Key differences exist in the epidemiology, pathophysiology, and clinical presentation of vascular lesions of the cerebellum in children versus adults. An understanding of these differences and an appreciation of the distinct imaging features of these lesions aid in distinguishing normal vascular variations from pathology, in predicting lesion etiology, and in directing effective treatment strategies. This paper reviews the embryogenesis of the normal vascular system of the cerebellum and brainstem and then discusses the clinical and imaging features of the common vascular lesions affecting these structures in the pediatric population.
Key points
- •
An understanding of the embryologic development of the intracranial vascular system helps to explain the common and uncommon variations in normal vertebrobasilar vascular anatomy.
- •
There are key differences in the etiology, clinical presentation, and imaging characteristics of vascular lesions in children as compared with adults.
- •
Cross-sectional imaging with computed tomography (CT) and MR imaging aids the clinician in planning proper interventional and operative therapies for the lesions displayed.
- •
Dedicated thin section CT angiography (CTA) or time-of-flight and time-resolved MR angiography (MRA) help to characterize arteriovenous malformations, arteriovenous fistulae, and intracranial arterial aneurysms.
- •
Catheter angiography remains the gold standard for lesion morphology, hemodynamic characterization, and therapeutic decision making.
Introduction
Neurovascular disease that involve the posterior fossa in the pediatric population include cavernous malformations, developmental venous anomalies (DVAs), capillary telangiectasias, intracranial aneurysms, and both arteriovenous (AV) malformations (AVM) and AV fistulae (AVF). Despite these conditions being uncommon, they can be associated with significant morbidity and mortality. In the case of intracranial aneurysms and posterior fossa AVMs/AVFs, their pathogenesis, clinical course, and angioarchitecture often differs significantly from the same condition in the adult population. An understanding of the epidemiology and pathogenesis as well as the clinical and imaging findings is crucial to accurately diagnose these lesions and to assess for, or exclude the presence of, any affiliated disease processes. Additionally, the variation in vascular anatomy provides insight into neurovascular embryogenesis. The study of these variations may also provide guidance for treatment of malformations that may result from dysembryogenesis.
In this article, we provide a synopsis of the early development of the posterior fossa vasculature and its variations and review the clinical and imaging findings associated with neurovascular disorders of the posterior fossa in the pediatric population.
Introduction
Neurovascular disease that involve the posterior fossa in the pediatric population include cavernous malformations, developmental venous anomalies (DVAs), capillary telangiectasias, intracranial aneurysms, and both arteriovenous (AV) malformations (AVM) and AV fistulae (AVF). Despite these conditions being uncommon, they can be associated with significant morbidity and mortality. In the case of intracranial aneurysms and posterior fossa AVMs/AVFs, their pathogenesis, clinical course, and angioarchitecture often differs significantly from the same condition in the adult population. An understanding of the epidemiology and pathogenesis as well as the clinical and imaging findings is crucial to accurately diagnose these lesions and to assess for, or exclude the presence of, any affiliated disease processes. Additionally, the variation in vascular anatomy provides insight into neurovascular embryogenesis. The study of these variations may also provide guidance for treatment of malformations that may result from dysembryogenesis.
In this article, we provide a synopsis of the early development of the posterior fossa vasculature and its variations and review the clinical and imaging findings associated with neurovascular disorders of the posterior fossa in the pediatric population.
Development of the cerebellar and brainstem arterial supply
Much of our understanding of the embryologenesis of the cranial vascular system is based on the work of Dorkas Paget and George Streeter in the first half of the twentieth century. In brief, the intracranial arterial vascular network develops in response to changing metabolic and hemodynamic demands within the developing brain. As the primitive brain enlarges, it no longer receives adequate nourishment by diffusion from the amniotic fluid. Increasing metabolic demands then initiate sequential—and broadly overlapping—changes that are conceptualized as a “4-step model” for the development of the cerebral arterial system :
- 1.
During the first 2 to 4 weeks, the exposed neural groove, neural plate and the open neural tube are fed by diffusion from the amniotic fluid.
- 2.
At week 4, the neural tube is closed and is covered by a dense connective tissue called the meninx primitiva, the precursor of the future meningeal coverings of the brain. The meninx primitiva contains a vascular plexus that supplies the neural tube during weeks 5 to 7 via the primitive dorsal aorta and the cardinal veins.
- 3.
Between weeks 5 and 7, the primary brain vesicles enlarge and differentiate. The meninx primitiva invaginates into the developing neural tube to create the primordial choroid plexus and the associated choroidal vasculature, including the choroidal arteries.
- 4.
With further enlargement, the neural tube becomes too thick to be supplied by diffusion alone. Intrinsic capillaries then develop from the vascular plexus of the meninx primitiva, first at the areas of highest metabolic demand (the ventricular/subventricular proliferative germinal zone), and then everywhere.
In greater detail, Dorkas Paget defined 7 stages in the development of the cerebral arterial system from the early (undifferentiated) stage to the morphologically mature pattern. These were summarized by Dr. Charles Raybaud in 2010.
- 1.
During stage 1 (4–5 mm, embryonic day 28–29), the blood supply to the developing forebrain is provided by the primitive carotid arteries. The developing hindbrain is supplied by 4 transient caroticovertebral connections arising from the developing carotid arteries. These connections comprise 3 presegmental branches (named trigeminal, otic, and hypoglossal for their association with the 5th, 8th, and 12th cranial nerves, respectively) and 1 intersegmental arterial branch (the proatlantal artery). The trigeminal artery and the otic artery are thought to arise from the proximal developing internal carotid artery (although the existence of the otic artery is debated). The hypoglossal artery and the proatlantal artery (first intersegmental cervical C1 artery) arise from the paired dorsal aortae. These 4 primitive caroticovertebral connections supply the hindbrain via the paired ventral longitudinal neural arteries. The ventral longitudinal neural arteries are the precursors to the basilar artery during this stage of development.
- 2.
During stage 2 (5–6 mm, embryonic day 29), the posterior communicating arteries are formed. The primitive internal carotid arteries extend caudally to join the bilateral longitudinal neural arteries to form the posterior communicating arteries. During this stage, both the trigeminal and hypoglossal arteries start to involute at their carotid origins. The longitudinal neural arteries begin to fuse in the midline to form the primitive basilar artery. However, the developing hindbrain still relies heavily on the caudal supply derived from the first intersegmental (proatlantal) arteries.
- 3.
During stage 3 (7–12 mm, embryonic day 32), the basilar and vertebral arteries are completed. A mesencephalic artery now extends toward the midbrain from the caudal end of the developing posterior communicating arteries. The basilar artery is better developed and the paired vertebral arteries are in the process of forming bilateral paired longitudinal anastomoses that link the intersegmental cervical arteries between C1 and C7. The trigeminal arteries have typically disappeared, and the anterior superior cerebellar arteries become evident ( Fig. 1 ).
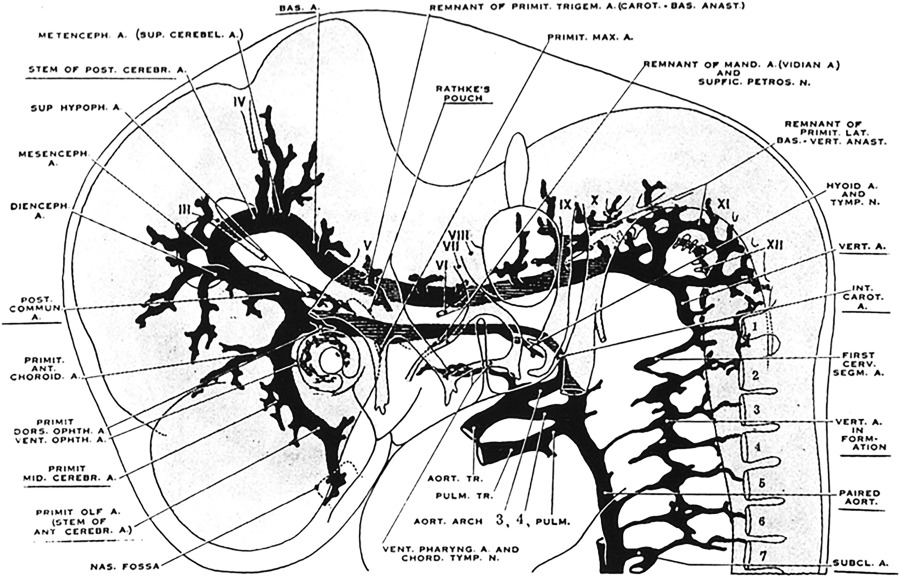
Fig. 1
Cranial arteries of the left side in a 9 mm embryo (lateral view). Note that the vertebral arteries are being formed from elements of the upper cervical segments.
( From Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1948;12(pub no: 575):205–61.)
- 4.
During stage 4 (12–14 mm, embryonic day 35), the morphology of the cerebellar arterial system becomes more mature. The basilar and vertebral arteries develop further, and early branches supplying the rhombencephalon now become recognizable ( Fig. 2 ).
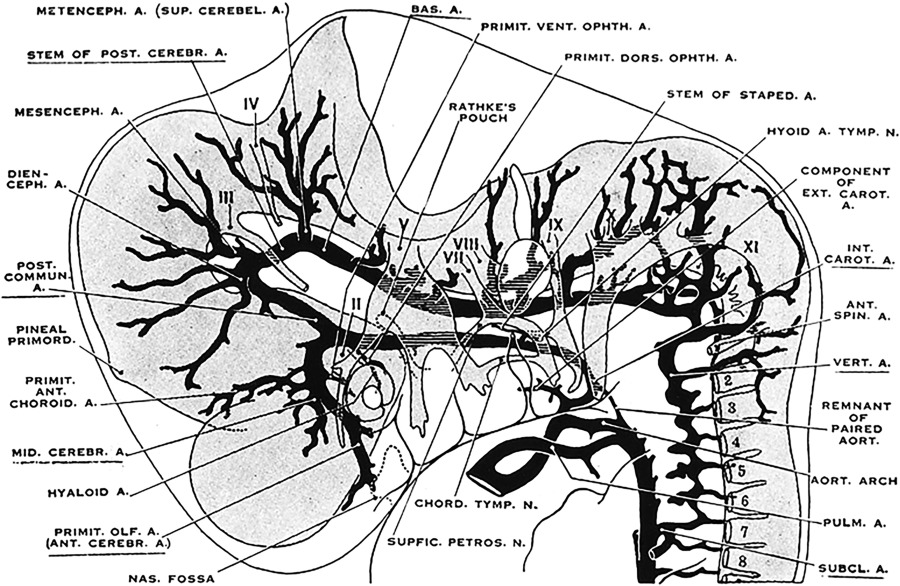
Fig. 2
Cranial arteries in an 12.5-mm embryo. The developing vertebral arteries have a more advanced conformation.
( From Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1948;12(pub no: 575):205–61.)
- 5.
During stage 5 (16–18 mm, embryonic day 40), the arterial system has a nearly adult morphology. During this stage primitive lateral, third and fourth ventricles form by invagination of the meninx primitiva. The mesencephalic artery, which develops from the primitive posterior communicating arteries, forms a rich vascular plexus over the tectal plate ( Fig. 3 ).
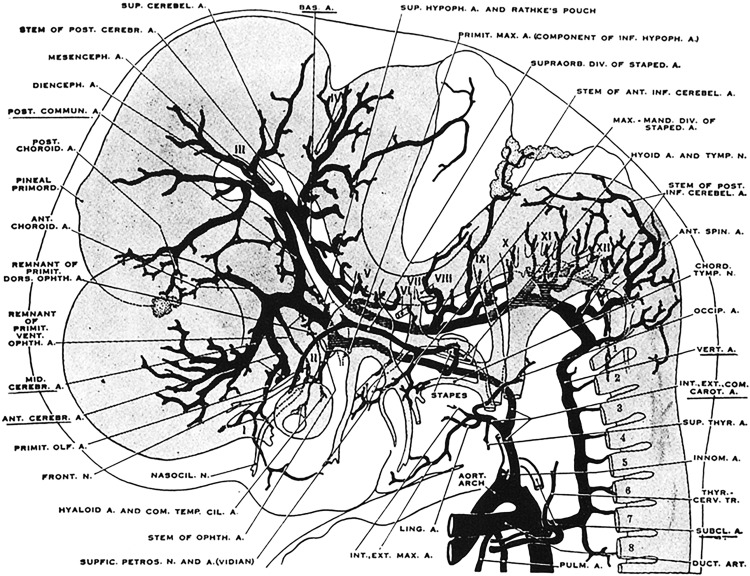
Fig. 3
Cranial arteries in an 18-mm embryo. Most of the intracranial arteries are now recognizable.
( From Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1948;12(pub no: 575):205–61.)
- 6. and 7.
During stages 6 (20–24 mm, embryonic day 44) and 7 (40 mm, embryonic day 52), the circle of Willis is completed and the vertebrobasilar arteries now supply the posterior cerebral hemispheres. The anterior inferior and posterior inferior cerebellar arteries become better developed and play a larger role in supplying the caudal hindbrain ( Fig. 4 ).
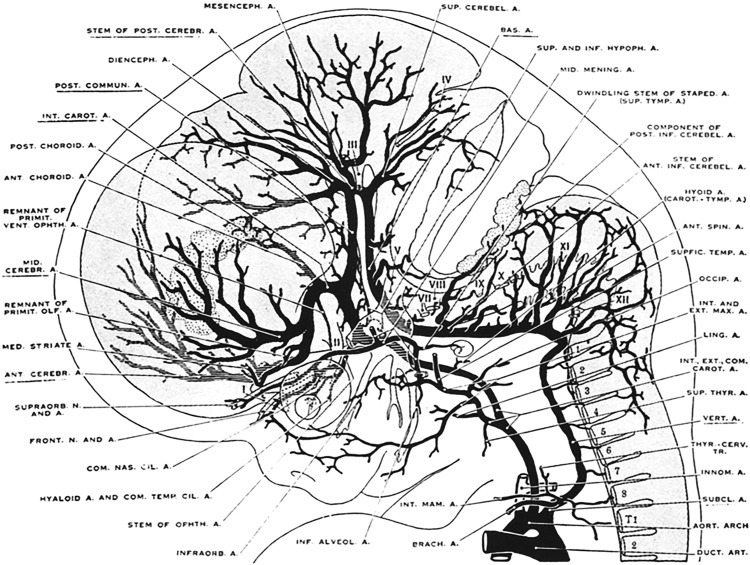
Fig. 4
Cranial arteries in a 24-mm embryo. The vertebrobasilar arteries now supply the posterior cerebral hemispheres. The anterior inferior and posterior inferior cerebellar arteries are better developed.
( From Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1948;12(pub no: 575):205–61.)
Anatomic Variations
Normal variations in the vertebrobasilar arteries are understood best as variations in the pattern by which the early arterial plexus condenses into mature vascular channels. Because arteries develop in response to the increasing metabolic demands of specific tissues, there are few variations in patterns of the smaller, distal “end territory” vessels. That is, the distal, intraparenchymal vasculature is more nearly constant. Because the proximal portions of the early vascular plexus may receive adequate supply through many different routes, successful embryogenesis tolerates far greater variation in the pattern of the larger, proximal vessels that feed into the developing networks. This process explains the development (“tolerance”) of fenestrations, anomalous origins of defined vessels, absence or duplications of the major cerebellar arterial branches and interruption, partial fusion, or duplication of the basilar artery ( Fig. 5 ).
Persistent Caroticovertebral Connections
The existence of persistent trigeminal, hypoglossal, and proatlantal arteries is well-documented, as are variations among them ( Figs. 6–9 ). The existence of a persistent otic artery is debated. Some authors suggest that the “otic artery” is simply a variant form of persistent trigeminal artery that arises unusually caudally. The “otic” artery has no homologue in other species, whereas the other presegmental and intersegmental arteries do, further fueling contention about the existence of a real persistent otic artery.
Venous development and drainage of the cerebellum and brainstem
The ventral mesencephalic vein is the most prominent venous structure within the posterior fossa of the developing 60 to 80 mm embryo. This vessel drains the ventral aspect of the developing brainstem and cerebellar plate, and is the precursor of the petrosal vein and superior petrosal sinus.
Slightly later, before the cerebellar plate forms the cerebellar hemispheres, a conspicuous dorsal mesencephalic vein begins to drain the dorsal aspect of the developing cerebellum. Once present, the dorsal mesencephalic vein drains into the straight and lateral sinuses via the primitive tentorial venous plexi. This flow from the superior surface of the cerebellum is then captured by the Galenic venous system.
The veins draining the posterior fossa have been classified in diverse ways. In 1 system, these veins are divided into 3 major groups: (1) the superior or “Galenic” group, (2) the posterior or “tentorial” group and (3) the anterior or “petrosal” group. In a second system, the veins of the posterior fossa may be classified, instead, into 4 separate groups, designated: (1) superficial veins that drain the cortical surfaces of the cerebellum, (2) deep veins that drain the tissue within the depths of the cerebellar folds, (3) veins of the brainstem, and (4) bridging veins that cross the subarachnoid space and dura to drain the parenchyma into the dural venous sinuses.
Superficial Veins of the Cerebellum
The superficial veins drain the cortical surfaces of the cerebellum. They are named for the specific surfaces they drain, as superior (synonym: tentorial), anterior (synonym: petrosal), and posterior (synonym: suboccipital) veins. These superficial veins are then subclassified by whether they drain the vermis or the cerebellar hemispheres.
Superior (tentorial) veins drain through the superior vermian and the superior hemispheric veins. Superior vermian veins that are situated anteriorly drain to the vein of Galen. Superior vermian veins that are situated posteriorly drain to the torcular. Superior hemispheric veins are considered in anterior, posterior, and lateral groups. The anterior superior hemispheric veins typically drain the anterior tentorial surface of the cerebellum. The posterior superior hemispheric veins typically form a common trunk with the inferior hemispheric veins from the suboccipital surface and drain together into the adjacent sinuses. The lateral superior hemispheric veins typically drain the lateral aspect of the tentorial surface of the cerebellum.
Anterior (petrosal) surface veins are subdivided into superior, middle, and inferior groups. These converge in the region of the flocculus to form the vein of the cerebellopontine fissure, which then drains into the superior petrosal sinus.
Posterior (suboccipital) surface veins include the paired groups of superficial tonsillar veins, the paired inferior vermian veins, and the paired groups of inferior hemispheric veins. The superficial tonsillar veins from the medial, lateral, and posterior surfaces of the tonsil converge into retrotonsillar veins that give rise to the more caudal inferior vermian veins. The paired inferior vermian veins drain the tonsils and the surface of the vermis into the torcular or medial portions of the transverse sinuses. The paired groups of inferior hemispheric veins run transversely to drain to the tentorial sinuses or longitudinally to drain into the inferior vermian veins.
Deep Veins of the Cerebellum
The deep veins of the cerebellum are divided into (1) fissural veins that course longitudinally within the cerebellomesencephalic, cerebellopontine, and cerebellomedullary fissures, and (2) peduncular veins that course along the superior, inferior, and middle cerebellar peduncles.
Veins of the Brainstem
The veins of the brainstem are named for the territories they drain and the direction in which they course. Veins coursing along the brainstem are called longitudinal veins and are considered to belong to the midline and lateral groups. The direction of venous flow within these veins may be superior or inferior. Veins coursing across the brainstem, perpendicular to the longitudinal veins, are called transverse veins. From cranial to caudal, the transverse veins are designated peduncular veins, transverse pontine veins, pontomedullary sulcal veins, and transverse medullary veins.
Bridging Veins
The bridging veins cross the subarachnoid space and dura to drain the brainstem and cerebellum into the dural venous sinuses. In the posterior fossa, these veins drain superiorly to the vein of Galen, laterally to the transverse petrosal vein and superior petrosal sinus, and posterosuperiorly to the torcular and tentorial sinuses.
Vascular lesions of the cerebellum and brainstem
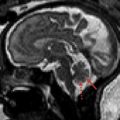
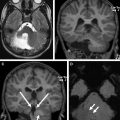
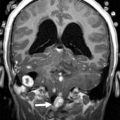
Cavernous Malformations
Cavernous malformations (cavernomas) account for approximately 20% to 25% of vascular malformations within the central nervous system in children. Posterior fossa cavernomas represent between 8% and 36% of all cases, with the vast majority being located within the brainstem. Histologically, cavernomas are rounded collections of cavernous or sinusoidal vascular spaces of variable size, with no intervening brain parenchyma. The malformations are formed by dilated and hypertrophied capillary beds, which contain regions of both clotted and liquid blood. The different states of blood within the malformation account for their characteristic signal and susceptibility on MR imaging scans. Most cavernous malformations are supratentorial (∼80%), but they can be found anywhere within the brain and may be distributed throughout the brainstem and cerebellar tissue ( Figs. 10 and 11 ). Cavernomas may be solitary or multiple, “eloquent,” or often clinically silent. These lesions are typically seen in adults, but may be associated with familial syndromes and are seen both in children and adolescents.