Fig. 1
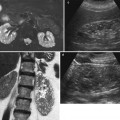
(a, b) Ultrasound (US) appearance of a normal neonatal kidney. US image – obtained using a high-resolution linear transducer – of a normal neonatal kidney in longitudinal (a) and axial (b) plane. Note the physiologic hyperechogenicity of the distal medullae and papillae, as well as the slight wall thickening (+…+1) of the nearly, though depictable, collapsed renal pelvis (+…+2). The axial kidney diameters as used for volume calculations are marked (+…+3,4) in image (b)
Finally, the entire environment as well as patient handling during an examination must be adapted: sick children are often afraid and in pain reducing their intrinsically restricted ability to cooperate in the hospital environment; communication may be difficult or even impossible; parents or other assisting persons are necessary as well as, sometimes, immobilization or sedation; and other practical needs such as pacifiers, swaddling facilities, a heater, blankets, and toys have to be provided. Note that due to these factors, all the investigations in children usually take considerably longer than the same study in adults!
All these amounts to the evident necessity for specifically trained and experienced pediatric (uro)radiologists, child adapted environment and equipment, as well as different scheduling of investigations when imaging the child’s urinary tract. Some basic procedural recommendations concerning the proper use of imaging in pediatric uroradiology have been published (Riccabona et al. 2008a, 2009, 2010, 2011, 2012; Darge et al. 2011). The proper use of renal scintigraphy in children has been outlined by European Association of Nuclear Medicine (Mandell et al. 1997; Piepsz 2002; Gordon et al. 2006; Piepsz and Ham 2006; Gordon 2008). The main messages of these recommendations are:
US: Use high-resolution (linear) high-frequency transducers; always perform a comprehensive study of the entire urinary tract and renal parenchyma in well-hydrated patients including a postvoid assessment (Riccabona et al. 2008a). Try to exploit the potential of modern techniques such as (amplitude-coded) color Doppler US or harmonic imaging (Riccabona et al. 2001; Bartram and Darge 2005; Riccabona 2002b, 2009). Always use volume calculations and age-related growth charts – kidney length measurements are less reliable in children (Riccabona et al. 2002b, c). Note that, particularly in the urinary bladder, the correction factor for the commonly used ellipsoid equation may vary depending on the bladder shape. And remember that – as the fetal development is closely linked – all particularly female children with a urinary tract malformation should undergo a detailed assessment of the genital tract (Riccabona 2013a, b; Riccabona 2014).
CEUS: Only two contrast agents are available in Europe and the USA, SonoVue (Bracco) and Optison (GE). Both are not registered for pediatric use. However, numerous off-label applications are reported in children and in the pediatric urogenital tract, particularly for VUR assessment by contrast-enhanced voiding urosonography (ce-VUS). European questionnaires have demonstrated a good safety profile (Riccabona 2012; Darge et al. 2013), and dose and procedural recommendations are available for both the intravesical use (i.e., for SonoVue® 0.5–1 % of actual bladder filling volume, for Optison® 0.5 % of the actual bladder filling volume) and the intravenous CEUS (Papdopoulou et al. 2012, 2013). However, recently some more severe reactions to the intravenous application of SonoVue® have been reported, so justified indications and precautions to mange potential (severe) allergic reactions are mandatory (Riccabona 2013a, b; Darge et al. 2013).
Radiographs of the KUB: Age-adapted dose as well as the proper use of filters, grid, and shutters are mandatory; also consider the need for the adaptation of digital radiography equipment to pediatric needs (i.e., different electronic filters and postprocessing, sufficient spatial resolution of the detector).
IVU: There are very few indications left. If necessary, use age-adapted CM and radiation dose as for KUB, and avoid multiple films – usually two or three well-timed films suffice for retrieving all therapeutically necessary information (Riccabona et al. 2010). To enable proper planning of the investigation and deciding on the optimal timing of films, a previous detailed US study is mandatory (Fig. 2).
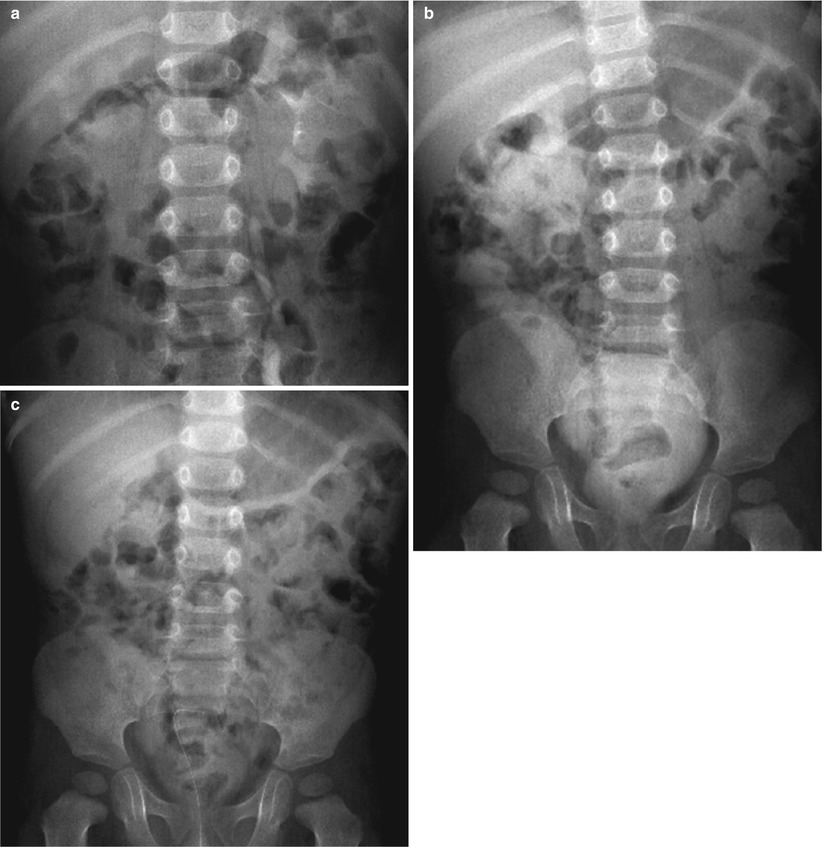
Fig. 2
(a–c) Focused intravenous urography (IVU) in childhood urolithiasis. Focused IVU after ureteral reimplantation with sonographically suspected postoperative obstruction and huge dilatation of the right collecting system as well as the ureter. Three well-timed exposures, defined according to the clinical query and the findings of the US examination: (a) an early kidney image at 7 min after contrast medium injection demonstrating delayed excretion into the operated and dilated right collecting system, but revealing all of the collecting tract anatomy of the left unobstructed system. (b) A delayed image showing the now homogeneously opacified dilated pelvocalyceal system and the diverted megaureter after ureterocutaneostomy on the right side, with complete drainage of CM from the unobstructed left kidney. (c) A final image after furosemide i.v. and upright positioning (to allow for gravity-dependent drainage) demonstrating the complete washout of contrast medium from the dilated right system, thus proving a dilated, but not obstructed, right system obviating any further intervention
VCUG: It is an invasive investigation with relatively high radiation burden particularly to the ovaries, although modern equipment and skilled investigation technique help to bring down the effective applied dose significantly (Lebovic and Lebowitz 1980; Hernandez and Goodsitt 1996; Ward 2006; Ward et al. 2008). Always assure a reliable justification for this invasive and radiating study. Use only digital pulsed fluoroscopy with last image hold for documentation reducing spot films to the minimum. Try to fill the bladder at physiologic filling pressure and perform cyclic VCUG in the first year(s) of life for the best yield, once you have decided to perform this study (Gelfand et al. 1999; Paltiel et al. 1992). Always dedicate your attention to the ureterovesical junction (oblique/lateral views mandatory!), the urethra (particularly in boys, lateral view!), the renal parenchyma (searching for intrarenal reflux), as well as the residual urine and the postvoid CM drainage dynamics (if refluxed) (Fernbach et al. 2000; Riccabona 2002a, b; Riccabona et al. 2008a, b, c).
Scintigraphy: Select the method properly, depending on age, kidney function, and clinical query (static or dynamic diuretic renography). A previous detailed US study is mandatory, also for enabling a correct interpretation of scintigraphic findings. The method has been standardized and is considered essential or even the present gold standard for various queries (Prigent et al. 1999; Gordon et al. 2000a, b; Piepsz 2002). Note that indirect cystoscopy should be performed only in toilet-trained patients. If you consider using single photon emission computed tomography (SPECT), adapt the technique in order to facilitate reliable results at the same dose; however, often – particularly in small children – this does not yield a lot of therapeutically relevant additional information.
CT: The most important rule is avoid CT whenever possible, and if indicated (e.g., no MRI available for tumor queries), avoid multiphase CTs (in the majority of cases, one adequate phase is sufficient for diagnosis) – or at least reduce their number! The adaptation of all other parameters such as CM dose, timing, tube settings, or scanning range varies with age, query, institution, and scanner (Tsapaki et al. 2006; Paterson and Frush 2007; Stöver and Rogalla 2008). Some orienting information can be retrieved from the recommendation for uro-CT in children (Riccabona et al. 2010) and on the website of the image gently campaign in the USA (www.imagegently).
MRU: At present, there are different approaches under evaluation – particularly, functional diuretic dynamic MRU is still considered a “work in progress.” For the use of contrast medium, the same rules apply as for CT or IVU, as prevention of nephrogenic systemic fibrosis has to be considered (Riccabona et al. 2008a, b, c). Immobilization and sedation are often necessary in the first years of life; however, this also applies to CT (one should never “try a CT” and repeat it in case the child had moved during the critical acquisition phase or use a field of view far larger than the targeted area to assure that everything is covered even if the child moves!). As MRI is a nonionizing imaging method that allows for simultaneous anatomic and functional assessment, it has practically replaced IVU for most queries in childhood and is increasingly considered the ideal one-stop-shop investigation in the child’s urinary tract; its implementation into routine diagnostics is strongly recommended and promoted, particularly for purely anatomic queries, where, in most instances, all relevant questions can be answered without the need for contrast administration and the safety issues of a potentially necessary sediation can be well addressed when respective guidelines are followed (Bluemke and Breiter 2000).
2 Vesicoureteral Reflux and Reflux Nephropathy
2.1 General Considerations
One of the conditions that may potentially need early treatment to prevent long-term renal damage is vesicoureteral reflux. This very controversial topic is constantly under discussion; knowledge and insight into the natural course of the condition has grown and is constantly growing. New knowledge has been and is influencing the management and treatment strategies leading to a constantly changing role and task of imaging. Whereas, initially, vesicoureteral reflux was seen as a separate entity that needs to be treated in any way, today, only the consequences of vesicoureteral reflux such as bladder dysfunction and renal damage give reason for concern or treatment. Additionally, long-established treatment strategies such as antibiotic prophylaxis are under discussion, particularly, as most congenital vesicoureteral refluxes spontaneously decrease and eventually resolve without any long-term sequelae. With this, the present confusion becomes obvious – whom and how to image for a condition that probably will vanish without any treatment or problems without missing those who might and will suffer from vesicoureteral reflux and its sequelae? And is there an impact of vesicoureteral reflux on renal transplants?
In order to answer these difficult questions, we need to consider that there are many different forms of vesicoureteral reflux: various grades; congenital or acquired; primary or secondary; high or low pressure; with or without intrarenal reflux; uni- or bilateral; with normal ureteral anatomy or with pathologic changes such as a gaping ostium or a duplex and potentially ectopically inserting ureter; as an isolated finding or part of a more complex urinary tract malformation such as posterior ureteral valve, duplex systems, or in combination with obstructive uropathy; and with or without renal parenchymal damage as in the typically newborn males with (bilateral) congenital high-grade vesicoureteral reflux and congenital renal dysplasia (“congenital reflux nephropathy”) (Riccabona 2002a, b, 2007). Additionally, one needs to acknowledge that vesicoureteral reflux itself probably does not cause renal damage, only early intrauterine high-grade high-pressure vesicoureteral reflux (potentially causing renal damage based on the water hammer effect theory, in addition to the associated primary developmental renal dysplasia) or when associated with other factors such as compound papilla (enabling intrarenal reflux), bladder outlet obstruction, functional bladder disturbance, or urinary tract infection. This leads to an increasingly reluctant indication for the imaging assessment of vesicoureteral reflux in childhood. Generally accepted indications are significant neonatal hydronephrosis in baby boys or infants with complex urogenital tract or cloacal malformations (see Sect. 4), infants and children with (recurrent) upper urinary tract infection (i.e., renal involvement and potential scaring in infection – see Sect. 3), for the differentiation of obstructive vs. refluxing dilatation, and with persisting bladder dysfunction (such as in neurogenic bladder, bladder outlet obstruction, or persistent dysfunctional voiding associated with recurrent urinary tract infection); additionally, vesicoureteral reflux into transplanted kidneys is increasingly becoming a major concern.
2.2 Methods for Vesicoureteral Reflux Assessment
2.2.1 Ultrasound
US is the workhorse in pediatric uroradiology. It has overcome its initial restrictions where it was considered just a rough initial orienting tool and now offers many reliable options that may help in diagnosing and assessing vesicoureteral reflux. For this, it is important to have a state-of-the-art US device with a variety of transducers; particularly, in infants and neonates, high-resolution linear transducers (15–5 MHz) with color Doppler US option and ability for harmonic imaging are indispensable. Every investigation should be performed on a sufficiently filled bladder and include a postvoid assessment of not only potential residual urine but also changes in upper tract dilatation that may indicate perimicturitional high-pressure vesicoureteral reflux. And one should aim at visualizing the distal ureters, usually achievable in every sufficiently hydrated child. Important findings that may hint toward vesicoureteral reflux are a thickened or trabeculated bladder wall, an unusual ostium position or anatomy (asymmetric, lateralized, gaping, duplex, etc.), a dilated distal ureter (particularly with a thickened urothelium) (“urothelial sign”), and changing width of the upper collecting system (Fig. 3). Other more advanced important options are the color Doppler US (which may directly depict vesicoureteral reflux as soon as there are some reflecting particles in the urine), assessment of ureteric bladder inflow jet (which exhibit specific jet forms that are associated with vesicoureteral reflux, Fig. 4), and various bladder filling techniques for direct vesicoureteral reflux visualization after bladder catheterization. Air, saline (shaken), and – most commonly – sonographic contrast agents have been successfully used for reflux cystosonography; particularly, contrast–enhanced voiding urosonography (ce-VUS) has been established as a reliable and nonirradiating imaging modality for vesicoureteral reflux detection, with an even higher sensitivity for vesicoureteral reflux detection than conventional fluoroscopic VCUG, particularly using modern agent detection techniques (Bosio 1998; Darge et al. 1999, 2001; Kenda et al. 2000; Berrocal et al. 2001; Riccabona et al. 2003a, b; Darge et al. 2005). Any contrast material detected in the ureter or the renal cavities indicates vesicoureteral reflux (Fig. 5). Recommendations on how to perform ce-VUS and sonographically grade vesicoureteral reflux have been established (Darge and Tröger 2002; Claudon et al. 2008; Riccabona et al. 2008a, b, c; Riccabona et al. 2014b). The major setback of this technique is that, currently, no sonographic contrast agent is approved for pediatric use; furthermore, a panoramic display and the assessment of the entire ureter (if not dilated) is impossible, or bladder diverticula that briefly pose during voiding as well as short-lasting low-degree vesicoureteral reflux into a nondilated ureter may be missed; the latter, however, will not have much therapeutic consequences and thus may be less important. Finally, the assessment of the urethra during voiding is a little cumbersome and time consuming. However, transperineal US is capable of reliably visualizing the urethra and the pediatrically important urethral anomalies (Good et al. 1993; Teele and Share 1997; Schöllnast et al. 2004; Duran et al. 2013). Additionally, US allows for early and noninvasive assessment of the inner genitalia that may also be affected in urogenital or cloacal malformations, particularly important in baby girls with some urinary tract malformations such as single kidneys (Gassner and Geley 2004; Geley and Gassner 2008; Riccabona et al. 2014a).
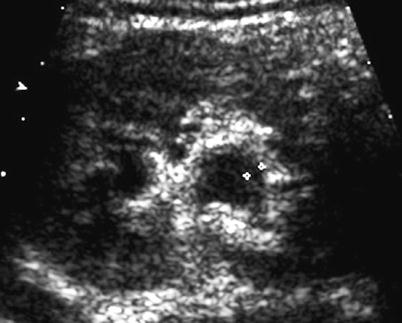
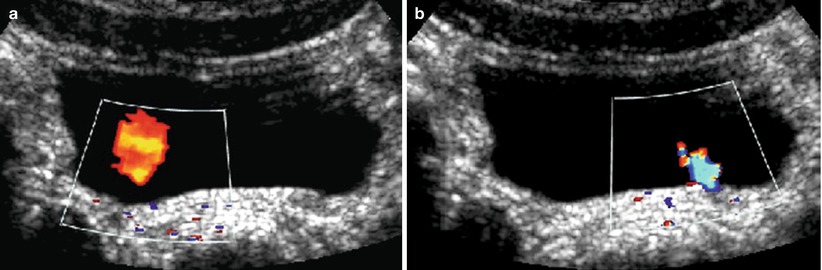
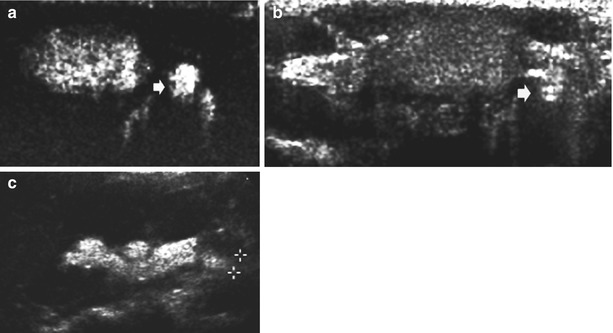

Fig. 3
US image – thickened urothelium (“urothelial sign”). Axial section of the right kidney in a girl with vesicoureteral reflux and recurrent urinary tract infection demonstrating the thickened urothelium (+…+) of the slightly prominent renal pelvis, a nonspecific sign seen with vesicoureteral reflux, obstruction, or infection as well as other causes of pelvic wall edema, necessitating further workup and, today, included in the sonographic “extended US criteria” which indicate urinary tract pathology and justify additional workup

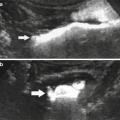
Fig. 4
(a, b) Ureteric inflow jet. Ureteric inflow jet using color Doppler US. Asymmetric jet with a lateralized ostium on the right side (a) in a child that then showed medium degree vesicoureteral reflux on the right side. The blue color of the jet from the normal positioned left ostium (b) is due to aliasing

Fig. 5
(a–c) Contrast-enhanced voiding urosonography (ce-VUS) in dilating vesicoureteral reflux. (a, b) Urinary bladder (axial plane) filled with the microbubble contrast agent, using a dual image-specific contrast imaging technique (a) and an orienting baseline grayscale B-mode image (b). Note the excellent depiction of the contrast-filled, dilated, and refluxing left ureter (small arrow) on the contrast image. c Longitudinal image of the left kidney using the same technique and contrast harmonic imaging showing the dilated ureter (+…+) and pelvocalyceal system, filled by the echogenic microbubble contrast agent consistent with grade IV vesicoureteral reflux
The other important aspect in suspected vesicoureteral reflux is imaging of the renal parenchyma – US is the established imaging tool that reveals information on renal size, parenchymal structure, and focal lesions such as dysplastic cysts or large scars, particularly if associated with scared and clubbed calices (Fig. 6). Power Doppler sonography (= amplitude-coded color Doppler sonography) additionally allows for the assessment of peripheral renal perfusion and vasculature, improving the depiction of diffuse or focal perfusion disturbances associated with diffuse renal parenchymal damage or focal scaring. Again, restrictions have to be acknowledged – small or minor and diffuse scaring may be missed by US; thus, additional renal assessment by static Tc99mDMSA scintigraphy is presently considered essential and the gold standard in children after 3–6 months of age. In the future, MRI may play a bigger role also for this query (Chan et al. 1999; Thoeny et al. 2005). US remains the routine imaging for regular follow-up of children with symptomatic vesicoureteral reflux and reflux nephropathy (an old term that is used more reluctantly for postnatally acquired renal scaring after urinary tract infection, even in children with vesicoureteral reflux).
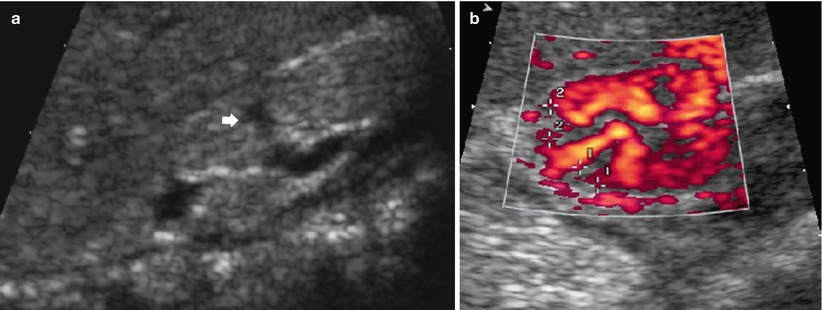

Fig. 6
(a, b) US image of a scarred kidney (“reflux nephropathy”). Longitudinal scan of a small, echogenic, nondifferentiated kidney with clubbed calices (arrow) (a) and focal perfusion defects demonstrating scars depicted by amplitude-coded power Doppler US (+…+) in an axial section of the upper pole area of the right kidney (b)
2.2.2 Voiding Cystourethrography
VCUG is considered the gold standard of vesicoureteral reflux imaging. It is invasive and burdening as it requires bladder catheterization or suprapubic puncture and uses radiation. On the other hand, it offers a panoramic overview and allows for exquisite anatomic display of the urethra and the bladder and – if refluxing – the entire ureter as well as the renal collecting system, also enabling the depiction of intrarenal reflux. With modern fluoroscopic techniques using age-adapted grids, pulsed fluoroscopy, last image hold for documentation, and skilled device handling that allows for short fluoroscopy time, the effective radiation burden can be significantly reduced, even when performing cyclic investigation as recommended in the first years of life (Lebovic and Lebowitz 1980; Gelfand et al. 1992; Paltiel et al. 1992; Hernandez and Goodsitt 1996; Ward 2006; Ward et al. 2008). The entire procedure and grading (grade I–V) have been standardized; recommendations exist and have been published (Lebowitz et al. 1985; Fernbach et al. 2000; Riccabona 2002a, 2005; Riccabona et al. 2008a) (Fig. 7; Table 1). VCUG was and is the base for most international vesicoureteral reflux studies, also with less investigator dependency than US. Furthermore, modified protocols that use a physiologic filling pressure of the contrast infusion – which is monitored to detect the phases of increased bladder pressure with consecutive infusion stops that guide fluoroscopy – have enabled a simultaneous functional assessment (Fotter et al. 1986). This is increasingly important, as functional bladder disturbances are being recognized as an important aspect in dealing with patients who have vesicoureteral reflux and recurrent urinary tract infection (Fotter 2008) – unrecognized and untreated bladder dysfunction not only will perpetuate urinary tract infection and clinical symptoms but may also lead to the reoccurrence of operated vesicoureteral reflux. On the other hand, successful treatment of bladder dysfunction will often result in the resolution of vesicoureteral reflux, without the need of cystoscopic treatment or open surgery.
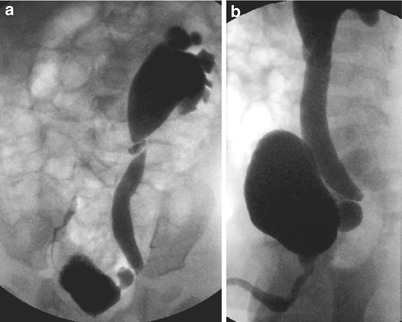

Fig. 7
(a, b) Vesicoureteral reflux shown on voiding cystourethrography (VCUG). Cyclic VCUG in an infant after urinary tract infection with US signs of a left-sided megaureter and hydronephrosis III° taken as last image hold without the need for additional exposures. Image (a) demonstrates a dilating high-degree vesicoureteral reflux (vesicoureteral reflux IV°) on the left side with a kink at the ureteropelvic junction and a low-degree vesicoureteral reflux into the distal right ureter (vesicoureteral reflux I°) that had occurred only during previous voiding. Image (b) during voiding (lateral oblique projection) demonstrates the diverticula at the ureterovesical junction of the left side, nicely posing during voiding (due to the perimicturitional higher intravesical pressure)
Table 1
Grading of vesicoureteral reflux according to international radiographic reflux grading system (Lebowitz et al. 1985)
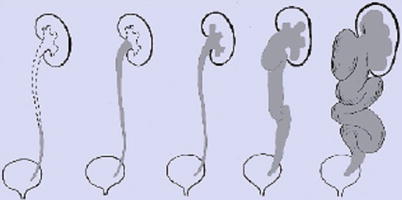 | |
Grade I | Vesicoureteral reflux only into a normal distal ureter |
Grade II | Vesicoureteral reflux reaching the renal pelvis |
Grade III | Vesicoureteral reflux into the renal calices |
Grade IV | Vesicoureteral reflux with dilated calices and dilated, tortuous ureter |
Grade V | Gross vesicoureteral reflux into a markedly dilated and clubbed pelvocalyceal system with a huge and tortuous (mega)ureter that may also exhibit kinking with secondary (intermittent) upper obstruction |
Indeterminate grade | Vesicoureteral reflux into a partially obstructed dilated ureter, with urine-contrast levels (no image) |
2.2.3 Radionuclide Cystography (RNC)
RNC is another option for VUR diagnosis.
Direct RNC uses – instead of radiopaque contrast – a Tc99m-labeled tracer that is directly instilled into the bladder via a catheter; any activity observed in the projection on the ureter or the kidney is indicative of vesicoureteral reflux. The radiation dose is slightly lower than in VCUG, and – partially due to the longer observation period – the yield is higher. The disadvantage is lack of anatomic details, especially of the urethra (Mandell et al. 1997; Piepsz 2002; Gordon 2008).
Indirect RNC consists of the delayed phase of a dynamic renography using intravenously applied Tc99m MAG3 that is excreted by the renal tubular cells into the collecting and draining system – thus, it avoids catheterization and offers a more physiologic approach (Gordon et al. 2000a, b; Piepsz 2002; Gordon 2008). After the activity has passed into the bladder during the early phases of the investigation, any reoccurrence of activity in the upper collecting system indicates vesicoureteral reflux, particularly during and after voiding. However, it can be performed only in toilet-trained patients, and anatomic resolution is low too.
2.3 Imaging Algorithm: When to Perform What in Whom
It is difficult to propose an imaging algorithm for children with suspected vesicoureteral reflux; in general, VCUG is preferred in male neonates and for preoperative assessment as well as in complex malformations, particularly with suspected (additional) obstruction. If available, ce-VUS is recommended for screening conditions, for vesicoureteral reflux assessment in girls, as well as for bedside or follow-up investigations. RNC is used for screening purposes and for following up children with symptomatic vesicoureteral reflux as well as postoperatively. Renal imaging in children with significant vesicoureteral reflux should start with US and a baseline static renal scintigraphy (usually after the age of 6 months, except for severe reflux nephropathy or malformations); follow-up usually relies on US, with a repeated scintigraphic study in case of deterioration or equivocal US findings, if these results potentially impact further patient management. In the future, MRI may become an alternative imaging tool to assess split renal function (dynamic diuretic contrast-enhanced MRU; see Sect. 4) or to visualize scars, particularly on T2*-weighted inversion recovery sequences.
3 Childhood Urinary Tract Infection (Including (Acute) Pyelonephritis)
Importance and management of, and thus also imaging for, childhood urinary tract infection are different from adults, particularly in early childhood. As with vesicoureteral reflux, the imaging in childhood urinary tract infection has changed. Initially, vesicoureteral reflux was considered the major condition that endangered the kidney and caused infection and scaring; therefore, aggressive search for vesicoureteral reflux was performed in all infants and children with urinary tract infection. Today we know that there are many factors that cause urinary tract infection as well as renal involvement and that vesicoureteral reflux itself is harmless and regresses spontaneously in the majority of cases; even high-grade vesicoureteral reflux may spontaneously regress. Thus, today, vesicoureteral reflux is considered as just one other point in a long list of risk factors that cause renal harm in children with urinary tract infection. The focus of imaging has moved toward the kidney, as only diffuse or segmental renal involvement in urinary tract infection causes long-term sequelae, particularly during the first 2 years of life, when the still somewhat immature kidney is prone to severe scaring, also affecting the kidney’s growth potential and thus endangering renal function, particularly if bilateral. Today, the task of imaging in pediatric urinary tract infection is to diagnose or rule out upper urinary tract infection (i.e., acute pyelonephritis or diffuse renal involvement) and to assess for potentially endangering preexisting urinary tract conditions or malformations. This new approach has impacted the imaging algorithms for children with urinary tract infection, with some age as well as local variations; in the UK, for example, the “NICE guidelines” suggest not to perform any imaging at all in children with urinary tract infection as long as they respond well to antibiotic treatment.
3.1 Which Imaging Modalities Are Available and How Do Things Look Like?
Again, the major imaging tool is US. It is easily available, nonirradiating, relatively inexpensive, and quite reliable if performed with professional skills by specifically trained, experienced, and knowledgeable investigators. US always includes the assessment of the entire well-hydrated urinary tract; an additional “sonoscopic” overview of the entire abdomen for other potential reasons of the child’s symptoms is recommended (Riccabona et al. 2008a; Riccabona 2009). The aim of this initial US investigation in urinary tract infection is to find signs that enable the differentiation of “upper” from “lower” urinary tract infection, to detect potentially undiagnosed preexisting urinary tract malformations early, to search for potential complications such as abscess formation or pyonephrosis, or to depict unusual disease such as infected urolithiasis or xanthogranulomatous pyelonephritis. US findings that indicate renal involvement are (Fig. 8):
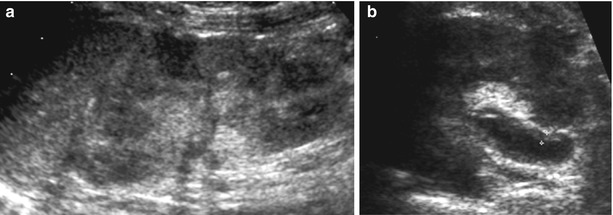

Fig. 8
(a, b) Renal US findings in upper urinary tract infection. (a) Longitudinal display of an enlarged, swollen, diffusely and inhomogeneously echogenic kidney with irregular echotexture; the cortical echogenicity is partially interrupted by focal hypoechoic regions that eventually became necrotic and scars. (b) Axial view of a slightly distended renal pelvis in a swollen kidney with increased echogenicity and hazy differentiation demonstrating a gross thickening of the pelvic urothelium (+…+) due to upper UTI. Note some sludge-like echoes in the collecting system
Kidney enlargement – they often become more spherical; therefore, volume calculations using the ellipsoid equation and comparison with age/size correlated growth charts are mandatory.
Focally or diffusely altered renal parenchymal echogenicity, with impaired corticomedullary differentiation.
Increased peripyelonal echoes and/or thickened urothelium/pelvic wall (“urothelium sign”) (Alton et al. 1992; Sorantin et al. 1997; Riccabona and Fotter 2004).
Echoes within the upper collecting system.
Focal or diffuse perfusion impairment on color and/or power Doppler sonography US (Dacher et al. 1996; Riccabona et al. 2001).
Potential remnants of old infections with scaring such as clubbed calices and parenchymal narrowing.
Note that these changes only gradually decrease after successful treatment even without any scaring – reestablishment of a normal US appearance can take up to 8 weeks.
Typical complications are necrosis and abscess formation: US will visualize the focal hypoechoic patchy destruction of the renal parenchyma with the gradual development of complicated fluid in an unphysiologic cavity; US may also depict edema and hyperemia of the perirenal tissue, and (amplitude-coded) color Doppler Sonography will help visualize the necrotic nature of the abscess (Fig. 9). Usually, these abscesses resolve under antibiotic treatment; rarely, (US-guided) drainage may become indicated (Riccabona 2004a). Note that, sometimes, infected or hemorrhaged cysts/tumors or obstructed calices/calyceal diverticula may mimic abscesses. Chronic pyelonephritis and particularly xanthogranulomatous pyelonephritis are rare; however, when observing the key diagnostic features such as more centrally located complex cystic masses that may be confluent, with a more or less destructed renal parenchyma in the presence of a central, stag horn-shaped stone, the diagnoses can be assumed sonographically (Fig. 10). A special entity is fungal infection: in the pediatric population, it typically occurs in children with dilated or obstructed cavities under antibiotic prophylaxis and in preterm infants or other immunocompromised children. The typical US features are echogenic (floating) masses in the collecting system that may cause dorsal shadowing and can cause obstruction (Fig. 11). All other features do not differ significantly from any other urinary tract infection manifestations. Although these fungus balls cannot be reliably differentiated from urolithiasis, the history, the urine findings, and the clinical details will enable the assumption of this condition. The signs of and findings in other complicating conditions in conjunction with urinary tract infection such as obstructive uropathy, urolithiasis, or tumors will be described in the respective chapters.
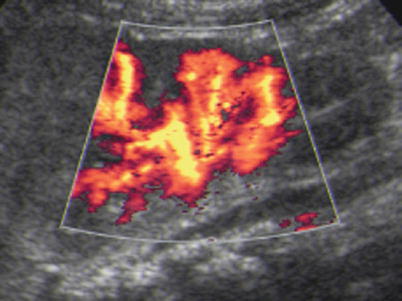
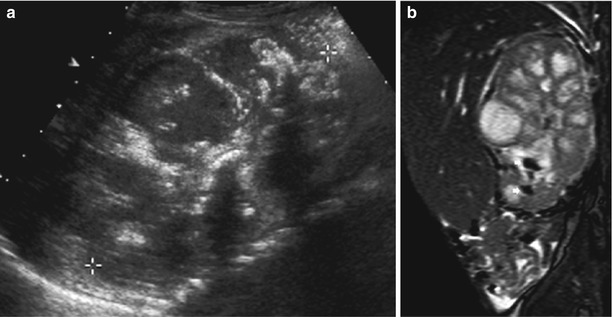
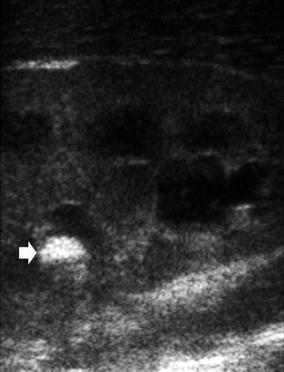

Fig. 9
Power Doppler in acute pyelonephritis: the same kidney as in Fig. 8a – power Doppler US depicts the focal perfusion defect in acute pyelonephritis well

Fig. 10
(a, b) US and magnetic resonance imaging (MRI) appearance of chronic xanthogranulomatous pyelonephritis. (a) Longitudinal US section through a grossly distorted and enlarged kidney (+…+) with irregularly dilated calices, destructed medullary parenchyma, only rim-like residual cortical parenchyma, atypical echoes within the collecting system, and a central hyperechoic structure (= concrement in the pelvis) with acoustic shadowing in a child with xanthogranulomatous pyelonephritis. (b) A sagittal MRI image (SSFP sequence) of the same child as in (a) demonstrates similar findings; even urolithiasis/calcifications can be discriminated as patchy areas with signal void in the lower calices (arrows)

Fig. 11
Fungus ball – US appearance. Sagittal US image demonstrating an echogenic clot-like material (arrow) within the dilated collecting system of an enlarged and swollen kidney in a preterm neonate – without acoustic shadowing – in renal candida infection after long-time antibiotic treatment
On the other hand, lower urinary tract infection (i.e., cystitis) can be assumed when changes are seen only in the bladder such as a hypervascular thickened bladder wall or floating echoes in the bladder without alteration of the renal collecting system or parenchyma (Fig. 12). Note that cystitis can sometimes produce impressive changes such as fibrous clots (e.g., in hemorrhagic cystitis) that may be difficult to be differentiated from bladder tumors such as rhabdomyosarcoma or a very pronounced echogenic wall thickening as observed in schistomatosis. And also consider that since renal changes may take some time to develop, early US, particularly, may miss the developing upper UTI.
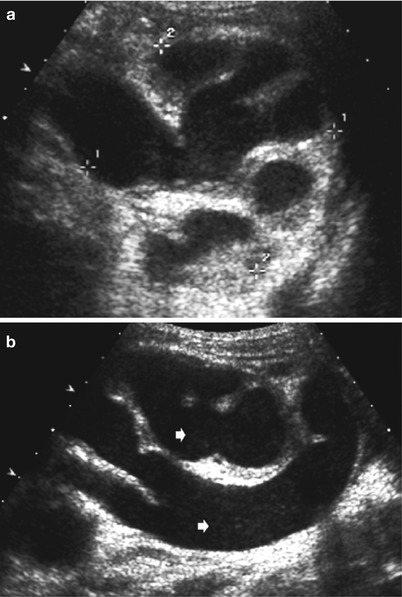

Fig. 12
(a, b) US in pyonephrosis. (a) Axial US image of a pyonephrosis seen as echogenic sedimentations in the grossly dilated pelvocalyceal system with narrow, echogenic residual parenchyma in a child with known hydronephrosis IV and megaureter. (b) The same child as in (a): longitudinal US section demonstrating diffuse floating inflammatory particles (arrows) on the ureter and the collecting system
The major alternative imaging tool is static renal Tc 99m DMSA scintigraphy (Piepsz et al. 1999, 2000; Craig et al. 2000). Though it may also be false positive and negative in rare cases, static renal scintigraphy is considered the gold standard for the assessment of renal involvement. Note that for differentiating reversible impaired parenchymal function in acute inflammatory involvement from persisting scars, a delayed scan 6–9 months after the infection is necessary. Furthermore, due to the restricted anatomic resolution of scintigraphy, some sort of anatomic study (usually US) is necessary for differentiating other focal lesions such as cysts or dysplastic moieties from focal infectious defects.
In the future, DMSA may be increasingly replaced by MRI which demonstrates reduced contrast uptake and diffusion disturbances in acute pyelonephritis well (Lonergan et al. 1998b; Kavanagh et al. 2005). However, today, due to sedation needs, restricted availability, as well as high costs, MRI is used rather reluctantly for this query.
Note that IVU – which was the major imaging requisite for decades – does not any longer play a role in the assessment of urinary tract infection and scaring in children, as it applies considerable radiation and needs intravenous contrast media with far less information than alternative modern imaging by US and scintigraphy or MRI.
3.2 Imaging Algorithm in Pediatric Urinary Tract Infection
In our experience, comprehensive US in conjunction with clinical and laboratory information can reliably establish the diagnosis of lower or upper urinary tract infection in the majority of cases, thus obviating the need for further investigations in a large number of children (Brader et al. 2008).
In patients where US is not clear, no high-end US or amplitude-coded color Doppler sonography is available or performable, or in patients where US findings do not match clinical and laboratory results, additional imaging by acute renal scintigraphy or MRI for establishing the diagnosis or ruling out upper urinary tract infection will become necessary, particularly if only those with proven renal involvement will undergo further studies (for the assessment of vesicoureteral reflux and scarring, the latter by delayed renal scintigraphy), as suggested by many local and international recommendations (Riccabona and Fotter 2004; Riccabona et al. 2008a). According to literature, the reduction of US indications in pediatric patients with acute UTI as suggested by the British guidelines (NICE 2007) is seen critically, as many children with significant pathology may be missed and thus renal integrity it as risk (Wong et al. 2010).
At present, MRI is recommended for assessing urinary tract infection complications in patients with equivocal US findings and has practically replaced CT for this indication in children, as CT is a highly irradiating modality and should be avoided in children whenever possible. If you need to perform a CT in a child with complicated urinary tract infection, use age-adapted protocols at lower radiation and contrast dose and avoid multiphase acquisitions – in the majority of children, a single scan is sufficient for diagnosis.
After the resolution of the acute infection, all those with (persisting) renal involvement will need vesicoureteral reflux assessment (see Sect. 1) and clinical as well as US follow-up (further renal development, scaring, development of hypertension). As US has limitations in diagnosing particularly small scars with little anatomic changes, a delayed DMSA scan is often recommended to prove scaring. But one has to acknowledge that what we see today is far more than what the original work of Smellie – who used IVU for the assessment of scars when establishing the importance of renal scaring for long-term outcome – was based upon; therefore, we, at present, do not know the long-term implication of small peripheral scars and whether these already justify more invasive imaging.
Increasingly, the importance of bladder dysfunction is recognized as a major contributing factor to childhood urinary tract infection particularly in slightly older children, mostly girls. Therefore, bladder function assessment is becoming very important, and imaging is urged to provide information on potential bladder dysfunction. This can be achieved by observing bladder shape and bladder neck form and activity as well as potential residual urine on US and doing voiding protocols in infants; in older toilet-trained children, uroflow measurements and pelvic floor electromyography are the first additional tests. If VCUG is performed, the modified protocol will reveal most of the necessary functional information similar to video-VCUG (simultaneous fluoroscopy with bladder manometry and pelvic floor EMG) (Fotter 2008).
4 Congenital Hydronephrosis and Urinary Tract Obstruction
4.1 Congenital Hydronephrosis
Congenital disorders and urinary tract malformations which often present with prenatally diagnosed hydronephrosis are addressed in chapter “Congenital and development disorders of the kidney.” Due to prenatal US (screening), there are many infants submitted for the assessment of prenatally recognized hydronephrosis. It is important to understand the history of the term hydronephrosis and its implications: this expression derives from early days when any distension of the renal central echo complex detectable on obstetric US suggested pathology. Today, the resolution of modern US has significantly increased and the normal distension of the physiologic renal cavities can routinely be visualized. This implies that not every fetally visible pelvocalyceal system needs postnatal assessment. An axial diameter of the fetal pelvis of 5–9 mm has been suggested as the cutoff value, with some variance depending on the gestational age and bladder filling. Furthermore, fetal and, accordingly, neonatal hydronephrosis have been graded; low-degree hydronephrosis (i.e., hydronephrosis 0–II, with no or only little pelvic distension less than 7–10 mm pelvic diameter and normal calyceal configuration) is considered normal; only moderate (hydronephrosis III, with rounded calices) or severe (hydronephrosis IV, with parenchymal narrowing) and gross dilatation (sometimes also called hydronephrosis V, when with only a thin residual parenchymal rim) indicate further postnatal assessment (Fernbach et al. 1993; Riccabona et al. 2008a).
4.2 Imaging Algorithm for Congenital Hydronephrosis
The postnatal imaging algorithm depends on these prenatal findings, with a delayed US investigation (after at least 1 week after birth) only in moderate-degree hydronephrosis and early comprehensive imaging workup in particularly bilateral high-grade hydronephrosis. Only those patients with low to moderate degree of fetal hydronephrosis who exhibit persisting sonographic abnormalities as defined by “extended US criteria” (i.e., pelvic dilatation ≥10/12 mm, hydronephrosis ≥III°, renal parenchymal or bladder wall abnormalities, urothelial sign, etc.) need further assessment (Alton et al. 1992; Sorantin et al. 1997; Riccabona et al. 2008a). Note that high-grade hydronephrosis is usually associated with obstructive uropathy, posterior urethral valve, or severe vesicoureteral reflux – in some of these patients, an early urinary tract infection may have devastating effects justifying early diagnosis by comprehensive US and VCUG (Riccabona et al. 2008a, 2009). Renal scintigraphy can be used to estimate residual renal function early in the most severe cases; otherwise, scintigraphy or MRU should always be postponed until at least after the sixth week of life, better the third month of life, as the immature kidney will not provide reliable results (Gordon and Riccabona 2003). Note that today there is no indication for IVU for these queries.
4.3 Obstructive Uropathy
The most common form of obstructive uropathy is ureteropelvic junction obstruction. This is usually a congenital condition often recognized in utero by high-grade fetal hydronephrosis; there are cases of late manifestation detected only during childhood (“juvenile type” of ureteropelvic junction obstruction). The other important entity is (primary/obstructive) megaureter. Additionally, there are acquired forms of obstruction by space occupying lesions and after surgery or trauma. Acute secondary obstruction as in urolithiasis will be described in Sect. 5. Posterior urethral valve, prune-belly syndrome, and megacystis–microcolon–hypoperistalsis syndrome are conditions primarily of the lower urinary tract and ureters which – although potentially impacting the kidney as mentioned in Sect. 1 – will not be discussed, as the renal consequences primarily are dysplasia, hydronephrosis, and secondary infection, with the same renal pathology and imaging aspects as detailed above or discussed in chapter “Congenital and development disorders of the kidney.”
There are different definitions of urinary tract obstruction. While, initially, obstruction was defined as “any stenosis that impairs urinary drainage from the pelvo-caliceal system and leads to increased pressure and reduced urine flow rate” (Whitaker et al. 1982), already in the same year, the importance of future renal function was highlighted by defining obstruction as “any restriction to urine flow, that left untreated will cause progressive renal deterioration” (Koff 1982). The most recent and presently generally accepted definition is the modification that defines urinary tract obstruction as “any condition that, left untreated, endangers renal functional and growth potential” (Peters 1995). The role of imaging in obstructive uropathy is to assess the degree of obstruction and to help decide on which kidney needs treatment and which will maintain function and growth even if left untreated. This a very difficult task, as, by today, there is no imaging tool that allows a decisive a priori pro-futuro assessment that can reliably identify those kidneys that will benefit from treatment and thus may be protected against deterioration of function or loss of growth potential. This inability to prospectively grade obstructive uropathy induces serial investigations to monitor renal development (Riccabona 2010).
The major initial imaging modality, usually, is US. Initially, in ureteropelvic junction obstruction, any gross dilatation detected by US was operated. Today, this is considered rather urinary tract cosmetics – we learned that dilatation does not equal obstruction and that US is only able to demonstrate dilatation, but has severe restrictions in assessing function and drainage even when observing US prerequisites such as good hydration and standardized (axial) measurements. Furthermore, dilatation varies with position, hydration, bladder filling, and kidney function – sometimes, constantly diminishing dilatation does not indicate improvement of obstruction, but is due to the deterioration of renal function with reduced urine production. The US intrinsically lacks the fundamental need to observe and quantify renal excretion and drainage, though modern US approaches such as Doppler sonography (i.e., asymmetrically elevated resistive indices in acute deterioration), diuretic US (serial investigations after diuretic stimulation by furosemide to observe and monitor renal response to diuretic stress based on pelvocalyceal dilatation and Doppler sonography findings), and 3D US (enabling reliable relative renal parenchymal size assessment even in gross hydronephrosis) have improved the US potential (Riccabona et al. 2003a, b, 2005).
Additional functional imaging is needed which is usually provided by dynamic diuretic Tc 99m MAG3 scintigraphy. Here, an intravenously applied radioactive tracer is taken up and excreted by the kidney; thus, renal perfusion and uptake (function) can be measured, and split/relative renal size can be calculated (Prigent et al. 1999; Gordon et al. 2000a, b; Piepsz 2002; Gordon 2008; Koff et al. 1979). Urinary drainage is assessed after diuretic stimulation using standardized drainage patterns to divide disturbances into normal, nonobstructive dilatation, and partial- or complete obstruction (O’Reilly et al. 1978, 1979, 1981). The major restriction of this technique is that the immature neonatal kidney with a very elastic (extrarenally) dilated pelvis creating a “wind kettle” effect does not allow accurate grading during the first months of life, often exhibiting equivocal results; sometimes, even a persisting normal and symmetrical renal function is observed in spite of a (initially) completely obstructive drainage pattern (Gordon 2001). This leads to repeated investigations with consecutive radiation burden. Furthermore, indications for surgery vary – some (particularly in America) focus in renal function and operate when split renal functions have deteriorated; in Europe, one more often relies on drainage patterns trying to indicate surgery before renal functional impairment occurs, and some even reject to operate infants under 1 year of age. And scintigraphy lacks anatomic details as sometimes necessary, e.g., for planning surgery; therefore, other anatomic imaging is usually required.
For decades, this additional anatomic imaging has been IVU – however, today, IVU has been replaced by (“anatomic”) MRU wherever available (see below). If IVU needs to be performed, adapted pediatric protocols with reduced number of well-timed and properly focused films should be applied. It should be avoided before 6 weeks of age, and contrast amount as well as radiography dose settings needs to be adapted to the patient’s age and weight (Riccabona et al. 2010).
There is no role for CT in infants and children with obstructive uropathy due to its high radiation burden, particularly if multiphase acquisitions are necessary because of the delay in the opacification of the collecting system.
Over the last years, MRU has been introduced into pediatric imaging. Using fast heavily T2-weighted (3D) sequences with breathhold or diaphragmatic triggering, the (dilated) pelvocalyceal system and ureter can be exquisitely demonstrated; note that previous hydration as well as the administration of furosemide is essential for an optimal filling (Sigmund et al. 1991; Nolte-Ernsting et al. 2001, 2003; Borthne et al. 2000, 2003; Grattan-Smith and Jones 2006). Additionally, gadolinium (Gd) can be administered intravenously which then allows the assessment of renal and vascular anatomy, renal parenchymal contrast uptake, as well as excretion and drainage of Gd in a single nonionizing investigation; this investigation has practically replaced IVU for most pediatric queries (Avni et al. 2002; Caldair et al. 2007; Riccabona et al. 2002b, 2004b; Perez-Brayfield et al. 2003; Rohrschneider et al. 2003; Boss et al. 2006; Riccabona et al. 2004b). A further refinement of this technique is diuretic dynamic functional Gd-enhanced MRU which now allows assessing renal perfusion, function, and drainage (see chapter “Functional imaging of the kidney”). This offers an ideal “one-stop-shop” imaging approach which – using dedicated software and protocols – enables quantification of (split and overall) renal function and (to a certain extent) urinary drainage as well as ureteral peristalsis (Rohrschneider et al. 2000a, b; Grattan-Smith et al. 2003; Jones et al. 2004; Vivier et al. 2009). The base for this assessment is continuously and repeatedly acquired T1-weighted 3D gradient-echo sequences that need to have signal linearity with contrast concentration. The calculations are based on similar models as scintigraphy (“area under the curve technique” – also applicable for drainage assessment) (Rohrschneider et al. 2000a, b, 2002). Alternatively, the equation is based on an unidirectional 2-compartment model, using the abdominal aorta as the reference to define perfusion and thus defining parenchymal Gd handling after the deduction of the intravascular component (“Patlok plot” – restricted for drainage assessment) (Hackstein et al. 2003, 2005; Grattan-Smith et al. 2008a, b). However, availability of these methods is still restricted, they are not standardized or homogenized and have not spread out yet, and infants as well as small children will need sedation for the MR study. Currently, efforts are undertaken to overcome some of these restrictions; software is being distributed on the Internet, and recommendations for standardization are being prepared (Darge et al. 2009; Riccabona et al. 2010). Therefore, many envision MRU to be the major imaging tool for pediatric uroradiology – still, a comprehensive initial US and the differentiation of refluxive vs. obstructive disease (particularly, also in patients with megaureter) will remain indispensable. Due to its restrictions and availability, MRU is at present often used only for complex cases with complicated disease or scintigraphically equivocal findings with repeated follow-up examinations.
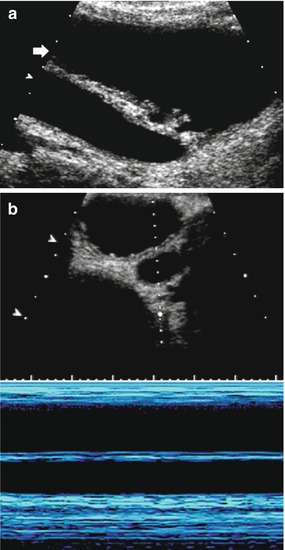
4.4 Imaging Algorithm in Obstructive Uropathy
In megaureter, US and ce-VUS or VCUG usually constitute the initial assessment. MAG3 scintigraphy is then used for the quantification of renal function and urinary drainage. Usually, these conditions do not need early intervention; thus, scintigraphy should be delayed until reliable values can be obtained (third to sixth month of life). Follow-up heavily relies on US – all investigations however need to be performed under standardized hydration (enabling conclusive comparisons). Besides measuring ureteral and pelvocalyceal width with full and empty bladder and assessing potential renal parenchymal changes (such as parenchymal narrowing, cystic/dysplastic areas, increased parenchymal echogenicity, lack/vanishing of corticomedullary differentiation), observation of ureteral peristalsis can be crucial for therapy decisions (Fig. 13). Video-clip cine-loop documentation or m-mode US improves the comparison of peristalsis during follow-up and thus improves the detection of impairment or deterioration (i.e., increasing hyperperistalsis in the aggravation of stenosis, lack of peristalsis in adynamic-dysplastic megaureter segments or in ureteral exhaustion) (Riccabona et al. 1998b). In complex conditions (e.g., duplex kidney, ectopic insertion), MRU is the modality of choice for additional imaging of (preoperative) anatomic and – by additional delayed acquisitions – drainage details (Avni et al. 1997, 2001, 2002; Gylys-Morin et al. 2000; Riccabona et al. 2004a). Only rarely – in infected systems that do not respond to intravenous antibiotic treatment or in bilateral obstruction with renal failure – percutaneous nephrostomy under US guidance needs to be performed (Riccabona et al. 2002b, c). This access can then (after successful treatment) be used for antegrade pelviureterography sometimes helping to decide between primary ureteral reinsertion and temporary diversion via ureterostomy. Conditions such as ureteroceles, segmental dysplasia, or severe cystic dysplastic kidneys that resemble multicystic dysplastic kidneys (MCDK) and are sometimes difficult to differentiate from chronically decompensated high-grade obstructive uropathy (and probably are just the other end of the same spectrum) are addressed in chapter “Congenital and development disorders of the kidney” (Fig. 14).