Lower respiratory tract infection (LRTI) remains a major cause of morbidity and mortality in children. Various organisms cause LRTI, including viruses, bacteria, fungi, and parasites, among others. Infections caused by 2 or more organisms also occur, sometimes enhancing the severity of the infection. Medical imaging helps confirm a diagnosis but also plays a role in the evaluation of acute and chronic sequelae. Medical imaging tests help evaluate underlying pathology in pediatric patients with recurrent or long-standing symptoms as well as the immunocompromised.
Key points
- •
Lower respiratory tract infections (LRTIs), caused by various pathogens, either alone or in combination, remain a substantial cause of morbidity and mortality in children.
- •
Medical imaging can demonstrate both acute and chronic complications of LRTI in children.
- •
Chest radiograph is usually the initial imaging tool in the evaluation of LRTI in children. Other imaging modalities, including ultrasound, computed tomography, and magnetic resonance imaging, all play important roles, depending on the clinical condition. It is important to understand the appropriateness of each imaging modality for a particular indication to maximize its utility.
- •
Cross-sectional imaging is recommended for further evaluation of patients with recurrent or persistent chest radiographic abnormalities to exclude underlying congenital lung and airway malformations or other disorders.
- •
There is a wide spectrum of imaging manifestations of LRTI in children, and, although some demonstrate typical imaging features, it is often that these imaging features do overlap.
Introduction
Lower respiratory tract infection (LRTI) in children is a spectrum of illness that may affect the peripheral airways (bronchiolitis) or the alveoli (pneumonia), but these can occur sequentially or simultaneously. There has been a considerable decrease in the incidence and mortality from LRTIs in the past 20 years; however, they remain the single largest cause of death in children worldwide. Pneumonia can cause substantial morbidity in children, with potential development of various acute and chronic complications. In 2017, approximately 15% of mortality among children below 5 years old was due to pneumonia. The disease burden remains substantial in low-income and middle-income countries, where more than 90% of pneumonia deaths occur.
Etiologies of LRTI include various organisms, such as viruses, bacteria, bacteria-like organisms, mycobacteria, fungi, and parasites. Mixed pathogens, however, also can occur. Among hospitalized children with pneumonia, approximately 26% to 35% had mixed pathogens of various combinations believed to function synergistically and likely enhancing the severity of the infection.
Medical imaging has been used to confirm or exclude the presence of pneumonia, but it also is helpful in the evaluation of recurrent or persistent abnormalities, in assessment of acute or chronic complications, and to exclude other underlying pathologic processes. This article aims to review the various imaging modalities utilized in the evaluation of LRTIs in children, present systematic and evidence-based diagnostic imaging recommendations and algorithms, and discuss the spectrum of acute and chronic imaging manifestations of these conditions.
Evidence-based imaging recommendations and algorithm
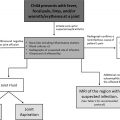
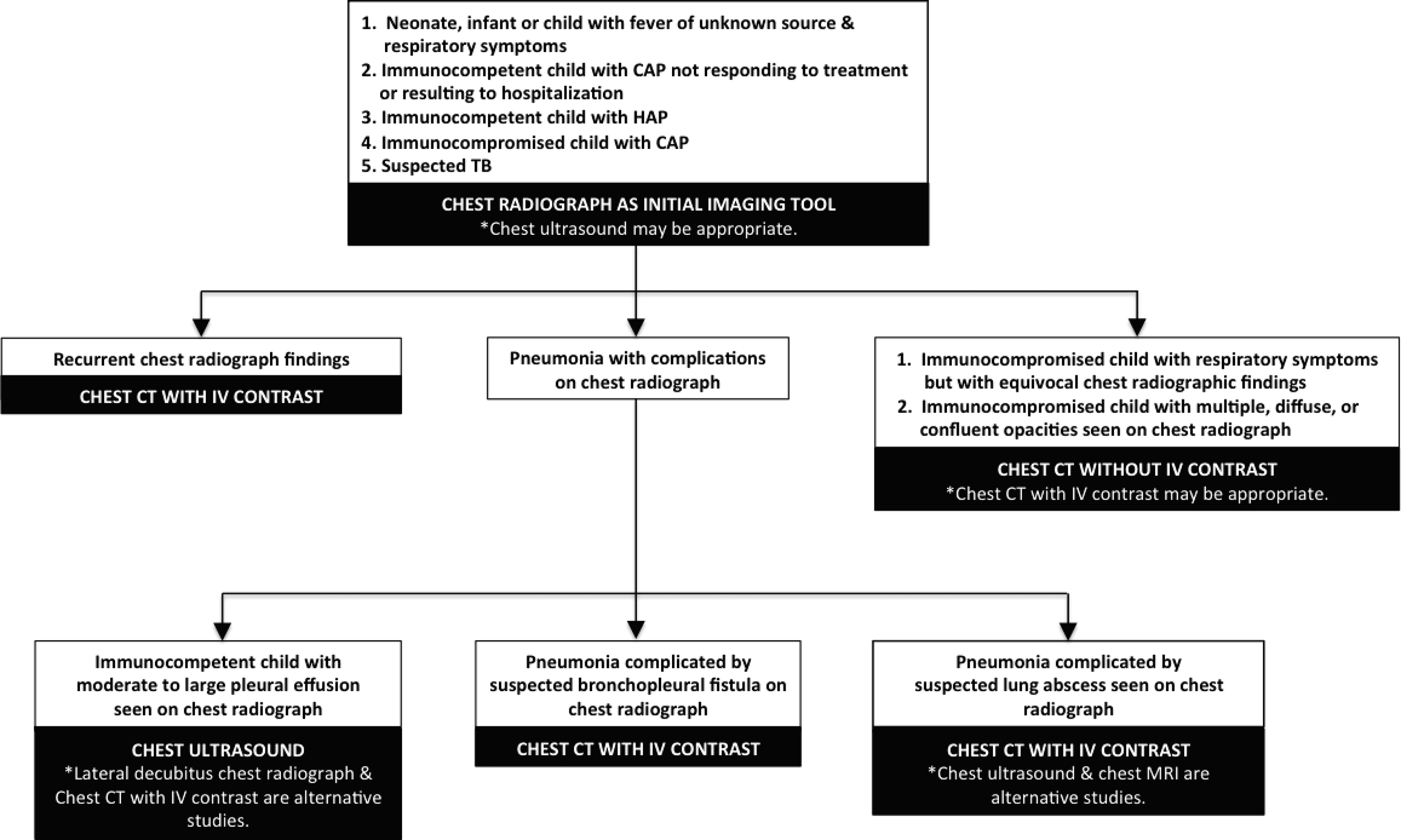
An extensive search of the medical literature, which includes peer-reviewed systematic reviews/meta-analysis, review articles, evidence-based guidelines, and consensus statements, was performed. In addition to American College of Radiology Appropriateness Criteria for pneumonia in the immunocompetent child, Appropriateness Criteria for acute respiratory illness in the immunocompromised patients, and Appropriateness Criteria for fever without source or unknown origin–child, many other references were utilized in order to generate various clinical scenarios in pediatric patients with LRTI along with corresponding recommended imaging tests ( Table 1 ). To analyze the strength of the references used, strength of recommendation (SORT) was utilized. The SORT category levels are the following: (A) recommendation is based on consistent and good quality patient-oriented evidence; (B) recommendation is based on inconsistent or limited quality patient-oriented evidence; and (C) recommendation is based on consensus, usual practice, opinion, disease-oriented evidence, or case series for studies of diagnosis, treatment, prevention, or screening. The SORT level of the references used in developing the recommendations falls under category C. After a careful synthesis of all the available information reviewed, which includes various clinical scenarios and recommended imaging tests, an evidence-based algorithm for children with LRTIs was developed ( Fig. 1 ).
| Imaging Recommendations | References | Comments |
|---|---|---|
| Chest radiograph is the initial imaging tool in the neonate, infant or child up to 36 mo with fever of unknown source and respiratory symptoms. | May disclose an occult pneumonia | |
| Imaging is not recommended in immunocompetent children with suspected uncomplicated community acquired pneumonia who does not require hospitalization. | , | Imaging does not change the management of these pediatric patients. |
| Chest radiograph is the initial imaging tool in immunocompetent children with community acquired-pneumonia that does not respond to initial outpatient treatment or requires hospitalization. | , | US may be appropriate. , |
| Chest radiograph is the initial imaging tool recommended in immunocompetent with suspected hospital acquired pneumonia. | US may be appropriate. , | |
| Chest radiograph is the initial imaging tool in immunocompromised children with cough, dyspnea, chest pain, or fever. | Chest CT without or with contrast may be appropriate. | |
| Chest radiograph is the initial imaging tool for a child when TB is suspected. | , | |
| US usually is appropriate in immunocompetent children with pneumonia complicated with moderate or large effusion on chest radiograph. | A decubitus view chest radiograph or a chest CT with IV contrast also may be appropriate. | |
| Chest CT with IV contrast is an appropriate imaging tool for immunocompetent children with pneumonia complicated by suspected bronchopleural fistula seen on chest radiograph. | , , , | Chest CT without IV contrast may be appropriate. |
| Chest CT with IV contrast is an appropriate imaging tool in immunocompetent children with pneumonia complicated by suspected lung abscess seen on chest radiograph. | , , , , | US of the chest or chest MRI with IV contrast , may be appropriate. |
| Chest CT without IV contrast is appropriate in immunocompetent children with nonlocalized recurrent pneumonia seen on chest radiograph. | , , | To evaluate underlying pulmonary disease. such as bronchiectasis and interstitial or diffuse lung disorders, HRCT is appropriate. |
| Chest CT with IV contrast or chest CTA is appropriate studies for immunocompetent children with localized recurrent pneumonia seen on chest radiograph. | , , | Causes of recurrent localized infections include congenital bronchopulmonary malformations or possible foreign body aspiration. |
| Chest CT is appropriate in immunocompromised children when chest radiographs are equivocal. | , , , , | Chest CT without IV contrast usually is appropriate, but IV contrast can be given depending on the clinical indication, which includes suspected TB. |
Practical imaging approach to lower respiratory tract infections in children
Radiography
The chest radiograph (chest x-ray) is widely available and the imaging examination performed most frequently not only in adults but also in children. This imaging modality can serve as a screening tool for the detection of lung parenchymal opacities or as a monitoring tool to evaluate response to therapy. , Prior to a radiologist’s interpretation of the radiograph, several parameters, including proper patient positioning, adequate inspiratory effort, and acceptable radiograph exposure, initially must be assessed and satisfied to determine the diagnostic quality of the films or images.
A frontal projection of the chest, either in anteroposterior or posteroanterior view, may be sufficient to demonstrate presence of lung opacities, but a lateral view also is recommended in children to better detect other associated findings, such as lymphadenopathy and minimal pleural effusion, among others. , , A lateral decubitus view, right or left, depending on the perceived abnormality on the frontal chest radiograph, also may be obtained in some cases: (1) to assess presence of minimal free pleural fluid in situations where the lateral costophrenic sulcus is blunted or (2) to differentiate a loculated from a nonloculated pleural effusion. Thelow specificity of the imaging findings, however, makes the evaluation of pediatric patients with suspected pulmonary infections challenging because of various potential causes that have similar radiographic characteristics. For example, a chest radiograph cannot accurately differentiate between viral and bacterial pneumonia. , , ,
Ultrasound
Ultrasound (US) is used primarily in the evaluation of pleural fluid, better characterizing the nature, type, presence of septations, and volume of pleural effusion. , In a radiographically opaque hemithorax, US also can differentiate pleural effusion from consolidation. Recent technological advancements in US also have improved the spatial resolution, tissue penetration, and characterization of the lung parenchyma. Moreover, the chest of infants and young children has less subcutaneous fat than adults with partially or unossified ribs and sternum, making it easier to perform transosseous scanning. ,
Chest radiographs are less sensitive in the detection of small pleural fluid, but US is able to detect even less than 10 mL of pleural fluid, especially when a patient is in an upright position. , , , US has other various advantages over the otherimaging modalities, including its portability and real-time scanning, without a need for sedation or exposure to ionizing radiation.
US may demonstrate lung consolidation as heterogeneous, low echogenicity similar to the liver, also known as lung hepatization. Bronchograms within the solid-appearing lung may be seen either as multiple, linear, branching bright dots within the lung (sonographic air bronchograms) or as hypoechoic branching structures filled with entrapped fluid or mucoid material within bronchi (sonographic fluid bronchograms) in pediatric patients with necrotizing or postobstructive pneumonias. Complications in pneumonia, such as abscess and empyema, also are readily visualized in US. Color Doppler imaging also may be helpful in detecting necrotizing pneumonia even without the use of intravenous (IV) contrast media.
Computed Tomography
Computed tomography (CT) is a highly useful imaging tool in the evaluation of the thorax, including the lungs, mediastinum, and chest wall in infants and children. Multidetector CT and dual-energy CT technologies with advanced multiplanar and 3-dimensional (3-D) reconstructions as well as artificial intelligence software currently are available, not only increasing image quality and lesion detection but also decreasing the sedation rates, scanning time, and radiation dose, which are of particular importance in infants and young children.
CT is considered an adjunct to conventional radiography , and often used in the evaluation of nonresponding pulmonary infection as well as its complications. , Unlike chest radiography, where there is superimposition of anatomic structures, CT provides cross-sectional images where pattern and distribution of pulmonary diseases are better visualized than chest radiography. , Various scanning protocols are available for different clinical indications, such as high-resolution CT (HRCT) or CT angiography (CTA) but usually are not appropriate for evaluation of acute respiratory illnesses.
Iodinated contrast agents may be administered IV and greatly improve the image quality by enhancing the differentiation of various tissues in the body, which could help radiologists distinguish normal structures from abnormal findings.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is an imaging modality that has no ionizing radiation. It uses strong magnetic fields and gradients as well as computer-generated radio waves to produce multiplanar images of various parts of the body. MRI, however, is less sensitive compared with CT in the evaluation of the lung parenchyma due to the lung’s inherent low proton density, high loss of signal due to susceptibility, and substantial cardiac and respiratory motion artifacts. , , Scan times also are longer, increasing the need for sedation in infants and young children as well as those pediatric patients with claustrophobia.
Even with disadvantages, pathologic processes with high fluid or proton content, such as pneumonia, pulmonary edema, and pleural and pericardial effusion, are readily depicted on MRI. In addition, various technological advances in the imaging protocols with electrocardiograph and respiratory gating as well as motion correction, the lungs now can be examined better than before. , , Gadolinium-based IV contrast media may be administered, similar to CT, for better detection of acute complications, such as necrotizing pneumonia, lungabscess, and empyema.
Spectrum of lower respiratory tract infections in children
Viral Infections
Demographics and clinical symptoms
Viruses are common causes of respiratory tract infection among children and are substantial causes of morbidity and mortality. , It often is difficult to determine the underlying etiology of pneumonia in a child, but a patient’s age can be helpful. , , Viral pneumonia is rare in neonates because of maternal antibody protection, but viruses are the most frequent cause of community-acquired pneumonia (CAP) in infants older than 4 months and in preschool-aged children (95%). For school-aged children, viral infections also are common but the incidence of bacterial infection increases. , , , In developed countries, infants and preschool-aged children experience a mean of 6 to 10 viral infections annually, and school-aged children and adolescents experience 3 to 5 illnesses annually. In hospitalized children with pneumonia, viral pathogens were detected in 73% of patients, and bacteria were seen in 15%.
The clinical signs and symptoms of viral pneumonia are highly variable, usually are nonspecific, and depend on the infectious agent, patient age, and state of immunity. , Affected pediatric patients typically present with cough, coryza, and wheezing, although severe respiratory distress that requires hospitalization also may occur.
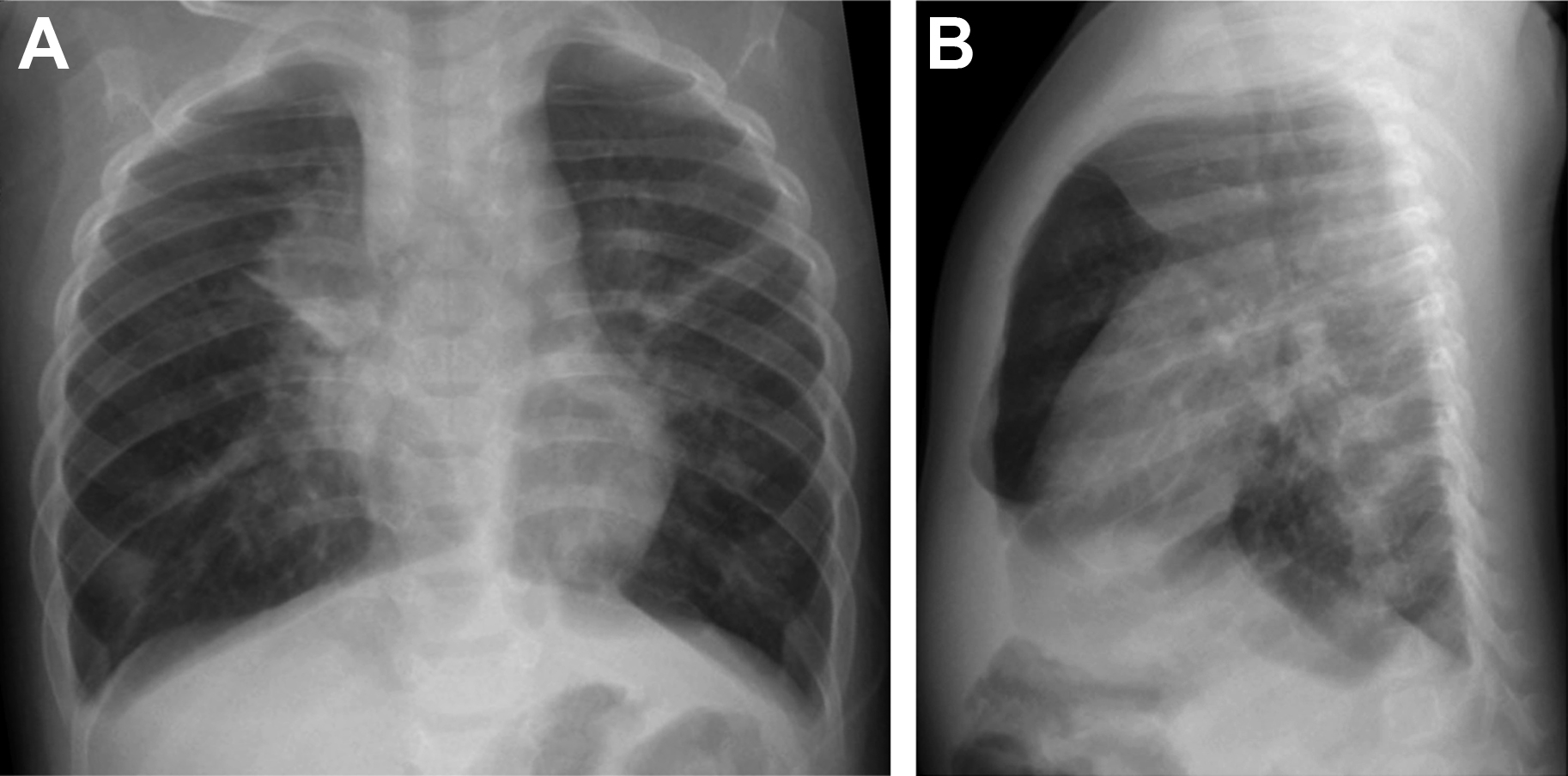
Following inhalation of the viral agent in the nasopharynx and upper respiratory tract, the virus migrates inferiorly to small airways and alveoli. , There is resultant inflammation and necrosis of the ciliated epithelial cells, goblet cells, and bronchial mucous glands that lead to bronchial and bronchiolar edema, often with involvement of the peribronchial tissue and interlobular lung septa. The affected small airway reacts with bronchoconstriction and increased mucous secretion, which narrows the already small airways causing peripheral atelectasis and hyperaeration ( Fig. 2 ). Both small airways and lung parenchymal changes do occur. , , Coinfection with 2 or more microbial agents can happen and a majority of mixed infections are viral-bacterial infections, but viral-viral coinfections also are seen. ,
DNA viruses
Adenovirus is an important cause of acute respiratory illness in children, accounting for 5% to 17% of all respiratory infections, and can occur endemically or in epidemics. , The peak incidence of infection in children is 6 months to 5 years. They can cause necrotizing bronchitis, bronchiolitis, bronchopneumonia, and severe respiratory disease in up to 20% of children, with fatality rates from 5% to 12%. , LRTI from DNA viruses can develop frequent sequela (22%–30%) as postinfectious bronchiolitis obliterans (PIBO), bronchiectasis, and Swyer-James-McLeod syndrome. , , , Varicella zoster virus is the etiologic agent of varicella or chickenpox and belongs to the herpesvirus family. The virus becomes latent in the neural tissue and can be reactivated later, causing herpes zoster. Infection generally is mild, but there is risk of complications in neonates and immunocompromised pediatric patients. , Varicella pneumonia is a serious complication, with an incidence from 5.6% to 30.3%. Cytomegalovirus (CMV) is the largest and most complex member of the herpes virus family, and can remain latent and with potential for reactivation. It is a major cause of morbidity and mortality in the immunocompromised pediatric patients. , ,
RNA viruses
Respiratory syncytial virus (RSV) is a common pathogen in children younger than 5 years old, , , , , and there is great risk of infection in premature infants and children with chronic lung disease and heart disease. Infection includes upper respiratory infection, bronchiolitis, pneumonia, and respiratory failure. , Measles is a contagious worldwide infectious disease transmitted by direct contact that causes substantial mortality in children. , , Typical symptoms with characteristic maculopapular skin rash and Koplik spots develop 10 days to 12 days after the exposure and last 7 days to 10 days. It may involve the airways, leading to bronchitis, bronchiolitis, and pneumonia (6%–15%). , RNA viruses also cause neurologic complications (encephalitis) and can suppress the immune system, which predisposes a child to other infections. , , Rhinovirus is a common etiology of childhood viral upper respiratory tract infections worldwide (18%–27%) but affects the lower respiratory tract less commonly, leading to bronchiolitis and pneumonia. , ,
There are 3 groups of influenza virus, types A, B, and C. Type A and occasionally type B can cause viral pneumonia. , One of the most severe outbreaks of this infection was the pandemic of 1918, Spanish influenza, resulting in approximately 50 million deaths. Influenza A can be classified in subtypes. Influenza A virus subtype H1N1 is a zoonotic virus of swine origin. It was discovered in Mexico and was known as the cause of the swine flu pandemic of 2009. Infection usually presents with nonspecific symptoms, but pneumonia and severe pulmonary complications as acute respiratory distress syndrome (ARDS) can occur, due to an exaggerated inflammatory response. , , , Influenza virus A subtype H5N1 is the causative agent of avian flu. Infection occurs after close contact with infected birds or their products. It first was isolated in Hong Kong in 1997 and later spread to other countries. The symptoms are fever, cough, diarrhea, shortness of breath, lymphocytopenia, and thrombocytopenia. Fatal and rapid progression to ARDS resulting in death could exceed 60%. ,
Coronaviruses (CoVs) have an approximately spherical shape with prominent spike projections resembling a crown. They can infect birds, humans, and others vertebrates. CoVs can cause mild to moderate upper respiratory tract infection in healthy individuals, but they also can infect lower respiratory tract, causing severe or fatal pneumonia and even ARDS. , The most highly pathogenic are, severe acute respiratory syndrome CoV (SARS-CoV), Middle East respiratory syndrome CoV (MERS-CoV), and SARS–CoV-2.
The SARS-CoV was detected in Guangdong province, China, in 2002. Chinese ferret-badgers, raccoon dogs, and masked palm civets are the animal hosts for this virus. During 2002 and 2003, the infection spread to 29 countries, with 8098 infected individuals and 916 deaths. The transmission was via respiratory droplets, and the infected patients presented with flulike symptoms. Affected pediatric patients can develop pneumonia and lymphocytopenia. Children, especially under the age of 10 years, have a mild clinical course, but adults can develop severe complications (ARDS). , , , The MERS-CoV was detected in Saudi Arabia, where camels and bats are reservoirs. It spread across the world, but 80% of cases occurred in Middle East Asia. A total of 2254 individuals were infected, and 800 deaths occurred between 2012 and 2018. In the literature, there are few MERS-CoV infections in children, and 42% are asymptomatic. In adults, the infection can progress to pneumonia, ARDS, multiorgan failure, and death. , , The SARS–CoV-2 was detected in Wuhan, China, in December 2019, and on March 11, 2020, the World Health Organization (WHO) declared this infection as a pandemic. The transmission is via respiratory droplets and fomites, with an incubation period of 5 days to 6 days. Children are affected less severely than adults, and most cases are asymptomatic or with mild moderate disease. , , General hospitalization is approximately 4% in children. Typical symptoms are fever, diarrhea, rash, conjunctivitis, and, in severe illness, edema, shock, myocardial dysfunction, and neurologic findings. Multisystem inflammatory syndrome also was reported in children. ,
Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome. Children represent 2% of the HIV reported cases. Most children are infected after vertical transmission from their mothers, but there are other causes, such as sexual transmission, illicit drug use, and blood transfusions. , HIV damages or destroys vital cells in the human immune system; therefore, pulmonary infections are a highly important cause of morbidity and mortality. , , These pediatric patients are highly susceptible to viral, bacterial, fungal, protozoal, and opportunistic infections. , , , Lymphocytic interstitial pneumonia can occur in these pediatric patients. , Imaging findings of lymphocytic interstitial pneumonia are diffuse, symmetric, reticular, or round opacities, which can coalesce and form pulmonary opacifications. , , , These imaging findings should be distinguished from TB infection. , , ,
Imaging of viral lower respiratory tract infections
Chest radiograph remains the mainstay of pneumonia imaging, supplemented with US and CT for specific indications. , , It can be normal in children with mild disease, but peribronchial inflammation manifests as symmetric and bilateral peribronchial interstitial opacities radiating from the hila and extending peripherally, with segmental and subsegmental areas of atelectasis. There also is hyperaeration due to the narrowing of the distal airway lumen due to bronchial wall edema, and mucous plugging, resulting in air trapping and subsequent hyperinflation. This is shown well on the chest radiographs as hyperlucency, increased transverse and anteroposterior chest diameter, and flattening of the diaphragms , , (see Fig. 2 ). The radiographic pattern of viral pneumonia, however, can be diverse and nonspecific because it can be seen in nonviral LRTIs as bacterial pneumonia and other diseases, such as asthma. , , Viral infection also can mimic the radiological findings of bacterial infection, with bilateral areas of consolidation. , This can occur in pandemic virus infections, such as influenza A H1N1, influenza A H5N1, and CoVs, in which initially it can appear as focal or diffuse interstitial opacities but rapidly progress to bilateral areas of consolidation , ( Figs. 3–5 ). In varicella and CMV infection, the imaging findings are ground-glass opacities (GGOs) or diffuse nodular opacities that may progress to large segmental areas of patchy airspace disease. Total clearing is expected but punctate calcifications could be seen after a varicella pneumonia. So, viral pneumonia can have a diverse imaging appearance on chest radiograph depending on the infecting agent, and the host response that depends on age, immune status, and presence of underlying comorbidity. The chest radiograph alone is a poor indicator of specific causative agent of pneumonia, but, with inclusion of clinical and laboratory data, sensitivity can increase. , ,
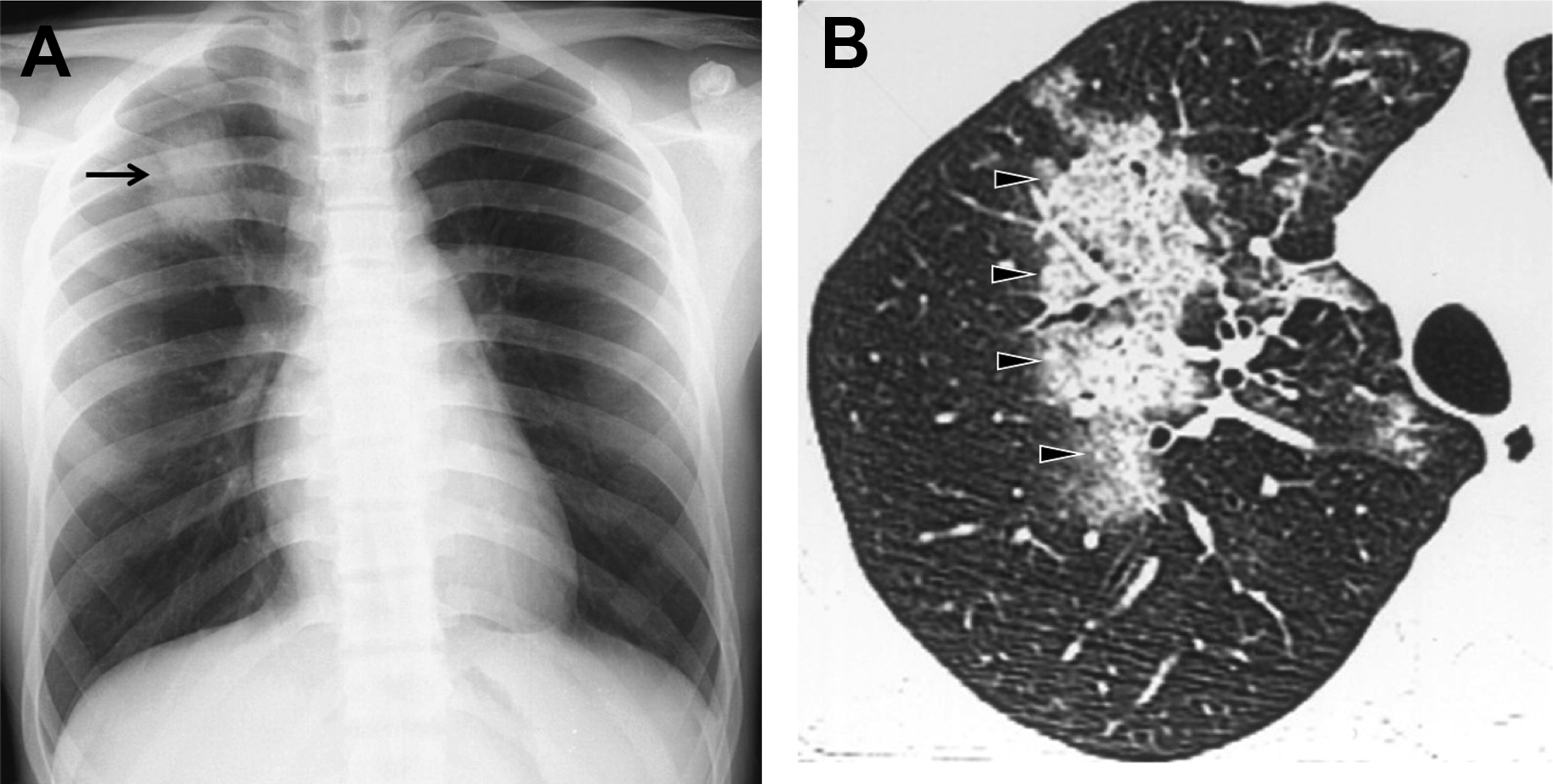
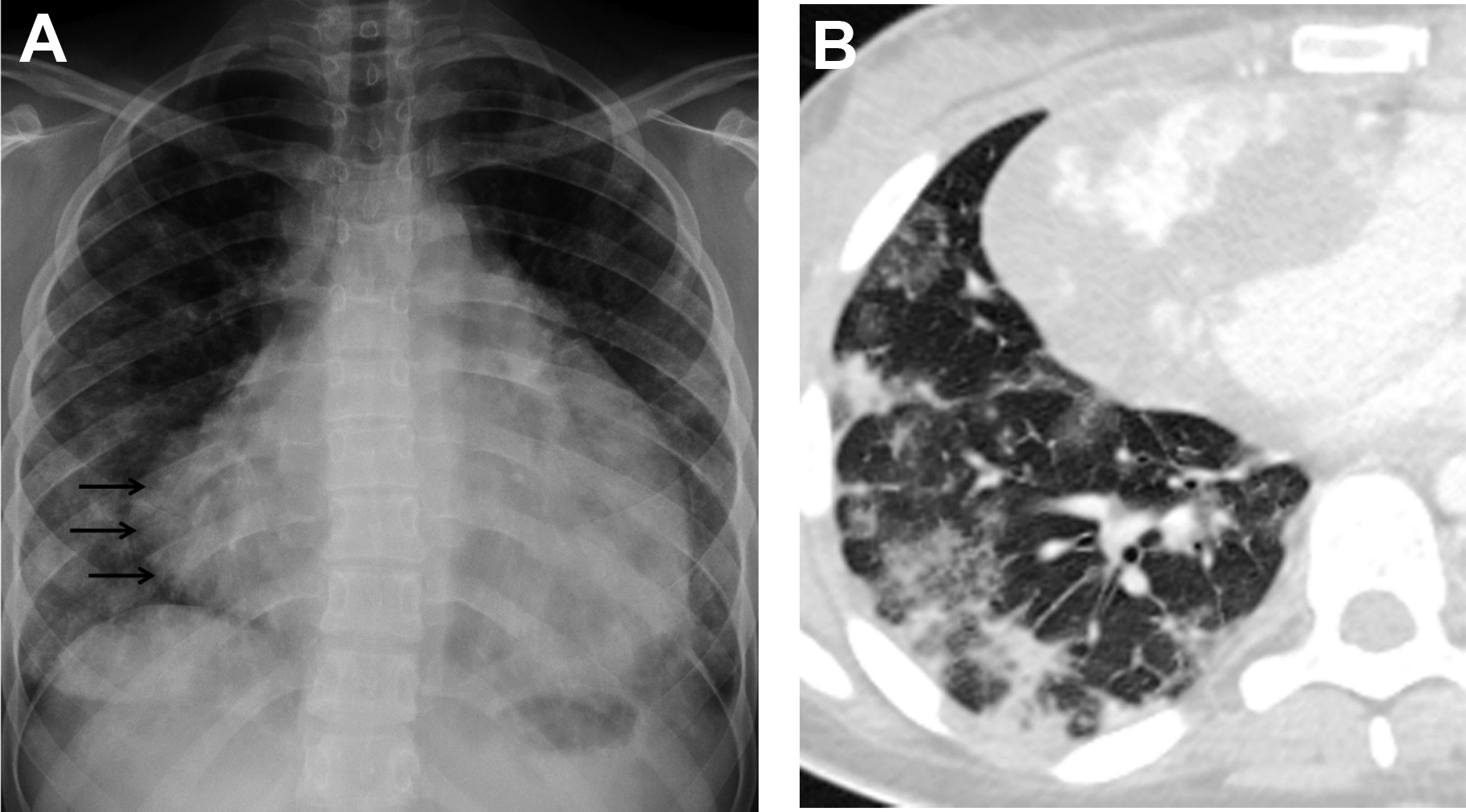
CT is a useful adjunct to chest radiograph in selected cases, and HRCT is the modality of choice to evaluate lung parenchyma. , , , The wide spectrum of CT findings of viral LRTIs includes patchy mosaic perfusion pattern, GGOs, consolidation, micronodules and tree-in-bud opacities, interlobular septal thickening, and bronchial and bronchiolar wall thickening with areas of hyperaeration and atelectasis. , GGOs with or without consolidation is the predominant finding in viral pneumonia , , , (see Figs. 3 and 4 ). The halo sign, which is seen as a focal consolidation with a rim of GGO, may be found in 50% of SARS–CoV-2 infection , ( Fig. 5 ).
Complication of viral lower respiratory tract infections
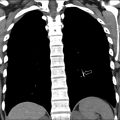
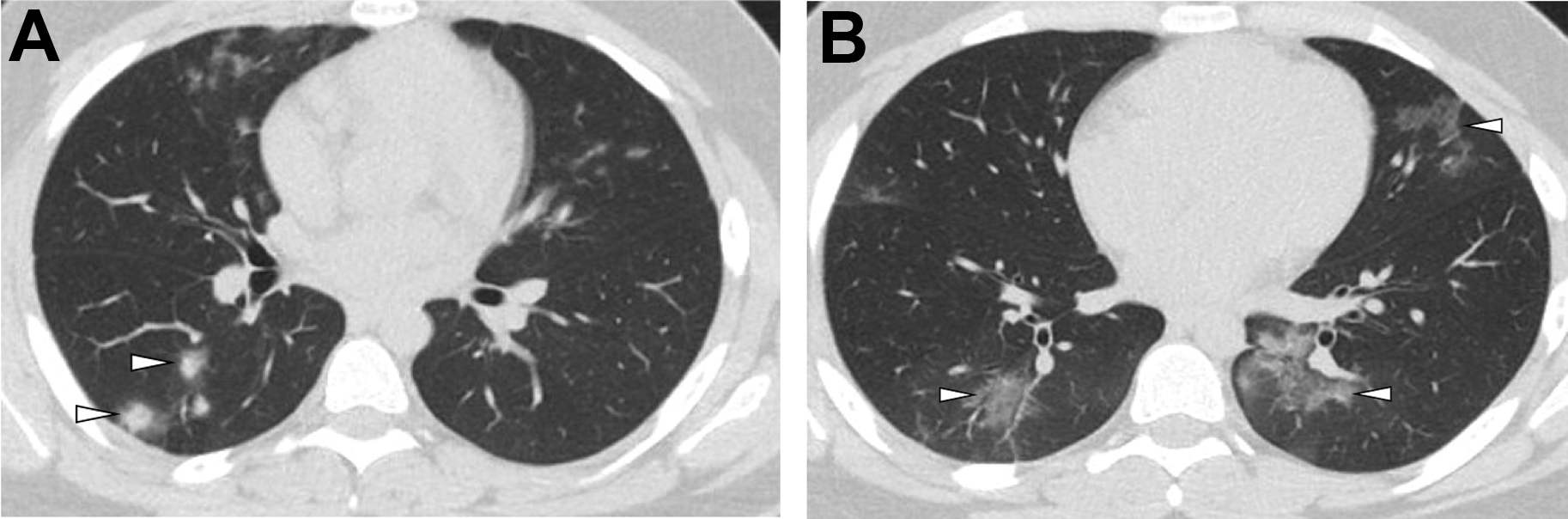
The most frequent acute complication of viral pneumonia in children is a superimposed bacterial infection and immune reconstitution inflammatory syndrome (IRIS) in HIV. , , , The most common chronic complications of viral pneumonia are bronchiectasis, PIBO, or Swyer-James-MacLeod syndrome. The radiological findings for bronchiectasis could be subtle on chest radiograph, but they are well shown on CT, where the bronchus is dilated and larger than its accompanying pulmonary artery. Chronic bronchiectasis commonly is seen with adenovirus infection ( Fig. 6 ).
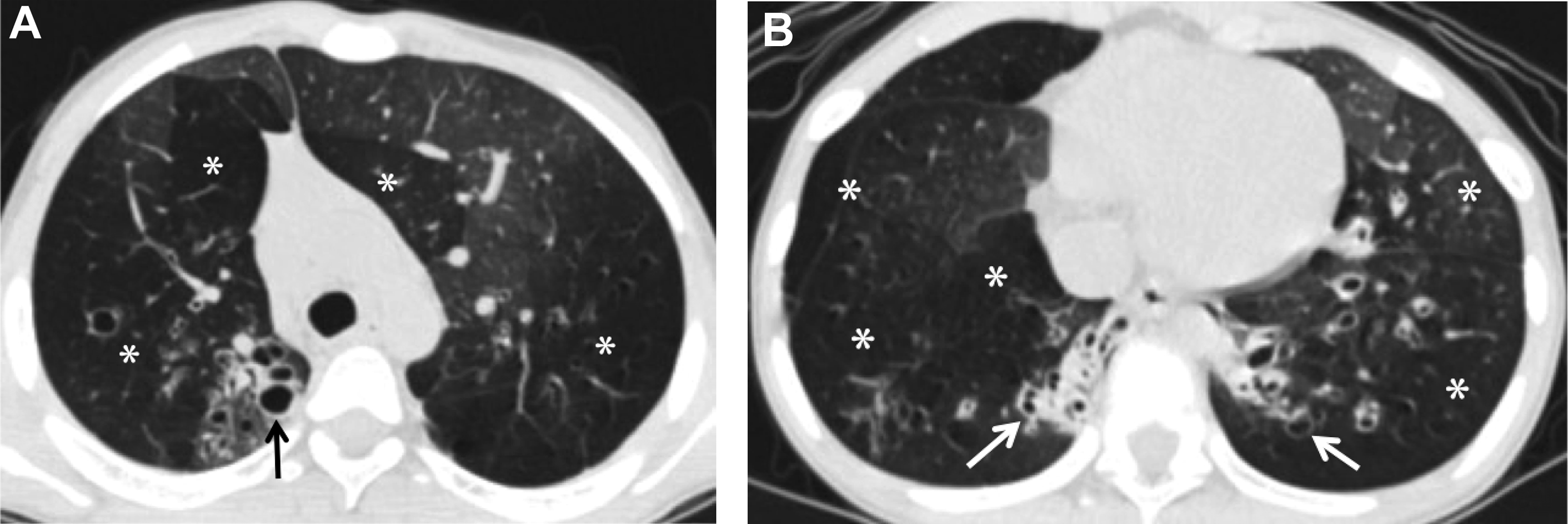
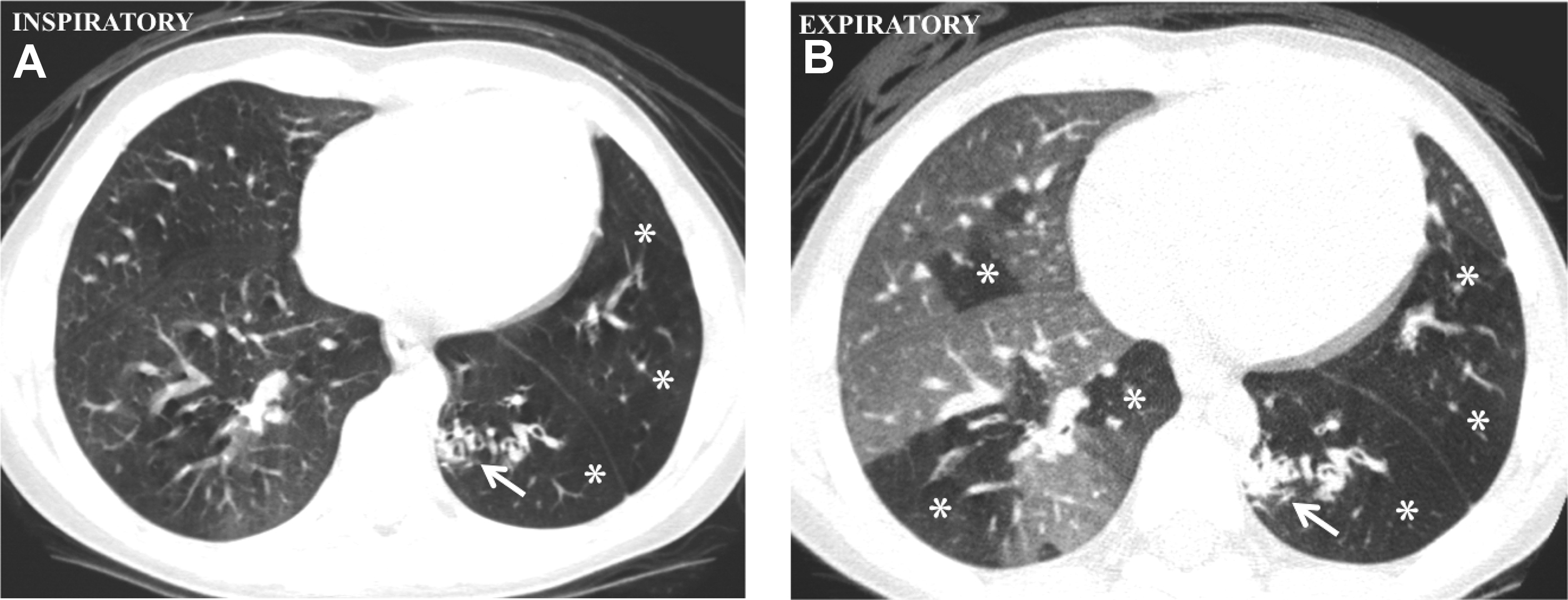
PIBO is an irreversible, chronic, and obstructive complication of viral pneumonia and can be seen as sequela of adenovirus, RSV, parainfluenza, measles, and CMV. , , , Radiographic findings of PIBO usually are nonspecific and even can appear normal but also can show hyperlucent lung, atelectasis, confluent densities, peribronchial thickening, and bronchiectasis. , , CT is the best radiological examination to analyze PIBO findings, demonstrating mosaic perfusion pattern, peribronchial thickening, bronchiectasis, atelectasis, and reduced vessels size in low-attenuation areas, indicating diminished perfusion, air trapping, and sometimes lung volume reduction , , , , (see Fig. 6 ). Swyer-James-MacLeod syndrome is a form of PIBO, where the lung involvement typically is unilateral, affecting the entire lung or lobe, although it also can affect both lungs. Characteristic findings on chest radiographs are hyperlucency due to pulmonary hypoperfusion, small pulmonary artery with small hilum, reduced volume of the affected lung or lobe, and air trapping. CT shows these findings in more detail and precision. , , Expiratory CT imaging could be useful to better demonstrate the air trapping and hyperlucencies, especially in detecting bilateral lung involvement (30%) , ( Fig. 7 ).
Bacterial Infection
Demographic and clinical symptoms
Viral infections are encountered more commonly than pyogenic bacteria in CAP. Even after the new conjugate vaccines have become more widely available, however, bacterial infections still result in severe and/or complicated pneumonia that sometimes requires hospitalization and even mortality. , , , In addition, viral-bacterial coinfections (7%–34.6%) of RSV and potentially pathogenic bacteria augment the disease severity. , , , , Common bacterial agents causing pneumonia in immunocompetent hosts vary by age, whereas in immunocompromised hosts bacterial agents also may vary depending on the types of immunologic defects as well. , , Children without fever or respiratory tract symptoms are unlikely to have pneumonia. Nonspecific and nonrespiratory signs and symptoms, such as irritability, malaise, pleuritic chest pain, abdominal pain, or stiff neck, are not uncommon, especially in infants and smaller children. , , , , , ,
Imaging of bacterial lower respiratory tract infections
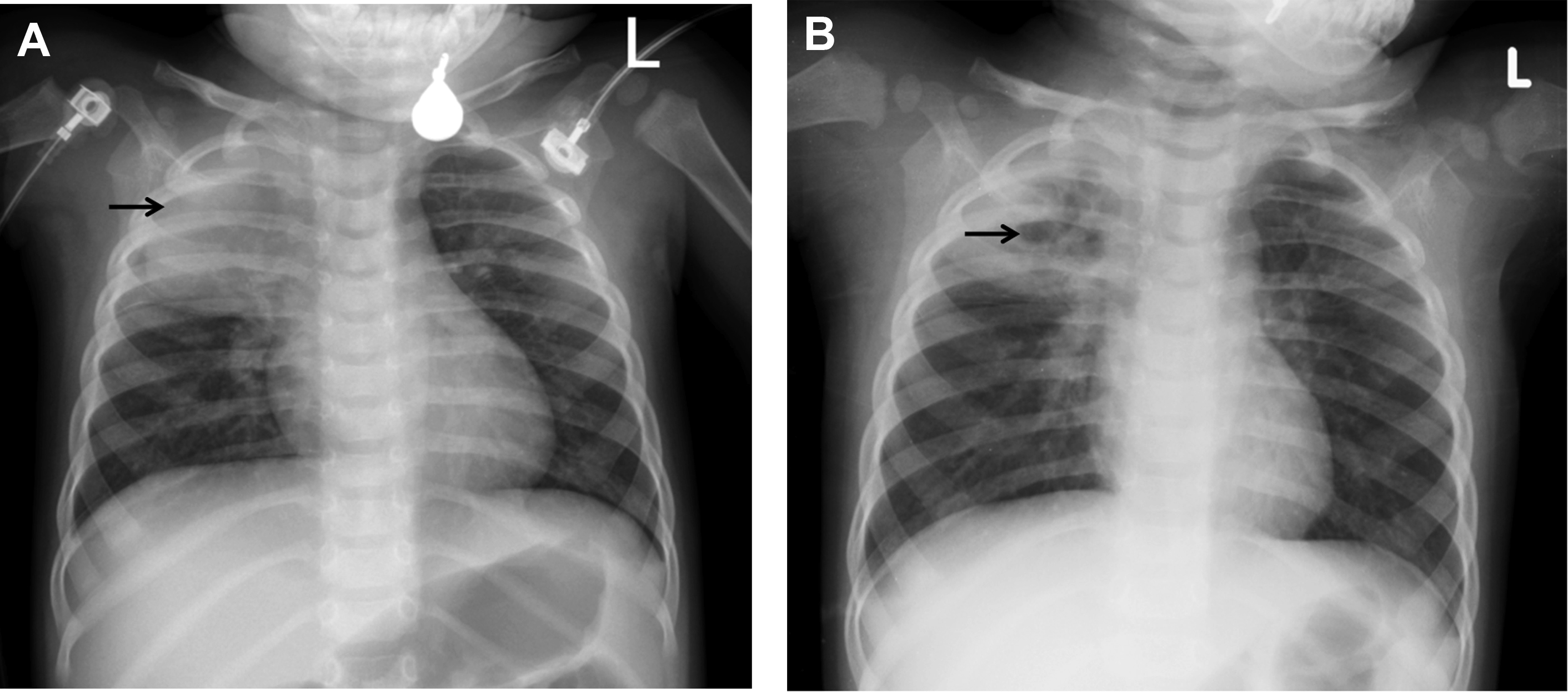
Bacterial pneumonia tends to have unilateral, segmental or lobar airspace opacification. , , , , , This alveolar pattern or endpoint consolidation, categorized by the WHO as radiographic definition for identifying pneumonia, has a high sensitivity (93%) and high negative predictive value (92%) in diagnosing bacterial pneumonia and high agreement in imaging interpretation , ( Fig. 8 ). Expansile pneumonia can be found in Klebsiella pneumoniae and Staphylococcus aureus infections. The chest radiographs, however, are not able to differentiate causative agent and interstitial (or nonendpoint) opacities seen in both viral and bacterial pneumonia. , , Round pneumonia, a unique pattern seen in children less than 8 years of age, can mimic tumor or developmental pulmonary malformations , , ( Fig. 9 ). The chest radiograph is not recommended in a child who appears well and can be treated as outpatient. Follow-up chest radiographs at 2 weeks to 4 weeks should be reserved for those with persistent or recurrent symptoms or patients with immunodeficiency. , , ,
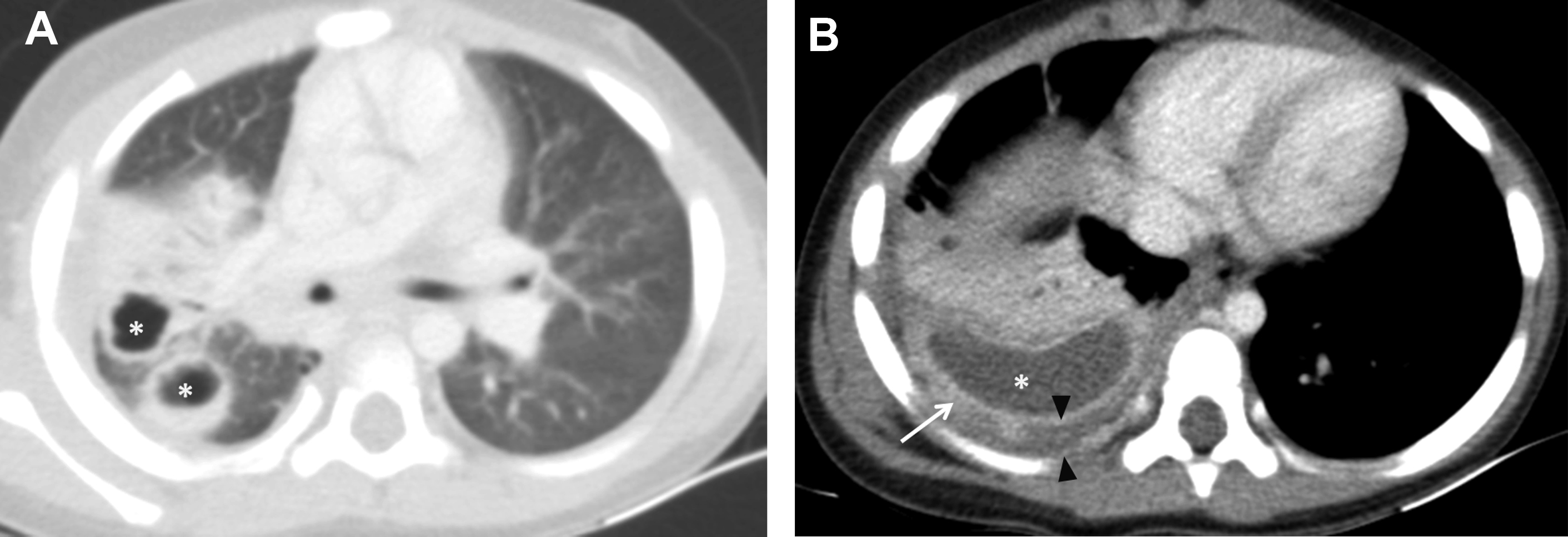
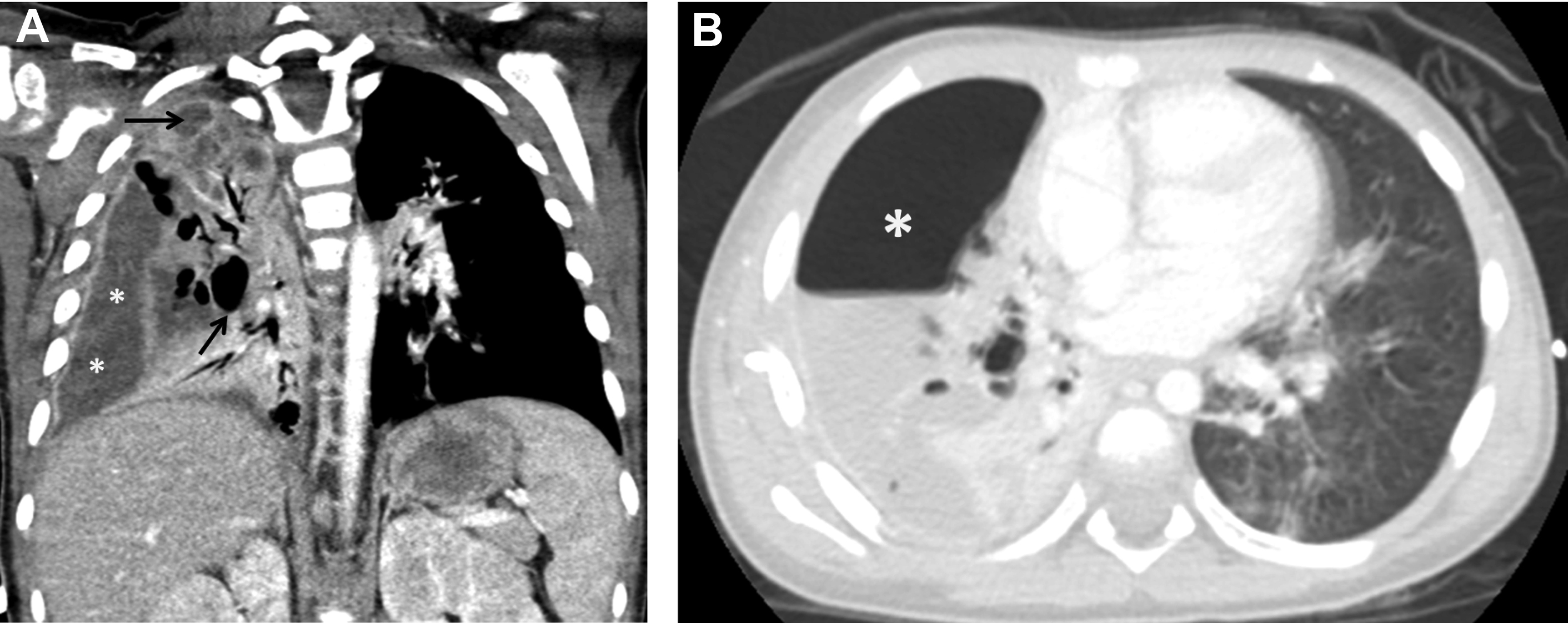
Complication of bacterial lower respiratory tract infections
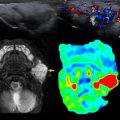
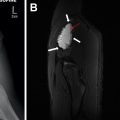
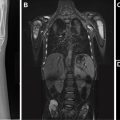
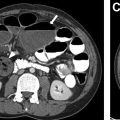
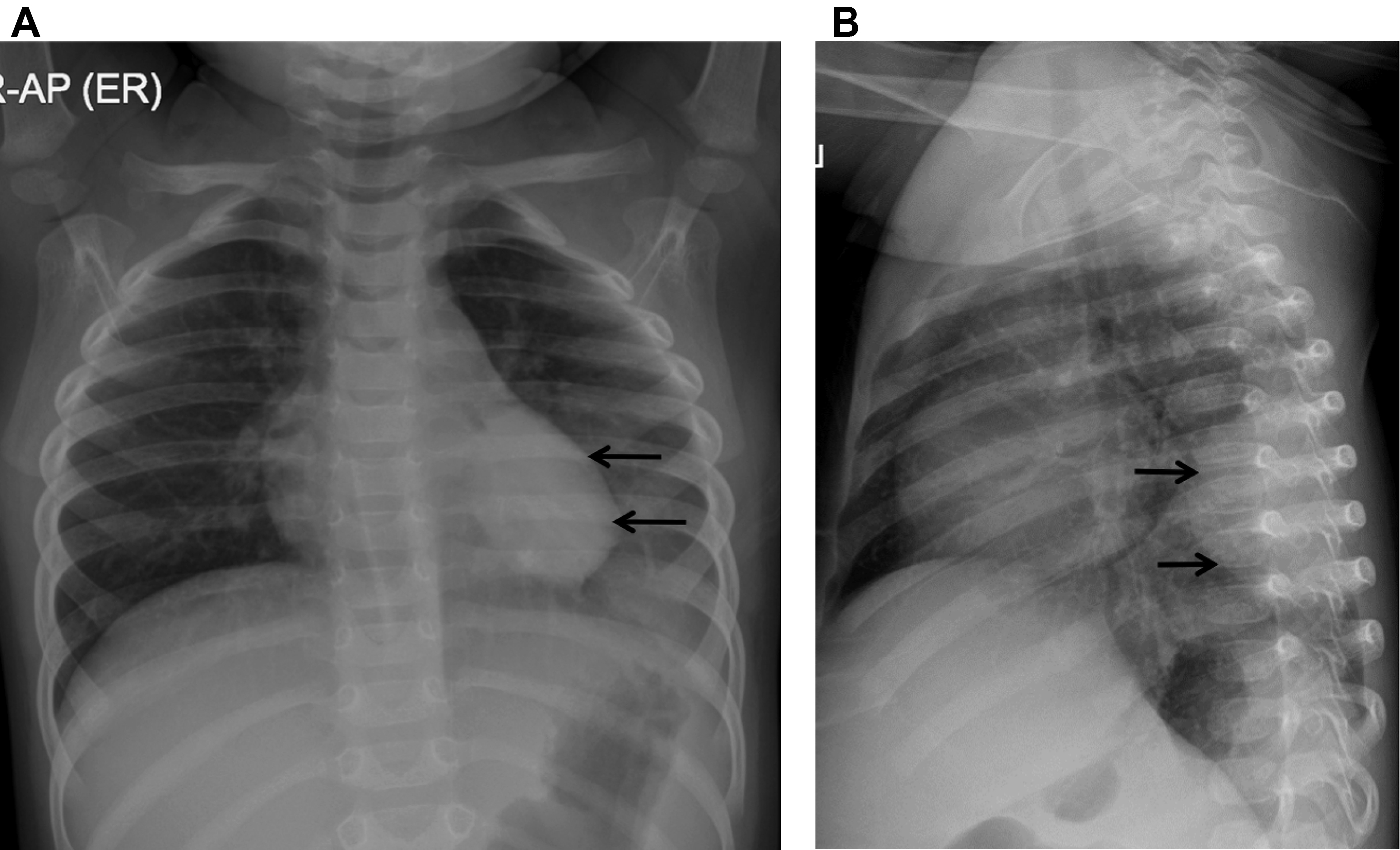
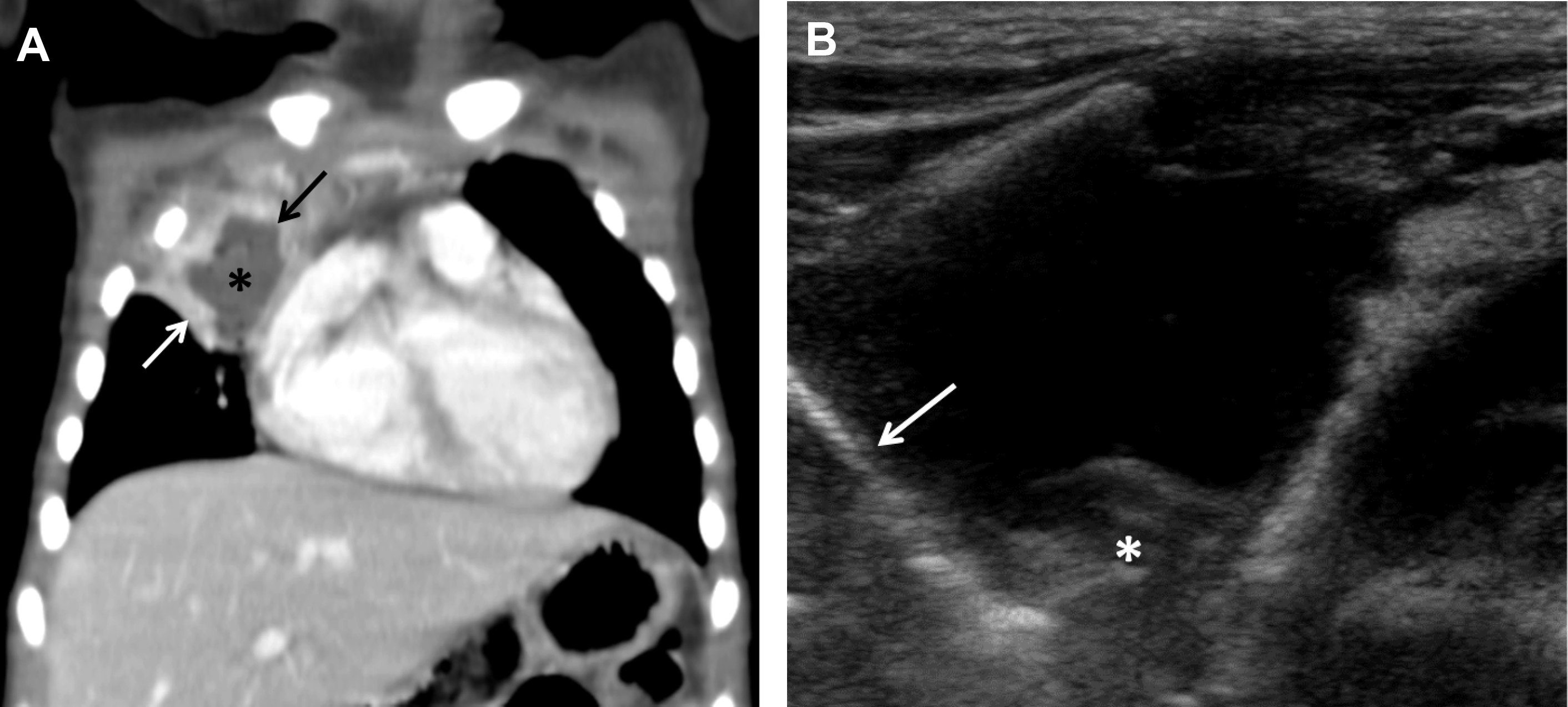
Complicated bacterial pneumonia is considered in healthy children who do not respond to treatment or in children with underlying comorbidities. Acute complications can be categorized as lung parenchymal complications (cavitary necrosis, lung abscess, pneumatocele, pulmonary gangrene, and bronchopleural fistula) and pleural complications (parapneumonic effusion and empyema) , , , , ( Figs. 10–12 ). Rare mycotic pulmonary arterial aneurysm may occur either as direct extension from necrotizing pneumonia or secondary septic emboli ( Fig. 13 ). Pneumatocele is caused most frequently by Staphylococcus aureus as the offending organism. , , Chronic complications of bacterial pneumonia include bronchiectasis and pulmonary fibrosis. US is appropriate in determining the echogenicity, presence of loculation, and septation of parapneumonic effusions. , , , Contrast-enhanced CT is helpful to evaluate lung parenchymal complications and plan for surgical treatments or interventions when appropriate. , , , Although MRI can characterize complicated pneumonia, it is advocated only for children with substantial radiation exposure from repeated CT scans. , , , ,

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree