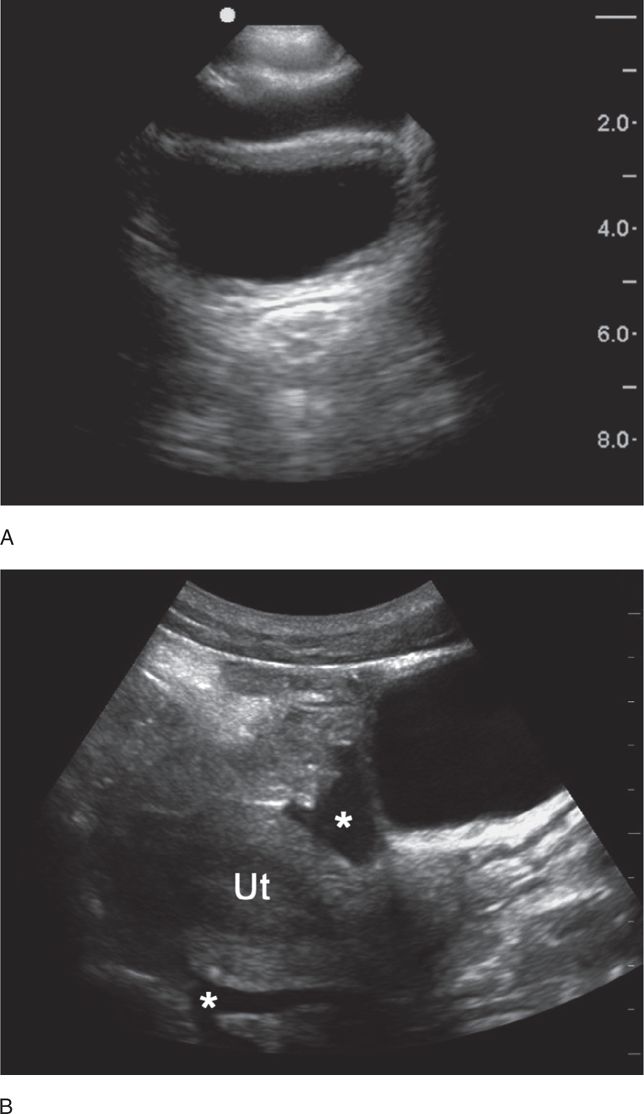
A small amount of intraperitoneal fluid may be visible just at the inferior portion of the liver, whereas a large amount of fluid will separate the liver from the kidney and produce a thick anechoic stripe in Morison’s pouch (Figure 20-16,B). Blood most often accumulates circumferentially around the spleen and below the left diaphragm because the splenorenal ligament maintains the integrity between the spleen and the kidney (Figure 20-17). Nonclotted blood in the pelvic region appears as anechoic free fluid posterior and cephalad to the bladder in boys (Figure 20-18A) and both anterior and posterior to the uterus in girls (Figures 20-18B).
Figure 20-16. Ultrasound: RUQ. Hemoperitoneum including example of small (A) fluid collection (arrow), and large hemoperitoneum (B).
Figure 20-18. Ultrasound: Pelvis hemoperitoneum. Transverse view (A) shows fluid anterior to the bladder. Longitudinal view (B) reveals fluid (*) in both the anterior and posterior cul-de-sac areas. Ut = uterus.
Hemopericardium
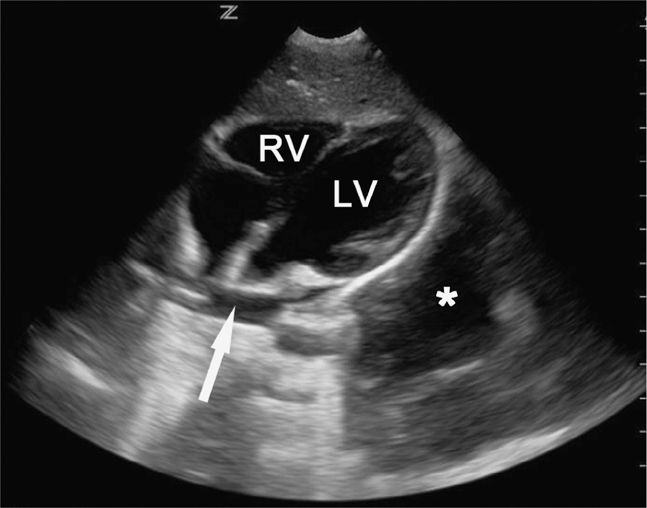
Unclotted blood in the pericardial space will appear as an anechoic stripe lying between the two brightly, echogenic layers of the pericardium (Figure 20-19). Clotted blood is more complex and echogenic. Small pericardial effusions may be visible anteriorly, posteriorly, or at the apex so it is important to scan through all of these areas, especially in patients who may have a penetrating cardiac injury (Figure 20-20).
Figure 20-20. Thoracic fluid. Subcostal four-chamber view demonstrates a small pericardial effusion at the base of the heart (arrow). Pt also has a larger hemothorax (*) adjacent to the left ventricle. LV = left ventricle, RV = right ventricle.
Hemothorax
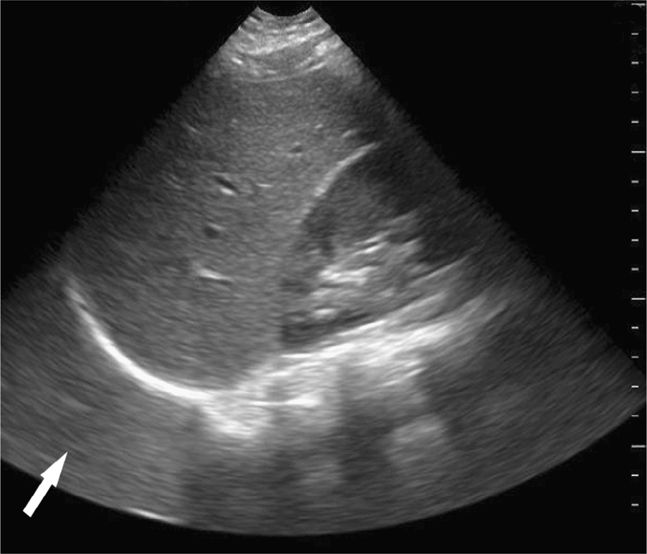
Blood in the chest appears as an anechoic area cephalad to the hemidiaphragm (Figure 20-21), and eliminates the mirror artifact. A large left sided hemothorax can sometimes be seen behind the heart on cardiac views (Figure 20-20).
Figure 20-21. Ultrasound: LUQ view. Fluid in the chest allows visualization of the posterior chest wall (arrowheads) or spine above the level of the diaphragm.
Pneumothorax
Absence of lung sliding, visualization of a lung point, and the “stratosphere” sign with M-mode may be seen when a pneumothorax is present (Figure 20-22). The lung point is the transition point between the pneumothorax and the normal lung and is highly specific for a pneumothorax. Absence of color Doppler scanning and linear, laminar lines deep to the pleura in M-mode resembling those of the overlying stationary anterior chest wall supports the diagnosis of pneumothorax (see Chapter 5, “Trauma,” for additional examples).
COMMON VARIANTS AND SELCTED ABNORMALITIES
Certain aspects of normal anatomy can be easily mistaken for positive findings. In the upper quadrant views, dark rib shadows must not be interpreted as anechoic blood. Since all fluid appears black (anechoic) on ultrasound, bile in the gallbladder and blood in the inferior vena cava (IVC) or aorta can be erroneously interpreted as free intraperitoneal fluid. In each of the abdominal views, and especially in the suprapubic view, fluid-filled loops of bowel can be mistaken for free intraperitoneal fluid.28 Holding the transducer still and observing for peristaltic movements often help differentiate free fluid from intraluminal fluid.
Perinephric adipose tissue, which may be present in pediatric patients of all ages, may be hypoechoic relative to surrounding structures and can be mistaken for free fluid or clotted blood. Comparison with the area around the other kidney may help distinguish this normal variant; additionally, perinephric fat does not move separately from the kidney with respirations and is more homogenous than clotted blood.
A subcapsular hematoma may be visible between the bright reflection of the splenic capsule and the homogenous parenchyma, which may be disrupted by injury. Blood is often clotted, appearing more echogenic, but may be distinguished from the splenic parenchyma. Intraparenchymal blood is often isoechoic with the splenic parenchyma on initial evaluation.44 Over time, a hematoma will become primarily hypoechoic.
Ascites is rarely an issue in children, and free fluid identified in the chest or abdomen in an injured child is regarded as blood until proven otherwise.
PITFALLS
1. There is no contraindication to performing an E-FAST exam unless it interferes with more important diagnostic tests or urgent patient management. Subcutaneous emphysema, gas-filled bowel, or morbid obesity may render the examination indeterminate.
2. The E-FAST examination is one data point in a continuum of clinical decision making. Repeat exams are often very helpful.
3. The E-FAST examination may not detect all intraperitoneal bleeding or significant injury. CT is generally used to characterize significant abdominal injuries, but most injuries will be managed nonoperatively in children.
4. Air scatters sound waves and makes images difficult to interpret. The inability to obtain standard views should raise the suspicion for free air or subcutaneous air.
5. Subtle free fluid may be missed if care is not taken to identify all the potential spaces. The view of Morison’s pouch is generally the easiest to identify and interpret. In children, however, this view is often more caudad than in an adult. In the left upper quadrant view, clinicians often fail to place the transducer far enough posteriorly. The perisplenic region is also more cephalad than Morison’s pouch. Missed subcapsular hematomas and blood between the spleen and the diaphragm are often the causes of false-negative scans; so it is recommended that at east 50% of the left hemidiaphragm be visualized to avoid missing blood in this area.
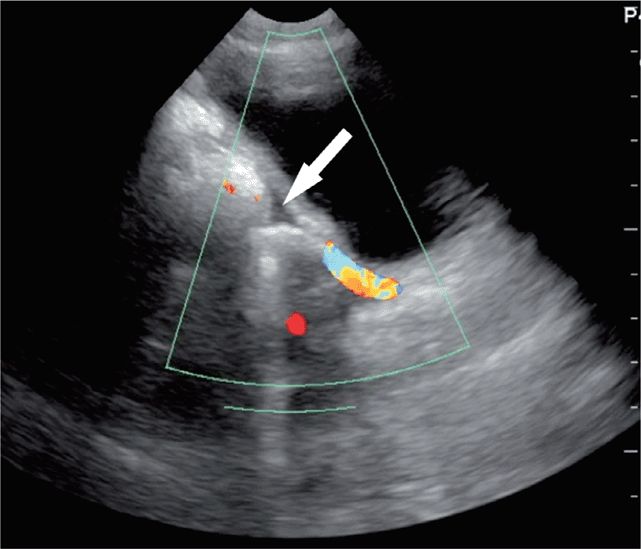
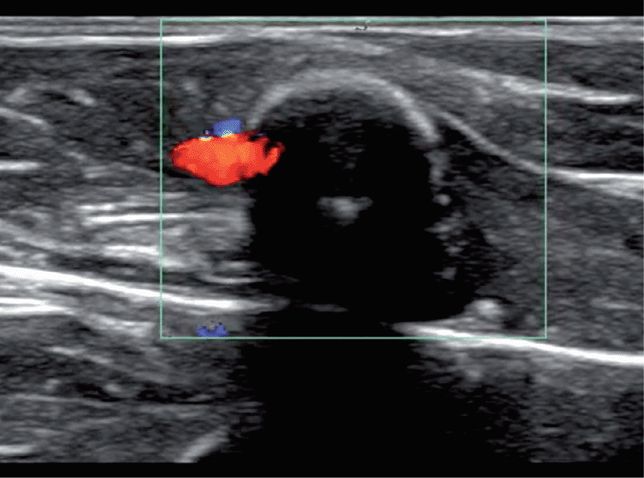
6. The pelvic views yield the most errors.28,45,46 A bladder containing little urine provides a poor acoustic window. The introduction of normal saline, in quantities appropriate for the patient’s age, into the bladder through a Foley catheter may be necessary to obtain good pelvic images. Also, care should be taken not to confuse free fluid with iliac vessels or other anechoic structures in the pelvis (Figure 20-23).
7. Adequate visualization of the heart in the subxiphoid view can be challenging, especially when the child is obese, crying, tachypneic, or has abdomen tenderness. Flattening the angle of the transducer and having the patient inspire may bring the heart into view. Another option is to move the transducer laterally toward the liver using the liver as an acoustic window. If adequate visualization is still not possible, a parasternal long- or short-axis view of the heart is recommended.
Figure 20-23. Ultrasound: Pelvic view. Free fluid (arrow) is distinguished from vessels by using color Doppler.
CASE STUDY
Patient Presentation
A 3-year-old healthy girl was transported to the ED by EMS after collapsing in her home. Her mother stated the girl came inside from playing in the yard and said, “Ow. Mommy, help,” and then fell to the ground. Her mother called 911 immediately. Per EMS the girl had altered mental status on scene and a serum glucose of 200 mg/dL. She was noted to be tachycardic and hypotensive.
Upon arrival, she had a blood pressure of 70/40 mm Hg, heart rate 150 beats perminute, respiratory rate 40 perminutes, and an oxygen saturation of 98% on room air. Her mental status waxed and waned, but she opened her eyes to painful stimuli. She had an unremarkable physical exam with no signs of trauma. Her abdomen was soft but moderately tender to palpation. Her repeat blood glucose was 500 mg/dL.
Management Course
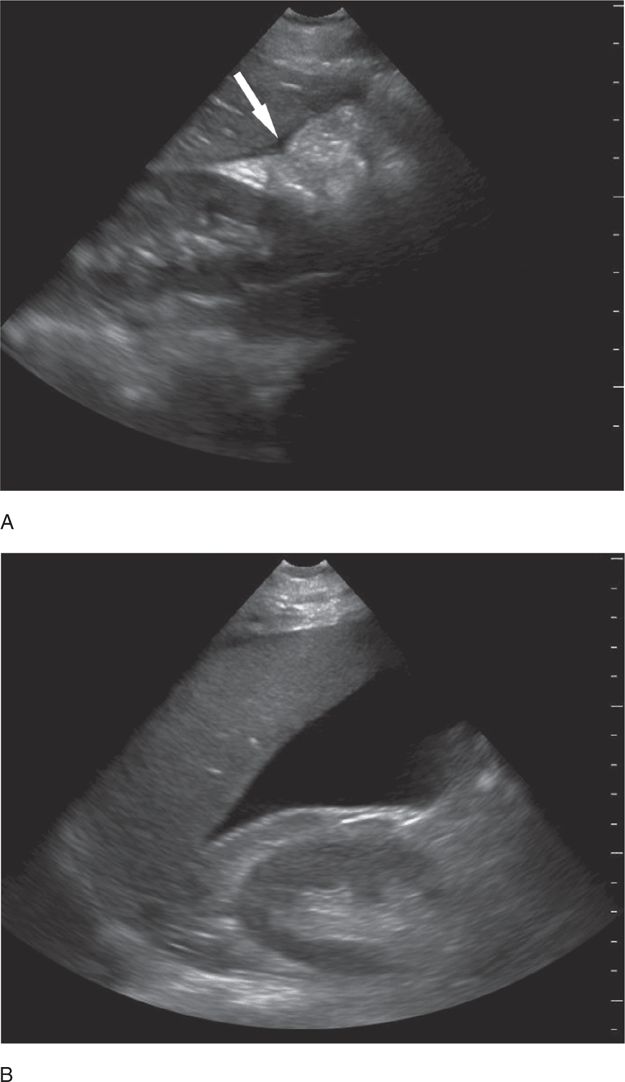
Access was established with intraosseous needles in both tibias and the team prepared to intubate the patient due to worsening vital signs and deteriorating mental status. An E-FAST exam was performed and revealed a small amount of free fluid at the inferior portion of the liver and a large amount of fluid in the pelvis (Figure 20-24). The remainder of the E-FAST exam revealed normal lung sliding (no pneumothorax) and mirror artifact above the diaphragm (no hemothorax) bilaterally. The cardiac exam revealed a hyperdynamic heart and no pericardial effusion. Surgery was immediately consulted. A repeat E-FAST 5 minutes later revealed free fluid accumulating in Morison’s pouch as well as the pelvis (Figure 20-25). The patient was taken to the operating room, where the surgical team found a liver laceration and controlled the bleeding.
Figure 20-24. Case study-Trauma. Ultrasound: Pelvis longitudinal view. Free fluid visible superior to the bladder (B).
Figure 20-25. Case study—Trauma. Ultrasound: RUQ. Repeat examination showed increased free fluid compared with the initial RUQ view.
Commentary
This case demonstrates the utility of the E-FAST exam in young children with altered mental status, abnormal vitals signs, and no history or evidence of trauma. This patient could have been mismanaged as a patient with presumed diabetic ketoacidosis had it not been for the identification of hemoperitoneum. Emergency ultrasound rapidly revealed the cause of her hypotension and directed her care appropriately to surgical intervention. Given that she was too unstable for a CT scan, emergency ultrasound was the only appropriate imaging modality.
 SKULL FRACTURE
SKULL FRACTURE
The role of neuroimaging for pediatric patients presenting to the ED after a closed head injury continues to evolve. Ultrasound has become an attractive alternative to skull radiographs and CT for imaging the skull, as it is rapid, reliable, inexpensive, and without ionizing radiation.
CLINICAL CONSIDERATIONS
The identification of an intracranial injury is critical to minimize morbidity and potential mortality. Pediatric patients, especially younger children, with intracranial injury may be asymptomatic on clinical exam.47 However, the presence of a scalp hematoma in asymptomatic infants with a head injury is predictive of skull fractures, which are in turn predictive of intracranial injury.48
Ultrasound has been shown to be an effective modality to identify skull fractures in a porcine model, with an accuracy of 97%.49 A recent case series highlighted the clinical value of this same technique in pediatric patients.50 The authors suggested three potential roles for emergency ultrasound: replacement of skull radiographs, rapid identification of a skull fracture prompting expedited management, and ruling out a skull fracture, hence potentially eliminating the need for CT. Further study is needed to fully assess the characteristics of this application and its integration with validated age-specific prediction rules.
CLINICAL INDICATIONS
An emergency ultrasound is indicated in stable pediatric patients with a scalp hematoma in whom the identification of a skull fracture will prompt further imaging or change clinical management.
ANATOMICAL CONSIDERATIONS
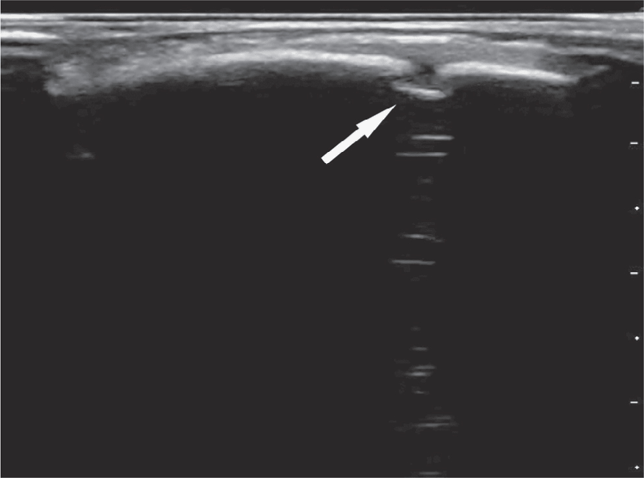
The cortex of the skull appears as a hyperechoic curvilinear line under the normal scalp soft tissue appearance (Figure 20-26). When overlying soft tissue edema is present, it will have a heterogeneous sonographic appearance that disrupts the normal fascial architecture.
GETTING STARTED
The presence of a skull fracture can be accurately identified with the patient in any position. For this reason, place the patient in a position of comfort, such as a caregiver’s lap, in order to decrease anxiety and facilitate image acquisition. Rarely the head may need to be gently restrained. Use copious amounts of gel to minimize painful contact with the hematoma and to maximize image quality. Warm gel may also be beneficial in keeping the patient comfortable and calm throughout the exam.
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
The ideal transducer for this application is a high-frequency linear array transducer. The transducer is placed over the area of swelling and scanned in two orthogonal planes (figure 20-27) The cortex should appear continuous with the exception of the cranial sutures and fontanelles.
Figure 20-27. The patient is placed in a position of comfort while the transducer is placed over the area of swelling and scanned in two orthogonal planes. A large amount of gel (not shown) may be needed to overcome air artifact associated with thick hair.
COMMON ABNORMALITIES
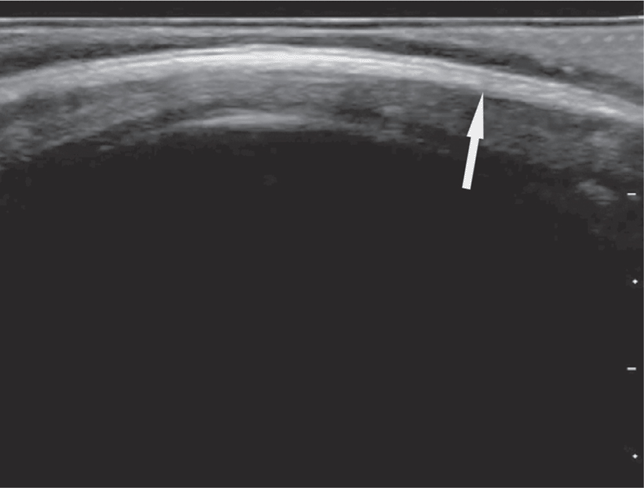
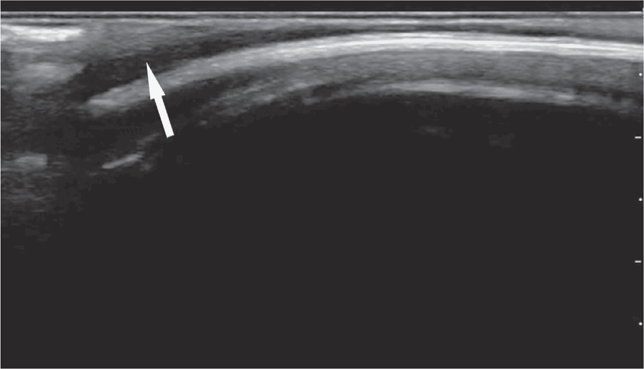
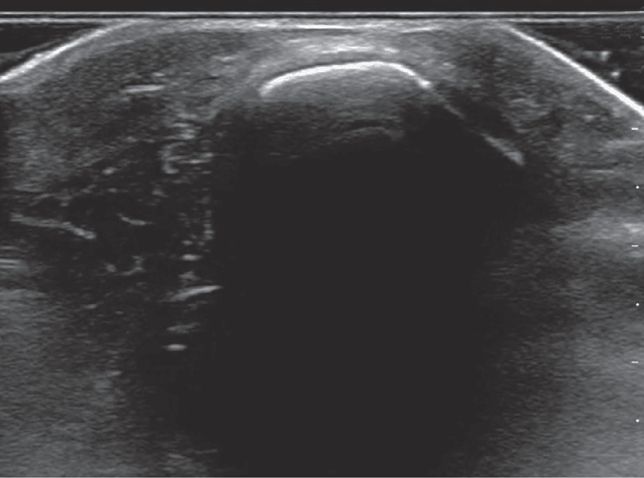
A scalp hematoma will be identified as a distinct, homogenous hypoechoic area adjacent to the cortex (Figure 20-28). A skull fracture appears as a hypoechoic break in the contour of the hyperechoic skull (Figure 20-29). The fractured bone may appear inline or depressed depending on the character of the fracture. Comparison with the opposite side of the skull can help to distinguish cranial sutures from linear fractures.
Figure 20-28. Overlying soft tissue may be edematous or demonstrate distinct hematoma formation (arrow) adjacent to the cortex.
Figure 20-29. A skull fracture appears as a hypoechoic break in the contour of the hyperechoic skull.
PITFALLS
1. The sensitivity and specificity of ultrasound to detect skull fractures are currently unknown. Although this application has been demonstrated to be effective in case series, its limitations have yet to be fully documented. Care should be taken when using this application for clinical decision making.
2. Cranial sutures may be misidentified as fracture. Understanding the position and normal appearance of the cranial sutures is needed to correctly distinguish pathology. Normal cranial sutures may have an end-to-end, beveled, or overlapping appearance.51 (Figure 20-30, Figure 20-31) A fracture or injury along a suture line must also be considered with a hematoma overlying a cranial suture and interpreted within the context of the clinical scenario.
CASE STUDY
Patient Presentation
An 8-month-old baby boy presented to the ED after a fall from his highchair onto hardwood floor 1 hour prior to admission. The mother saw the boy fall and strike the right side of his head on impact. She denied loss of consciousness, vomiting, or abnormal behavior since the fall. His blood pressure was 90/60 mm Hg, heart rate 90 beats perminute, respiratory rate 26 perminute, and an oxygen saturation of 100% on room air. The patient was alert and appropriate, with normal tympanic membranes and no Battle’s sign. He had a hematoma in the right temporoparietal area measuring 3 × 3 cm. Palpation of the underlying skull was limited due to pain and significant swelling at the site. The exam was otherwise normal.
Management Course
An emergency ultrasound confirmed the absence of a skull fracture under the area of swelling. The patient was appropriately observed in the ED and then discharged home with return precautions.
Commentary
This case demonstrates the advantage of using emergency ultrasound to assess for skull fracture in closed head trauma. Unnecessary imaging and radiation exposure can be avoided.
 INTRAOSSEOUS NEEDLE CONFIRMATION
INTRAOSSEOUS NEEDLE CONFIRMATION
Intraosseous access is widely used in the emergent resuscitation of pediatric patients. This life-saving technique allows for rapid fluid resuscitation and the administration of medications when peripheral IV access cannot be achieved.52 The traditional role of the intraosseous needle (ION) has been further expanded by automated technologies that facilitate its insertion. In addition, the ION is increasingly used in the prehospital setting53 as well as in the resuscitation of neonates.54
CLINICAL CONSIDERATIONS
A misplaced ION can create confusion and delay the delivery of essential fluids and medications during resuscitation. Although serious complications are rare,55, they include extravasation of fluid, compartment syndrome, and muscle necrosis.56 Conventional methods used to confirm needle position may be unreliable. These methods include observing a bone marrow aspirate, the presence of blood on the tip of the needle stylet, the ION standing firmly upright, and the ability to infuse fluid easily without visible extravasation or swelling. Based on current experience, ION confirmation by ultrasound is feasible and can be rapidly performed during resuscitation.57 Ultrasound confirmation of ION placement ensures reliable delivery of the therapeutic agents while minimizing the risk of complications from a misplaced needle.
CLINICAL INDICATIONS
All patients with an ION placed in the ED or in the prehospital setting should undergo ultrasound confirmation to ensure correct needle position. Patients transferring from one ED to another are at risk of having an ION dislodged during transport. They should have needle position determined on arrival and while en route if possible. Patients with ongoing resuscitative efforts, which rely on an appropriately positioned ION, should have needle position confirmed on a serial basis to ensure continued efficacy.
ANATOMICAL CONSIDERATIONS
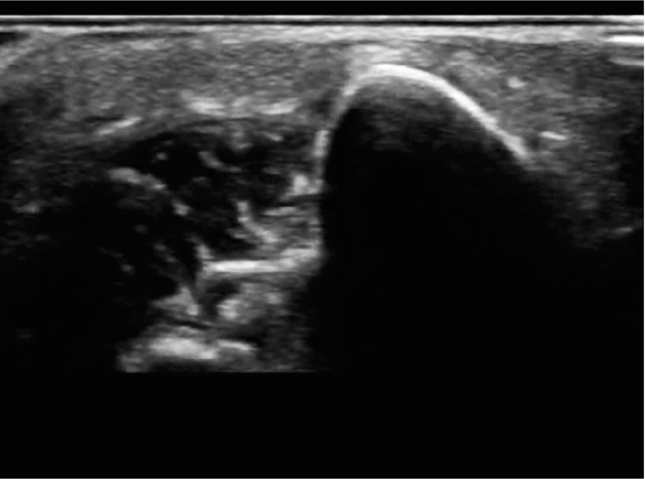
The cortex of the target long bone should appear as a hyperechoic linear structure present below the heterogeneous soft tissue (Figure 20-32). The hyperechoic character of the cortex increases as the bone matures. For this reason, an older patient’s cortex appears more distinct than a younger patient’s cortex (Figure 20-33).
Figure 20-32. Transverse view of the infant long bone. The cortex of the target long bone in an infant should appear as a hyperechoic linear structure present below the heterogeneous soft tissue.
Figure 20-33. Transverse view of adolescent long bone. The hyperechoic character of the cortex increases as the bone matures as demonstrated in this adolescent.
GETTING STARTED
The majority of ION confirmation occurs during resuscitation. Therefore, preparation is key as it minimizes disrupting the tasks of other team members. The ultrasound system should have preestablished settings, gel on the transducer, and a location at the bedside that is predetermined. A system preset for ION confirmation further improves efficiency by limiting the time needed to acquire adequate images.
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
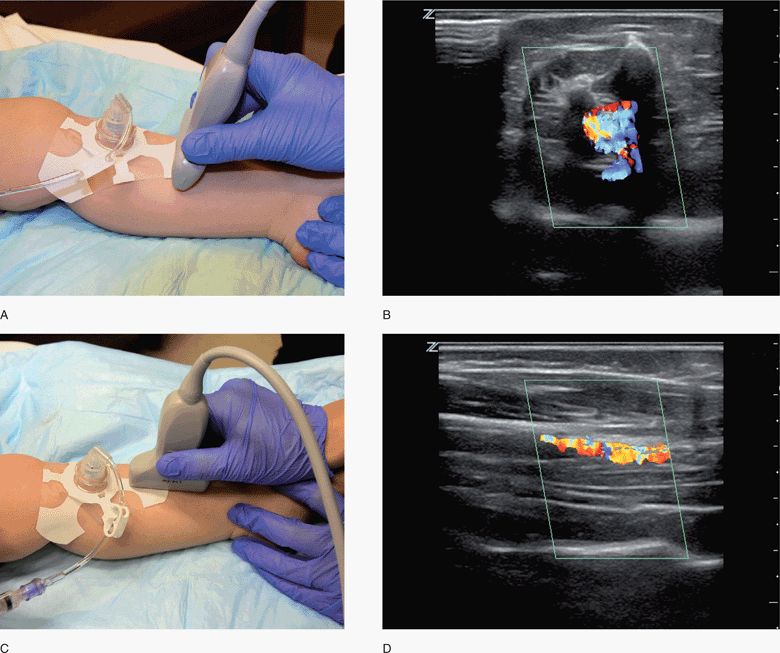
The ideal transducer for this application is a high-frequency linear array transducer, although needle confirmation may be determined with a variety of transducer types and frequencies. The transducer is placed proximal or distal to the ION in the transverse plane and the accessed long bone is then visualized in a short-axis view (Figure 20-34,C).58 The transducer may also be positioned in a sagittal or longitudinal plane, which enables visualization in the long-axis.
Figure 20-34. ION confirmation. (A) The transducer is placed proximal or distal to the ION in the transverse plane for visualization in a short-axis view. (B) Marrow flow is demonstrated in the transverse plane. (C) The transducer is placed in a sagittal or longitudinal plane for visualization in the long-axis view. (D) Marrow flow is demonstrated in the longitudinal plane. ION = intraosseous needle.
Color or power Doppler may be used to identify flow below the cortex of the target site and within the bone marrow (Figure 20-34B,D). An initial 5–10 mL flush given following ION insertion can be recognized using color Doppler. Serial examinations to monitor ongoing fluid or medication infusions may require the low flow velocity sensitivity of power Doppler.
COMMON ABNORMALITIES
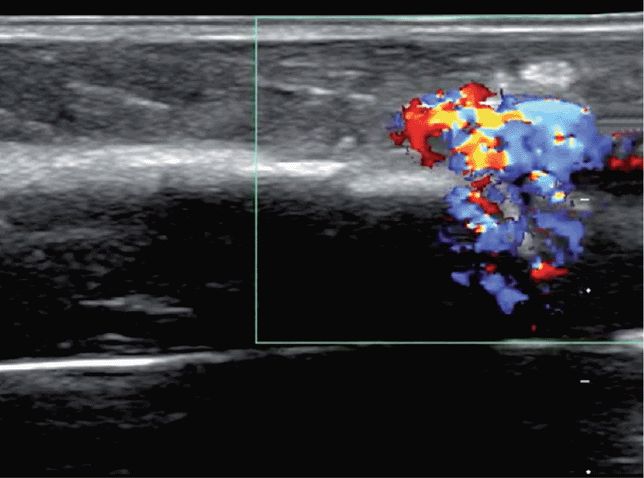
The identification of flow in the soft tissue and not in the marrow suggests a misplaced ION. Extraosseous flow in the soft tissue may appear between the cortex and the transducer or outside the lateral margins of the target long bone (Figure 20-35, Figure 20-36).
COMMON VARIANTS AND OTHER ABNORMALITIES
There is a theoretical risk of bony fracture or a partially placed ION. Although not reported in the literature, it is possible that flow could be present both within the marrow and in the soft tissue in both these scenarios. If recognized, an alternative site of access would be preferred to ensure proper fluid and medication delivery.
PITFALLS
1. Care should be taken while scanning the ION to reduce the risk of dislodging the needle during the ultrasound scan. The ION should be appropriately secured and efforts made not to bump the transducer against the needle.
2. A higher probability of error may exist when scanning with the transducer in sagittal or longitudinal view. Long-axis visualization of the cortex can lead to erroneous interpretation of flow due to inadvertent visualization off axis or visualization of the fibula.57
3. Low velocity flow should be considered if color Doppler fails to identify any flow during the initial manual push or subsequent infusion. A color Doppler scale adjustment or the use of power Doppler may be necessary to correctly assess needle position in this setting.59
4. A single determination of needle placement during resuscitation may not be adequate to ensure ongoing efficacy of the ION. The potential for needle dislodgement as a result of inadvertent movement may occur at anytime during the resuscitation. Serial examinations may be required.
CASE STUDY
Patient Presentation
A 6-month-old female child with a history of developmental delay, seizure disorder, and a gastric tube presented to the ED with 48 hours of severe vomiting and diarrhea. She had no urine output for 6 hours and had become increasingly lethargic despite ongoing hydration through the gastric tube.
Her initial blood pressure was 68/40 mm Hg, heart rate 200 beats perminute, respiratory rate 60 perminute, temperature 37.0 C, and an oxygen saturation of 96% on room air. The patient was minimally responsive to voice, mucous membranes were dry, and her eyes were sunken. Capillary refill was 3 seconds. There was severe diaper dermatitis on the buttocks. Large volume diarrhea was ongoing during the examination without blood or mucous.
Management Course
Two attempts to place peripheral IV access failed and an ION was immediately placed in the right tibia. No marrow or blood was aspirated following insertion. The ION was upright and appeared to be infusing without visible soft tissue swelling. The ION was secured and emergency ultrasound immediately confirmed correct needle placement by identifying flow within the marrow using color Doppler. Aggressive IV rehydration was immediately started and vital signs normalized.
Commentary
This case demonstrates the importance of fast and accurate ION placement in resuscitation. Emergency ultrasound confirms needle placement while conventional methods of confirmation prove unreliable. Hypovolemic shock can then be treated aggressively.
 APPENDICITIS
APPENDICITIS
Acute appendicitis is the most common surgical emergency in children. The diagnosis is made in 60,000–80,000 children each year in the United States and accounts for 0.6% of pediatric ED visits.60–64 While the appendix has appeared in anatomic texts for hundreds of years, the linkage with an acute inflammatory process was first noted in 1711 by Heister, followed by the intervention of an appendectomy by Melier in 1827.65 The incidence of appendicitis is highest in the adolescent years, which correlates closely with the peak amount of lymphoid tissue present within the GI tract.66 Appendicitis is thought to result from luminal obstruction by lymphoid hyperplasia, fecal impaction, or appendiceal calculi. The lumen subsequently distends, leading to venous congestion, wall ischemia, and bacterial translocation. Uncorrected this may lead to perforation, abscess formation, or peritonitis in 36–48 hours67; however, there are reports of spontaneously resolving appendicitis.68–70
Delays in diagnosis have been associated with higher rates of perforation and an increase in the rate of overall morbidity from 6% to 36%.71 Studies of preschool-aged children report a perforation rate of 30–60% at laparotomy,72–74 with 100% perforated when aged 3 and under.75 If the diagnosis is delayed, the perforation rate increases to 65% or higher, and mortality increases as well.76 Failure to diagnose appendicitis is one of the most frequent malpractice claims against emergency physicians.77–79 In pediatric patients, the diagnosis is often elusive for even the most astute clinician, with misdiagnosis rates up to 100% in infants, 57% for toddlers, 28% for school aged children, and 15% for adolescents.80
CLINICAL CONSIDERATIONS
Acute appendicitis should be considered in any patient with right lower quadrant abdominal pain, nausea, vomiting, anorexia, and fever. Together, these signs and symptoms have been found to be highly sensitive for appendicitis.81 However, children are often unable to adequately express themselves and the physical examination may be nondiagnostic. Children with equivocal findings represent 25–30% of all cases of acute appendicitis.82,83 Although frequently ordered, the WBC count84 and plain abdominal radiograph85,86 are neither sensitive nor specific for appendicitis and have a low positive likelihood ratio.87 Because of these limitations, negative laparotomy rates as high as 20% have been reported, with rates of 10–15% accepted historically.88–90 Negative laparotomies come at significant costs, both financially and in terms of morbidity. Morbidity includes adhesions, prolonged or return hospitalization, and time away from school.
Clinical scoring models have been proposed to identify patients who may appropriately receive surgical intervention without diagnostic imaging. Two studies proposed scoring models based on common clinical and laboratory features, with respective sensitivities of 75% and 100% and specificities of 84% and 92%.91,92 However, attempts to validate these models in other settings have been unsuccessful.93–97 A low-risk clinical screening model showed that patients with a low absolute neutrophil count, no nausea, and no right lower quadrant tenderness have a very low risk of appendicitis, but this model does not apply to most patients.98 As would be expected, patients who score at the extremes of these models pose little diagnostic dilemma. These studies, however, highlight that there are many patients with equivocal signs and symptoms who need diagnostic imaging.
Currently, ultrasound and CT are commonly used to help diagnose acute appendicitis in patients with equivocal presentations. The first demonstration of an inflamed appendix by ultrasound was reported in 1981.99 Development of the graded compression examination in 1986 established a technique and criteria for the diagnosis of appendicitis.89
The right lower quadrant examination of appendicitis is one of the more technically difficult ultrasound examinations. Studies of ultrasound use in children for detecting appendicitis have sensitivities ranging from 44% to 98% and specificities from 88% to 100%.62,89,100–110,238 The wide range of sensitivity reflects the operator-dependent nature of ultrasound, particularly for this examination. This is again demonstrated by the wide range of normal appendix visualization rates, from 2% to 99%.62,125
Nonradiologists have also looked at employing right lower quadrant sonography in their evaluation of acute appendicitis. Emergency physicians in Taiwan compared their evaluation of patients for appendicitis using ultrasound to the surgeons’ clinical impressions without sonographic examinations and found sensitivities of 94.6% and 86.2%, respectively, for the diagnosis of appendicitis.111 Another study found a sensitivity of 65% and specificity of 90% utilizing primarily emergency medicine residents in the evaluation of appendicitis.112 Swiss surgeons achieved a sensitivity of 91% with ultrasound for detecting appendicitis.113 An investigation used “specially trained pediatricians” in addition to a pediatric radiologist and achieved 90% sensitivity.101
Studies of the use of helical CT for the diagnosis of appendicitis in children have produced sensitivities of 84–97% and specificities of 89–98%.62,102,109,110,240 Although these ranges of sensitivity and specificity overlap with those of ultrasonography, the reliability of sonography is less consistent. When CT and ultrasonography are compared directly, CT has been superior in each study,62,83,102,109,110,40 except for one study in which patients undergoing noncontrast CT were compared to a historic cohort who underwent graded compression sonography.110 In a meta-analysis comparing ultrasound to CT in children, there was no statistically significant difference found in the specificity, but with a significant difference in sensitivity of 6% in favor of CT.114
While CT has overall greater accuracy than ultrasound, there has been growing concern over the increased use of diagnostic imaging tests with ionizing radiation exposure.115–119 This is particularly troubling for pediatric patients, given the greater risks of children exposed to ionizing radiation.7,120–123 While rates of CT use have dramatically increased, the reduction in negative appendectomies or missed appendicitis remains unclear.115–118,124–126 To address this concern, several authors have analyzed maximizing the specificity of the ultrasound examination in proposing a staged algorithm for diagnostic imaging for acute appendicitis. One protocol utilizing ultrasound prior to CT reduced negative appendectomies from 14% to 4% and had an accuracy of 94%.62,127 Another study found that the implementation of a staged protocol reduced CT usage by 50% without an increase in morbidity.128 It is because of this safety and accuracy that the American College of Radiology has reaffirmed that the initial imaging modality for the evaluation of acute appendicitis in patients under 14 years remain graded compression ultrasonography, with CT reserved for equivocal or negative studies.129
MRI has also been utilized in selected patient populations, such as pregnancy, and found to be a potentially useful adjunct.130,131 When used in a general population sensitivities and specificities have been reported as high as 95–100%.132,133 The limitations of utilizing MRI in children would include cost, equipment, technician availability, and patient cooperation. Modern MRI protocols utilizing ultrafast spin echo sequences require 20 seconds of breath holding by the patient to minimize motion artifact.132 MRI could eventually replace CT for patients with equivocal or negative ultrasound studies, but further research is needed, since trials to date have been small.134,135
CLINICAL INDICATIONS
An ultrasound examination is indicated when there is clinical suspicion for appendicitis. It should be considered in a child with all or part of a constellation of symptoms that includes migratory right lower quadrant pain, vomiting, nausea, anorexia, pain with cough or hop, rebound tenderness, abdominal distention, or peritoneal signs.
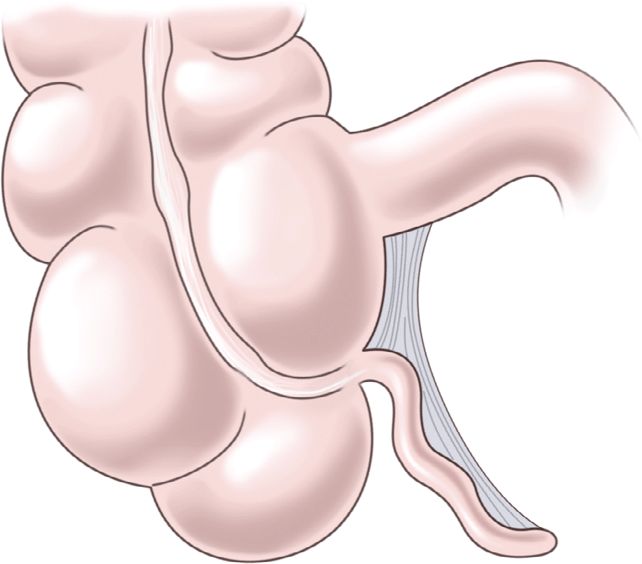
ANATOMICAL CONSIDERATIONS
The vermiform appendix is a hollow lymphoid organ whose function is not well understood. The blind-ended appendix typically arises from the cecum, 1–2 cm distal to the ileum in the right lower quadrant (Figure 20-37). It is rarely congenitally absent.136 The classic teaching is that the maximum pain from an inflamed appendix localizes to McBurney’s point, at the midpoint of an imaginary line between the umbilicus, and the anterior–superior iliac crest. However, a 1933 study reported that the classic pelvic orientation of the appendix occurred in only 31% of 10,000 autopsies and retrocecal in 65%.137 Also, in a retrospective review on appendiceal positioning using ultrasound visualization, only 39% of patients with appendicitis had the appendix found in the classic mid-pelvic region, with 26% retrocecal.238
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 TRAUMA
TRAUMA SKULL FRACTURE
SKULL FRACTURE INTRAOSSEOUS NEEDLE CONFIRMATION
INTRAOSSEOUS NEEDLE CONFIRMATION APPENDICITIS
APPENDICITIS HYPERTROPHIC PYLORIC STENOSIS
HYPERTROPHIC PYLORIC STENOSIS INTUSSUSCEPTION
INTUSSUSCEPTION HYDRATION STATUS
HYDRATION STATUS URINE COLLECTION
URINE COLLECTION