Cerebellar tumors are the most common group of solid tumors in children. MR imaging provides an important role in characterization of these lesions, surgical planning, and postsurgical surveillance. Preoperative imaging can help predict the histologic subtype of tumors, which can provide guidance for surgical planning. Beyond histology, pediatric brain tumors are undergoing new classification schemes based on genetic features. Intraoperative MR imaging has emerged as an important tool in the surgical management of pediatric brain tumors. Effective understanding of the imaging features of pediatric cerebellar tumors can benefit communication with neurosurgeons and neuro-oncologists and can improve patient management.
Key points
- •
Cerebellar tumors are the most common solid neoplasms in children, with the 3 most common entities including pilocytic astrocytoma, medulloblastoma, and ependymoma.
- •
Diffusion-weighted/tensor imaging plays a key role in preoperative characterization of pediatric cerebellar tumors, with lower apparent diffusion coefficient values correlating with higher-grade tumors.
- •
Genetic characterization is resulting in new understanding of medulloblastoma.
- •
The previous 4 histologic categories are in the process of being supplanted with 4 genetic groupings, in particular based on analysis of the WNT and Sonic Hedgehog pathway genes.
- •
This genetic characterization has allowed therapeutic options targeted to the specific tumor and improved prediction of tumor aggressiveness compared with histologic categorization.
Introduction
Cerebellar tumors are among the most common central nervous system (CNS) neoplasms, not to mention solid neoplasms, in children. These tumors include benign and malignant entities, tumors that have slow local spread, and others with leptomeningeal dissemination. Although there are a small number of tumor types that account for most of these tumors, recent work on the genetic origins of these lesions has created an understanding of a much broader landscape of tumors. The newer understanding of genetic origins has the potential for targeted therapy, and imaging features that may help determine the tumor type (or even subtype) take on an increasingly critical role.
Introduction
Cerebellar tumors are among the most common central nervous system (CNS) neoplasms, not to mention solid neoplasms, in children. These tumors include benign and malignant entities, tumors that have slow local spread, and others with leptomeningeal dissemination. Although there are a small number of tumor types that account for most of these tumors, recent work on the genetic origins of these lesions has created an understanding of a much broader landscape of tumors. The newer understanding of genetic origins has the potential for targeted therapy, and imaging features that may help determine the tumor type (or even subtype) take on an increasingly critical role.
Tumor types
Pediatric cerebellar tumors most commonly involve four entities. Pilocytic astrocytomas (PAs), ependymomas, and medulloblastomas are the key players, with atypical teratoid rhabdoid tumor (ATRT) representing a high-grade embryonal tumor most common in the first year or two of life. Initial clinical presentation for all of these lesions is typically due to mass effect, including headaches, nausea and emesis, cranial neuropathies, and obstructive hydrocephalus.
Pilocytic astrocytoma
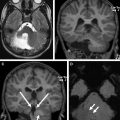
PAs, sometimes referred to as juvenile PAs, are benign neoplasms, classified as World Health Organization (WHO) grade I tumors. They account for more than two-thirds of all cerebellar astrocytomas and can be seen from birth to about 15 years of age. Other more aggressive astrocytomas, such as anaplastic astrocytomas and glioblastomas, are rare and usually seen in older children. There is an equal incidence of PA in boys and girls. PA of the cerebellum can arise from nearly any location in the cerebellum, including the hemispheres and vermis. PA of the cerebellum most classically has an imaging appearance of a cystic lesion with a mural nodule ( Fig. 1 ). The solid portions of the tumor have a large volume of interstitial space with a high water content, which results in a somewhat hyperintense appearance on T2-weighted (T2W) imaging, and results in facilitated diffusion. The solid portion of PAs typically has diffusion characteristics of greater than 1300 × 10 −6 mm 2 /s (or 1.3 × 10 −3 mm 2 /s). The solid portions will usually enhance after contrast administration because of leaky capillaries. Accordingly, there is delayed accumulation of gadolinium within the interstitial space, and the lesions will enhance more on delayed imaging. The contrast enhancement is not due to increased vascularity, as would be the case for a hemangioblastoma.
The cyst walls of a PA may or may not enhance. If the enhancement is thin and smooth, this is likely due to leaky capillaries as a result of the pressure from the cyst and not due to neoplasm. If the enhancement is irregular and nodular, then neoplasm is more likely. Inspection of the inside of the cyst under microscopic magnification and illumination will usually allow the surgeon to distinguish whether the wall of the cyst is neoplastic or not. Many PAs are amenable to gross-total resection (GTR) with appropriate surgical planning, but these can be challenging lesions. These tumors can be difficult to distinguish from normal adjacent cerebral tissue, and there can be a transition zone of tumor infiltration. When GTR is achieved, no adjuvant chemotherapy or radiation is typically indicated and patients will undergo imaging surveillance. A recurrence along the margins of the resection cavity may undergo continued surveillance, repeat surgical resection, or (if not amenable to resection) focal irradiation. If surgery is performed and there is a small focus of residual tumor, the neuro-oncologic team may still advocate for observation over radiation, especially if patients are young. Chemotherapy is typically reserved for young children with unresectable residual or recurrent disease, although it is less effective at achieving local disease control than radiation.
PAs tend to have local growth, both of the initial lesion and recurrences. Distant cerebral spinal fluid (CSF) dissemination of the disease, either intracranially or to the spine, is much less common than the other lesions discussed, seen in as little as 2% of these lesions. The 25-year survival rate of cerebellar PA is greater than 90%, and the 25-year survival rate of solid cerebellar astrocytomas is approximately 40% but may be improved with more recent therapy.
Recent work has shown that mutations in the BRAF gene are present in some PAs. The BRAF proto-oncogene makes a protein called B-Raf that is involved in the mitogen-activated protein kinase (MAPK)/Extracellular signal regulated kinases signaling pathway, which affects cell division and differentiation. BRAF alterations may be a point mutation (V600E) or various fusions (KIAA 1549); the pattern of such genetic alterations may be tumor location specific. MAPK (eg, tranetinib) and BRAF inhibitors (eg, dabrafenib and vemurafinib in patients who are V600E positive) are under investigation as a treatment option for PA. Interestingly, the initial studies with these inhibitors were done in patients with melanoma.
Medulloblastoma
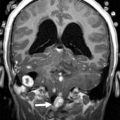
Medulloblastoma is a malignant tumor classified as WHO grade IV, which most commonly arises from the superior medullary velum in young children and cerebellar hemispheres in adolescents. Medulloblastomas are the most common posterior fossa tumor and the most common brain tumor overall in the 6- to 11-year age group. The median age at diagnosis is 6 years, and boys are affected more than girls. This highly cellular tumor is a small, round, blue cell tumor with 4 histologic subtypes, as defined by the 2007 WHO criteria: classic medulloblastoma, large cell, anaplastic, and nodular desmoplastic ( Fig. 2 ). Nodular desmoplastic medulloblastomas tend to occur in older patients, adolescents, and young adults and occur in the cerebellar hemispheres ( Fig. 3 ). Based on histologic criteria, medulloblastoma has been considered to be a subtype of a primitive neuroectodermal tumor (PNET) and is sometimes referred to as medulloblastoma-PNET to reflect this relationship.
Beyond histologic characteristics, molecular analysis has given rise to 4 subtypes. The Sonic Hedgehog pathway (SHH) and WNT pathway, in particular, correlate with the site of origin of these tumors, and importantly with treatment response and outcome. WNT medulloblastomas are thought to arise from the lower rhombic lip of the brainstem, whereas SHH tumors arise from the external granular layer and are found within the cerebellar hemispheres (overlapping with the histologic category of nodular desmoplastic). Given the different genetic origins and biological behavior, different therapeutic options exist for these tumors. Accordingly, active investigations are underway to determine clinical and imaging biomarkers to help stratify these tumors.
Because of the highly cellular nature of these tumors, there are low apparent diffusion coefficient (ADC) values, typically lower than 800 × 10 −6 mm 2 /s. ADC has not yet been shown to correspond to the genetic subtype of medulloblastoma or treatment responsiveness. Medulloblastomas usually demonstrate postcontrast enhancement, and approximately half of lesions show signs of calcification. The high nuclear to cytoplasmic ratio results in a high-density appearance on computed tomography (CT), even in the absence of calcifications, and a relative hypointense appearance on T2W imaging. Unlike ependymomas, medulloblastomas tend to displace rather than conform to margins and are described as being more spherical. This feature also explains why patients with medulloblastoma have a relatively short clinical history and come to medical attention more quickly than their ependymoma counterparts. Spinal metastatic disease is more common with medulloblastoma than ependymoma.
Like ependymoma, optimal management is multimodal, including maximal cytoreductive surgery in nonmetastatic patients, followed by chemotherapy and depending, on the extent of disease and age of patients, radiation.
Ependymoma
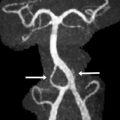
Ependymomas are of 2 types: well differentiated (WHO grade II) and anaplastic (WHO grade III). Anatomically, posterior fossa ependymomas can be thought of as either midline or lateral in origin ( Fig. 4 ). They have a bimodal age distribution, the first between 1 and 5 years of age and the second much later during the fourth decade of life. They account for up to one-fifth of the posterior fossa tumors in children with a slight increase incidence in boys.
Ependymomas are histologically defined by perivascular and ependymal pseudorosettes, among other features. Recent work suggests that the exact cell of origin of ependymoma and genetic origin may vary in lesions of different locations. The tumors that are predominantly midline, typically filling the fourth ventricle, represent the most commonly described location for posterior fossa ependymomas. Lateral ependymomas arise from the inferior margin of the middle cerebellar peduncle and can project into the cerebellopontine and medullary angles as well as through the foramen of Luschka into the fourth ventricle. Lateral ependymomas have a higher incidence of recurrence owing to the increased difficulty of achieving a gross total resection.
Ependymomas have a tendency to spread along subarachnoid planes, such as the foramina of Luschka and Magendie around the brainstem and into the cervical spine, encasing vessels and cranial nerves thereby earning the title of “plastic ependymoma.” This pattern of growth explains why ependymoma patients typically have a long clinical history of vague symptoms before coming to clinical attention.
Ependymomas have intermediate diffusion characteristics in the range of approximately 1000 to 1300 × 10 −6 mm 2 /sec, with ADC values lower than that of PAs but higher than that of medulloblastomas. Ependymomas may not enhance, complicating evaluation of metastatic deposits. Small internal cystic components are common, and approximately half of all lesions show signs of calcification.
Outcomes from ependymoma are best when a gross total resection is achieved. Therefore, characterization of metastatic deposits and tumor extent is critical. Ependymomas are not typically responsive to chemotherapy; radiation is critical in achieving long-term local disease control.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree