There is increasing evidence that the cerebellum is susceptible to prenatal infections and hemorrhages and that congenital morphologic anomalies of the cerebellum may be caused by disruptive (acquired) causes. Starting from the neuroimaging pattern, this report describes a spectrum of prenatal cerebellar disruptions including cerebellar agenesis, unilateral cerebellar hypoplasia, cerebellar cleft, global cerebellar hypoplasia, and vanishing cerebellum in Chiari type II malformation. The neuroimaging findings, possible causative disruptive events, and clinical features of each disruption are discussed. Recognition of cerebellar disruptions and their differentiation from cerebellar malformations is important in terms of diagnosis, prognosis, and genetic counselling.
Key points
- •
The complex and protracted duration of development (from 4 weeks’ gestation to 2 years postnatally) results in a high vulnerability of the cerebellum for acquired injury.
- •
In the last decades, progressing pre- and postnatal anatomic and functional neuroimaging techniques (ultrasound scan and MR imaging) have led to an improved recognition, classification, and understanding of the spectrum of cerebellar disruptions, which include various forms of cerebellar agenesis, unilateral cerebellar hypoplasia, cerebellar cleft, global cerebellar hypoplasia, and vanishing cerebellum associated with Chiari type II malformation.
- •
Similar disruptive processes may cause a spectrum of cerebellar disruptions as revealed by neuroimaging.
- •
Timing and chronicity of injury in relation to the stage of cerebellar development impacts the phenotype of cerebellar disruption.
- •
The recognition of cerebellar disruptions and differentiation from inborn errors of cerebellar development (so-called malformations) is important in terms of diagnosis, prognosis, treatment, and genetic/parental counselling.
Introduction
The embryogenesis/histogenesis of the cerebellum is a highly complex process that is programmed/determined by a large number of genes and can be summarized in 4 basic steps: (1) characterization of the cerebellar territory in the hindbrain, (2) formation of 2 compartments of cell proliferation giving rise to the Purkinje cells and the granule cells, (3) inward migration of the granule cells, and (4) differentiation of cerebellar neurons. The cerebellum develops over a long period, extending from the early embryonic period at approximately 4 weeks of gestation into the first postnatal years. The high complexity and long duration of development makes the cerebellum vulnerable for a wide range of pathologic conditions/injuries including inborn errors of development (primary malformations) and acquired/secondary disruptions. Both inborn errors of development (primary malformations) and acquired/secondary disruptions result in morphologic changes (malformed) of the cerebellum and brainstem and are usually named malformations independent on the cause and pathomechanism. The term malformation , however, has a more specific significance and implies an alteration of the primary developmental program as pathomechanism of the morphologic anomaly.
Disruptions are defined as nonprogressive, congenital morphologic anomalies caused by the breakdown of a body structure that had a normal developmental potential and was initially normally developing until injured. The timing and nature of the disruptive event/agent may either directly injure or destruct the cerebellum or impair/alter the subsequent sequences of development with resultant perturbation of normal growth and development. There are several possible causes of disruptions, including vascular (eg, hemorrhage and ischemia), infectious, teratogenic, and mechanical. In the prenatal period, the cerebellum is particularly vulnerable to infections and hemorrhages ( Table 1 ). In contrast to true malformations (inborn errors of development), disruptions are acquired lesions with low recurrence risk. The differentiation between malformations and disruptions is important for genetic counseling and for diagnostic and prognostic purposes. A genetic predisposition for disruptive lesions may, however, be present. For example, dominant mutations in COL4A1 lead to changes in the basal membrane of capillaries resulting in microangiopathy. Within the brain, the microangiopathy increases the risk for hemorrhage or ischemia and subsequent porencephaly or unilateral cerebellar hypoplasia on follow-up. Furthermore, homozygous mutations in NED1 are found to cause the fetal brain disruption sequence characterized by severe microcephaly, scalp rugae, and prominent occipital bone.
| Category | Cerebellar Vulnerability |
|---|---|
| Neonatal hypoxic-ischemic injury | + |
| Postnatal infections | + |
| Prematurity (<30 wk gestational age) | + |
| Prenatal infections (particularly CMV) | ++ |
| Prenatal hemorrhages | +++ |
| Toxicity/selected drugs | +++ |
| Metabolic disorders | ++++ |
High-quality anatomic and functional neuroimaging plays a key role in the early and correct diagnosis and differentiation of the many morphologic cerebellar abnormalities that may be seen in the pre-, peri- and postnatal period. In particular, neuroimaging may help differentiate between malformations and disruptions. For example, abnormalities (eg, hypoplasia or dysplasia) involving only 1 cerebellar hemisphere are most likely the sequela of a prenatal disruptive event such as a hemorrhage. High-resolution anatomic MR imaging sequences remain of essential importance for the evaluation/characterization of the normal or abnormal pre- and postnatal posterior fossa contents, which include the cerebellum and brain stem. Advanced, functional neuroimaging techniques such as diffusion tensor imaging (DTI) and susceptibility weighted imaging (SWI) may render additional crucial information to better elucidate certain aspects of the pathogenesis of the encountered cerebellar disruptions. DTI allows exploration of the internal derangement of the fiber architecture. SWI is highly sensitive for blood, blood products, and calcifications and may be of particular help in supporting the notion of a disruptive pathomechanism related to infections or hemorrhages. Additional, less frequently applied techniques include 1 H magnetic resonance spectroscopy, and perfusion weighted imaging. Finally, pre- and postnatal ultrasonography with dedicated posterior fossa imaging should not be forgotten as a valuable, widely available, safe, low-cost bedside alternative imaging technique that can be used in critically sick or unstable children. In skilled hands, advanced ultrasound units can give highly diagnostic imaging data, which, because of the ease of serial data collection, allow us to follow and explore the dynamics of cerebellar injury.
This article discusses the morphologic spectrum of prenatal cerebellar disruptions including various forms of cerebellar agenesis, unilateral cerebellar hypoplasia, cerebellar cleft, forms of global cerebellar hypoplasia, and vanishing cerebellum related to Chiari type II malformation. For each cerebellar disruption, the article focuses on the neuroimaging presentation, but the clinical presentation, the presumptive underlying disruptive pathomechanism, and the long-term neurologic outcome are also discussed.
Introduction
The embryogenesis/histogenesis of the cerebellum is a highly complex process that is programmed/determined by a large number of genes and can be summarized in 4 basic steps: (1) characterization of the cerebellar territory in the hindbrain, (2) formation of 2 compartments of cell proliferation giving rise to the Purkinje cells and the granule cells, (3) inward migration of the granule cells, and (4) differentiation of cerebellar neurons. The cerebellum develops over a long period, extending from the early embryonic period at approximately 4 weeks of gestation into the first postnatal years. The high complexity and long duration of development makes the cerebellum vulnerable for a wide range of pathologic conditions/injuries including inborn errors of development (primary malformations) and acquired/secondary disruptions. Both inborn errors of development (primary malformations) and acquired/secondary disruptions result in morphologic changes (malformed) of the cerebellum and brainstem and are usually named malformations independent on the cause and pathomechanism. The term malformation , however, has a more specific significance and implies an alteration of the primary developmental program as pathomechanism of the morphologic anomaly.
Disruptions are defined as nonprogressive, congenital morphologic anomalies caused by the breakdown of a body structure that had a normal developmental potential and was initially normally developing until injured. The timing and nature of the disruptive event/agent may either directly injure or destruct the cerebellum or impair/alter the subsequent sequences of development with resultant perturbation of normal growth and development. There are several possible causes of disruptions, including vascular (eg, hemorrhage and ischemia), infectious, teratogenic, and mechanical. In the prenatal period, the cerebellum is particularly vulnerable to infections and hemorrhages ( Table 1 ). In contrast to true malformations (inborn errors of development), disruptions are acquired lesions with low recurrence risk. The differentiation between malformations and disruptions is important for genetic counseling and for diagnostic and prognostic purposes. A genetic predisposition for disruptive lesions may, however, be present. For example, dominant mutations in COL4A1 lead to changes in the basal membrane of capillaries resulting in microangiopathy. Within the brain, the microangiopathy increases the risk for hemorrhage or ischemia and subsequent porencephaly or unilateral cerebellar hypoplasia on follow-up. Furthermore, homozygous mutations in NED1 are found to cause the fetal brain disruption sequence characterized by severe microcephaly, scalp rugae, and prominent occipital bone.
| Category | Cerebellar Vulnerability |
|---|---|
| Neonatal hypoxic-ischemic injury | + |
| Postnatal infections | + |
| Prematurity (<30 wk gestational age) | + |
| Prenatal infections (particularly CMV) | ++ |
| Prenatal hemorrhages | +++ |
| Toxicity/selected drugs | +++ |
| Metabolic disorders | ++++ |
High-quality anatomic and functional neuroimaging plays a key role in the early and correct diagnosis and differentiation of the many morphologic cerebellar abnormalities that may be seen in the pre-, peri- and postnatal period. In particular, neuroimaging may help differentiate between malformations and disruptions. For example, abnormalities (eg, hypoplasia or dysplasia) involving only 1 cerebellar hemisphere are most likely the sequela of a prenatal disruptive event such as a hemorrhage. High-resolution anatomic MR imaging sequences remain of essential importance for the evaluation/characterization of the normal or abnormal pre- and postnatal posterior fossa contents, which include the cerebellum and brain stem. Advanced, functional neuroimaging techniques such as diffusion tensor imaging (DTI) and susceptibility weighted imaging (SWI) may render additional crucial information to better elucidate certain aspects of the pathogenesis of the encountered cerebellar disruptions. DTI allows exploration of the internal derangement of the fiber architecture. SWI is highly sensitive for blood, blood products, and calcifications and may be of particular help in supporting the notion of a disruptive pathomechanism related to infections or hemorrhages. Additional, less frequently applied techniques include 1 H magnetic resonance spectroscopy, and perfusion weighted imaging. Finally, pre- and postnatal ultrasonography with dedicated posterior fossa imaging should not be forgotten as a valuable, widely available, safe, low-cost bedside alternative imaging technique that can be used in critically sick or unstable children. In skilled hands, advanced ultrasound units can give highly diagnostic imaging data, which, because of the ease of serial data collection, allow us to follow and explore the dynamics of cerebellar injury.
This article discusses the morphologic spectrum of prenatal cerebellar disruptions including various forms of cerebellar agenesis, unilateral cerebellar hypoplasia, cerebellar cleft, forms of global cerebellar hypoplasia, and vanishing cerebellum related to Chiari type II malformation. For each cerebellar disruption, the article focuses on the neuroimaging presentation, but the clinical presentation, the presumptive underlying disruptive pathomechanism, and the long-term neurologic outcome are also discussed.
Cerebellar agenesis
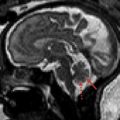
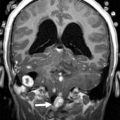
Cerebellar agenesis is a rare finding in pediatric neuroimaging and is defined by the near complete absence of cerebellar tissue ( Fig. 1 ). The definition of cerebellar agenesis is based on the morphologic pattern/neuroimaging findings and does not suggest the pathogenesis. Cerebellar agenesis may be seen as a primary malformation (eg, mutations in PTF1A ) or secondary to a disruption. Cerebellar agenesis caused by disruption may be seen in following scenarios: (1) pre- or perinatal hemorrhage, (2) vascular insufficiency/compromise in Chiari II malformation with cerebellar herniation, and (3) as a sequela of prematurity. The authors recently reported on a child with normal cerebellar anatomy on prenatal ultrasound scan at 18 and 22 weeks of gestation but isolated cerebellar agenesis at 12 months of age when the patient was referred for an MR imaging examination because of developmental delay. Huissoud and colleagues reported on a fetus with normal anatomy at 12 weeks of gestation but complete absence of cerebellar structures on ultrasound scan at 23 weeks of gestation. After termination of pregnancy at 25 weeks of gestation, neuropathology confirmed a complete and isolated cerebellar agenesis. In 2010, Mohila and colleagues reported on a newborn with cerebellar agenesis in the setting of neonatal alloimmune thrombocytopenia, diffuse body ecchymoses, petechial hemorrhages, and arthrogryposis at birth. She died on day 3 of life because of multisystem organ failure and disseminated intravascular coagulopathy. Neuropathology found complete loss of cerebellar tissue, marked reduction in size of the pons and medulla, acute and chronic germinal matrix hemorrhages, and deposition of blood degradation products and reactive gliosis in the posterior fossa. The neuropathology findings suggest a disruptive pathomechanism of cerebellar agenesis in this neonate. Recently, the authors reported the association of cerebellar agenesis and severe periventricular leukomalacia in a 7-year-old girl who was born at 25 weeks of gestation. The serial ultrasound studies performed at 4.5 and 6 weeks of life showed a normal structure of the cerebellum and brainstem, whereas an ultrasound study at 9 weeks of life found an abnormal hypoechogenic cerebellum. A computed tomography (CT) scan at the age of 12 weeks showed a cerebellar agenesis ( Fig. 2 ). In these cases, cerebellar agenesis is secondary to a pre- or perinatal acquired event/complication supporting the possible disruptive etiology of cerebellar agenesis.
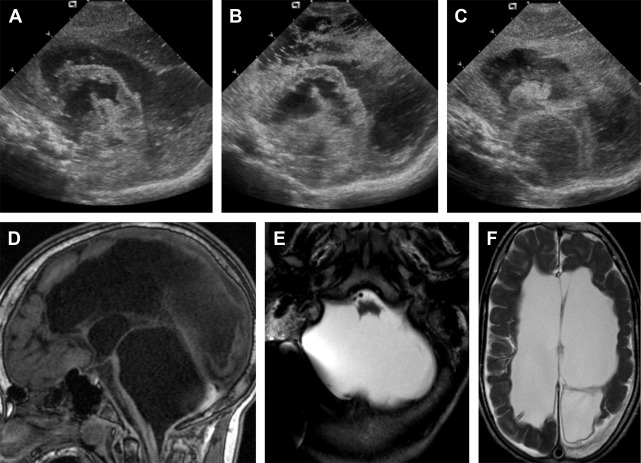
All children with cerebellar agenesis are symptomatic. Patients who survive infancy have variable degrees of cerebellar dysfunction (truncal and limb ataxia, dysarthria, and muscular hypotonia) and cognitive impairment including specific deficits in planning behavior, visuo-spatial abilities, visual memory, attention, speech com prehension, verbal learning, and declarative memory.
In cerebellar agenesis, the absence of cerebellar tissue is usually not complete, and remnants of the anterior vermian lobules (particularly vermian lobule III), flocculus, or middle cerebellar peduncles may be present (see Fig. 1 ). A secondary pontine volume loss/hypoplasia and absence of the normal protuberance of the inferior olives are typically seen. The volume of the posterior fossa is variable, mostly of normal or increased size. Cerebrospinal fluid (CSF) pulsations within an “empty skull” may result in the increased size of the posterior fossa as also seen supratentorially in children with hydranencephaly. However, reduced volume has been also reported. Recently, 2 studies applied DTI and fiber tractography to study 2 patients with cerebellar agenesis. One study applied probabilistic constrained spherical deconvolution tractography in a child with cerebellar agenesis. Findings showed absence of the transverse pontine fibers, which explains the marked pontine hypoplasia and is most likely caused by the absence of cerebellar tissue. In addition, the cerebellothalamic, fronto-cerebellar, and spinocerebellar tracts had aberrant courses. The second article reports on DTI and functional (resting state) MR imaging data in a 48-year-old right-handed man with cerebellar agenesis. Qualitative analysis of DTI data found absence of the transverse pontine fibers, superior cerebellar peduncles and their decussation (most likely secondary to Wallerian degeneration), and the fronto-pontine tracts in the mesial part of the cerebral peduncles. The fronto-pontine tracts are part of the executive control network. Resting-state functional MR imaging confirmed impairment of the executive control network. An empty posterior fossa on ultrasound scan may suggest cerebellar agenesis that needs to be confirmed by MR imaging.
Based on the neuroimaging findings, the diagnosis of cerebellar agenesis is straightforward. The differentiation between a malformative and disruptive nature of cerebellar agenesis may be more challenging but is important for prognosis and genetic counseling of the affected families. Dysmorphic features on the general examination, endocrine disorders (such as diabetes) on metabolic screens, and additional morphologic abnormalities of the central nervous system on neuroimaging may favor a malformative origin. Neonates with cerebellar agenesis should be evaluated for diabetes mellitus, as this association is suggestive of a mutation in PTF1A .
Global cerebellar hypoplasia
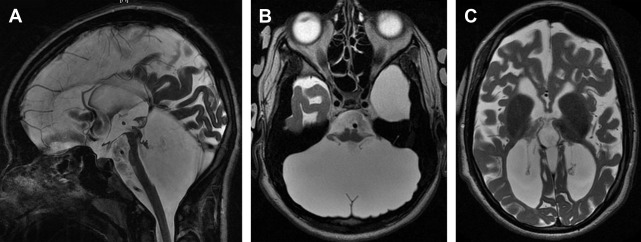
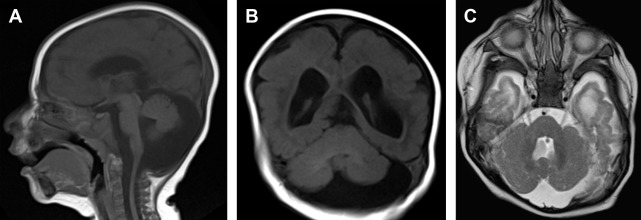
Global cerebellar hypoplasia refers to a cerebellum with globally (vermis and cerebellar hemispheres) reduced volume but a normal shape ( Fig. 3 ). Global cerebellar hypoplasia is associated with a broad group of etiologies including primary (malformative, genetic) lesions, such as chromosomal aberrations, metabolic disorders, genetic syndromes, and some brain malformations, but may also be secondary to disruptive processes.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree