In this article, the pathobiological, clinical, and treatment aspects of pediatric-onset multiple sclerosis (MS) are summarized, and the conventional magnetic resonance (MR) imaging (ie, T1-weighted, proton-density, and T2-weighted imaging) features of MS in children are discussed, as well as the application of MR imaging in the diagnosis of pediatric-onset MS and in prediction of MS in children with an incident central nervous system demyelination. Insights gained from studies comparing MR imaging features of pediatric-onset and adult-onset MS are presented.
Key points
- •
Multiple sclerosis (MS) is an immune-mediated disease that is largely viewed to be initiated when a genetically-susceptible host is exposed to environmental risk factors, resulting in a dysregulated immune response that is targeted at the central nervous system (CNS).
- •
Pediatric-onset MS is increasingly recognized, with 2-4% of patients diagnosed prior to age 18 years.
- •
Epstein-Barr virus seropositivity, vitamin D deficiency, and the presence of an HLA-DRB1 ∗ 15 allele are well-described environmental and genetic risk factors for pediatric MS.
- •
Essentially all children with MS follow a relapsing-remitting clinical course.
- •
MR features of MS in children are similar to that of adults; lesion features predictive of a subsequent MS diagnosis in children with acute demyelination have recently been defined.
Introduction
Multiple sclerosis (MS) is the most common autoimmune demyelinating disease of the central nervous system (CNS), characterized by immune-mediated inflammation and progressive neurodegeneration, causing intermittent and accumulating neurologic deficits. Pediatric-onset MS is being increasingly recognized worldwide, and it is estimated that approximately 2.2% to 4.4% of patients are diagnosed with MS before age 18 years. Although the worldwide incidence of pediatric-onset MS is unknown, an American study reported an incidence rate of 0.51 per 100,000 children per year. When considering acute CNS demyelinating events more broadly, 2 studies have reported an incidence ranging between 0.9 and 1.56 per 100,000 children per year.
Historically, the first documented patient with MS was a girl living in the fourteenth century who, at 16 years of age, experienced a first attack of MS characterized by gait impairment and later developed optic neuritis (ON), upper extremity paresis, facial weakness, and pain. However, it was not until 1958 that a cohort of patients with pediatric-onset MS was first described.
That the onset of pediatric MS occurs in close temporal proximity to the inciting events believed to play a role in disease initiation uniquely permits exploration of the earliest aspects of disease pathobiology. The use of magnetic resonance (MR) imaging as a paraclinical tool to detect disease-related changes in the CNS of pediatric patients with MS permits exploration of the earliest aspects of inflammation and neurodegeneration. The sensitivity of MR imaging provides valuable information for MS diagnosis, assessment of disease activity and progression, and monitoring of treatment response.
In this article, after a summary of the pathobiological, clinical, and treatment aspects of pediatric-onset MS, the conventional MR imaging (ie, T1-weighted, proton-density, and T2-weighted imaging) features of MS in children are discussed, as well as the application of MR imaging in the diagnosis of pediatric-onset MS and in prediction of MS in children with an incident CNS demyelination. Insights gained from studies comparing MR imaging features of pediatric-onset and adult-onset MS are presented.
Introduction
Multiple sclerosis (MS) is the most common autoimmune demyelinating disease of the central nervous system (CNS), characterized by immune-mediated inflammation and progressive neurodegeneration, causing intermittent and accumulating neurologic deficits. Pediatric-onset MS is being increasingly recognized worldwide, and it is estimated that approximately 2.2% to 4.4% of patients are diagnosed with MS before age 18 years. Although the worldwide incidence of pediatric-onset MS is unknown, an American study reported an incidence rate of 0.51 per 100,000 children per year. When considering acute CNS demyelinating events more broadly, 2 studies have reported an incidence ranging between 0.9 and 1.56 per 100,000 children per year.
Historically, the first documented patient with MS was a girl living in the fourteenth century who, at 16 years of age, experienced a first attack of MS characterized by gait impairment and later developed optic neuritis (ON), upper extremity paresis, facial weakness, and pain. However, it was not until 1958 that a cohort of patients with pediatric-onset MS was first described.
That the onset of pediatric MS occurs in close temporal proximity to the inciting events believed to play a role in disease initiation uniquely permits exploration of the earliest aspects of disease pathobiology. The use of magnetic resonance (MR) imaging as a paraclinical tool to detect disease-related changes in the CNS of pediatric patients with MS permits exploration of the earliest aspects of inflammation and neurodegeneration. The sensitivity of MR imaging provides valuable information for MS diagnosis, assessment of disease activity and progression, and monitoring of treatment response.
In this article, after a summary of the pathobiological, clinical, and treatment aspects of pediatric-onset MS, the conventional MR imaging (ie, T1-weighted, proton-density, and T2-weighted imaging) features of MS in children are discussed, as well as the application of MR imaging in the diagnosis of pediatric-onset MS and in prediction of MS in children with an incident CNS demyelination. Insights gained from studies comparing MR imaging features of pediatric-onset and adult-onset MS are presented.
Introduction to pediatric MS
Pathogenesis: Immunobiology and Risk Factors
MS is largely viewed as an immune-mediated disease that is initiated when a genetically susceptible host is exposed to environmental risk factors, resulting in a dysregulated immune response targeted at the CNS. T cells and B cells mature in the thymus and bone marrow, respectively, and enter the circulation as naive cells. These naive cells may encounter various antigens and contribute to memory and effector repertoires. Activation of these peripheral immune cells, such as CD4 + and CD8 + T cells, myeloid cells, and B cells, is dysregulated in patients with MS. Such aberrant immune cell activation leads to their more efficient adherence to the blood-brain barrier endothelium and increased trafficking into the CNS via matrix metalloproteinases, where the immune cells become reactivated. The episodic cycles of immune cell infiltration into and reactivation within the CNS contribute to the development of the multifocal inflammatory lesions within the white and gray matter of patients with MS. Pathologic and imaging studies have shown that the CNS injury extends beyond the perivascular cuffs of inflammation and includes extensive neuroaxonal damage within the normal-appearing brain tissue. Taken together, the underlying pathophysiology of MS involves a complex interaction of inflammatory and neurodegenerative processes that are interrelated, with chronic inflammation leading to foci of neuronal destruction and neuronal or axonal damage inciting an immune response.
Interactions between genetic and environmental factors contribute to MS risk. The most well-established genetic component of MS susceptibility resides in the major histocompatibility complex (HLA), and HLA-DRB1 has specifically been associated with MS in adults. A higher frequency of HLA-DRB alleles has been reported in patients with pediatric-onset MS. In a cohort of children with incident CNS demyelination, those diagnosed with MS were more likely to have HLA-DRB1*15 alleles compared with those who remained monophasic, and this association was driven mainly by the presence of European ancestry. In a multivariable analysis of HLA-DRB1*15 genotype, Epstein-Barr virus (EBV) seropositivity and 25-hydroxyvitamin D status in children with acute demyelination, the presence of at least 1 HLA-DRB1*15 allele was associated with a 2-fold increased risk of MS.
Vitamin D insufficiency, remote infection with EBV and exposure to cigarette smoke have been proposed as environmental risk factors for MS in both pediatric-onset and adult-onset patients. Studies in animal models of MS have shown that vitamin D has a role in regulating immune cell activity via the vitamin D receptor on T lymphocytes, suggesting the role of vitamin D as a mediator of immune-mediated inflammation. In a cohort of children with MS, every 10-ng/mL increase in serum 25-hydroxyvitamin D 3 (the active form of vitamin D) was associated with a 34% decrease in relapse rate. When considering several predisposing factors for MS in children with acute demyelinating syndromes (ADSs), a 10-nmol/L increase in serum 25-hydroxyvitamin D concentration was independently associated with a risk reduction of 0.9. Trials of vitamin D in children with MS are being planned. One study has reported that children exposed to parental cigarette smoke are at an increased risk for MS. When comparing serum titers of cytomegalovirus (CMV), herpes simplex virus type 1, varicella zoster virus, parvovirus B19, and EBV in children with MS to those of age-matched controls, seropositivity to EBV was associated with a 3-fold increased likelihood of MS. Several other studies have shown a higher EBV titer in children with MS compared with controls, and this association seems stronger for the Epstein-Barr nuclear antigen (EBNA1) than for the Epstein-Barr viral capsid antigen. In children with ADSs, risk of MS is increased by more than 2-fold in those with remote EBV infection. Intrathecal EBV antibodies have been reported in children with MS. One study reported that, in addition to the increased risk of MS in children seropositive for EBV, remote CMV infection lowers the odds of MS, suggesting that risk for MS depends in part on the viral infections acquired during the early-life window of exposure.
The pathobiology of pediatric-onset MS has many resemblances to that of adult-onset disease. When compared with adults with MS, inflammatory responses in children with MS, measured in studies of T-cell proliferation and B-cell-derived antibodies, are as robust and in some cases stronger than those observed in adult patients. With respect to genetic and environmental risk factors, presence of HLA-DRB1*15 alleles, remote infection with EBV, vitamin D insufficiency, and exposure to cigarette smoke have all also been implicated as risk factors for adult-onset disease.
Chronic Cerebrospinal Venous Insufficiency
Beginning in 2007, the Italian vascular surgeon Paolo Zamboni proposed the theory of chronic cerebrospinal venous insufficiency (CCSVI) as the underlying cause of MS. It was hypothesized that MS results from chronically impaired venous drainage of the CNS caused by stenoses in the neck. The investigators implied that this state of CCSVI led to venous reflux, causing extravasation of iron and its accumulation in the CNS parenchyma; they proposed that iron accumulation may represent the inciting event in MS pathobiology, triggering immune-mediated inflammatory injury. CCSVI was defined as the presence of at least 2 of the following criteria: (1) reflux in the internal jugular veins (IJVs) or vertebral veins (VV) in the upright and supine positions, (2) reflux in the deep cerebral veins, (3) high-resolution B-mode evidence of IJV stenoses, (4) lack of Doppler-detectable flow in the IJVs or VVs, and (5) reverted postural control of main cerebral venous outflow pathways.
Using transcranial color-coded Doppler sonography and extracranial color Doppler sonography, Zamboni and colleagues found that MS was associated with venous outflow anomalies and that patients with MS met the criteria for CCSVI with 100% sensitivity, 100% specificity, 100% positive predictive value (PPV), and 100% negative predictive value (NPV). Interpretation of these results is challenged by the possibility of spectrum bias (sampling conditions for evaluation of the diagnostic test are not clinically representative), lack of blinding of the sonographer to patient diagnosis, uncertain reproducibility of ultrasonography results given the dependency on patient positioning and angle of insonation, and the possible risk of pulsation artifact from adjacent vascular structures.
Led by Professor Zamboni, an open-label study was conducted in which patients underwent a percutaneous transluminal angioplasty to treat the neck venous stenoses. During the 18-month period of follow-up after the procedure, improvement in clinical outcomes was noted in relapsing-remitting patients, although such improvements were not clearly defined. However, the small sample size, lack of a control group, unblinded neurologic evaluations, significant IJV restenosis rate (47%), and the confound that treated patients remained on disease-modifying therapies limit interpretation of these findings and make tenuous the interpretation of efficacy.
Using ultrasound and MR imaging techniques, several international studies have been performed to compare venous structure between patients with relapsing-remitting or progressive forms of MS, those with a first attack of CNS demyelination, patients with other nondemyelinating CNS disorders, and healthy controls. The findings challenge the theory that CCSVI plays a causative role in MS pathogenesis. Iron accumulation and iron-mediated CNS injury are not unique to MS and have been reported in many neurologic disorders, and cerebrospinal ferritin levels have been found to be normal in patients with MS and do not change over time, suggesting that CCSVI is not an inciting factor in MS. For CCSVI to be causative in MS pathobiology, it should be observed in the earliest stages of disease. The presence of anomalous venous drainage patterns in children with MS is being evaluated.
Several centers worldwide are conducting endovascular procedures for treatment of CCSVI. Anecdotal reports indicate serious injury and in some cases death as a result of endovascular procedures to treat CCSVI in patients with MS. It is critical that patient safety remain foremost and that the findings of CCSVI be rigorously replicated in independent cohorts before the implementation of trials to evaluate the efficacy of balloon angioplasty as a treatment of MS.
Demographic and Clinical Characteristics
Onset of MS before the age of 10 years occurs in only 17% of all pediatric-onset patients. Gender ratios vary according to age at onset. The ratio of girls to boys in children younger than 6 years is 0.8:1 and increases to 1.6:1 between the ages of 6 and 10 years. The female preponderance, similar to that seen in adults with MS, emerges only in adolescence, at about 10 years of age which may indicate a hormonal influence on MS risk or a gender-defined genetic influence on immunologic activity.
An ADS represents the first presentation of MS in 20% to 30% of children; for the remaining 70% to 80%, the demyelinating event occurs as a monophasic illness. An ADS can be classified into one of several clinical presentations: ON, acute transverse myelitis (TM), monofocal or polyfocal neurologic deficits extrinsic to the optic nerves or spinal cord, or acute disseminated encephalomyelitis (ADEM). Whereas monofocal presentations are more common in adolescents and adults, children typically present with polyfocal symptoms. A polyfocal presentation of MS, characterized by neurologic symptoms referable to multiple areas within the CNS, occurs in 50% to 70% of children, whereas 30% to 50% present with monofocal symptoms. Approximately 10% to 23% of children with MS present with ON, and bilateral ON carries an increased MS risk compared with unilateral ON. Acute isolated TM occurs as a first attack of MS in only 2% to 14% children ; more frequently, acute TM represents a monophasic illness, or when co-occurring with ON, may represent the heralding features of neuromyelitis optica. Monofocal presentations of motor dysfunction occur in 30% of children with MS, sensory symptoms in 15% to 30%, ataxia in 5% to 15%, and brainstem symptoms in 25% to 41%.
ADEM, defined by the International Pediatric MS Study Group (IPMSSG) as multifocal neurologic deficits and encephalopathy (change in behavior or consciousness), is the most frequent ADS, occurring in 25% to 40% of children, and represents a monophasic illness in up to 80% of children, with symptoms lasting approximately 3 months. Recurrent and multiphasic forms of ADEM have been described but are rare. Because younger children are more likely to experience a first attack of MS characterized by multifocal neurologic deficits, the distinction from monophasic ADEM can be difficult, but is important for prognosis. ADEM has been reported as a first attack of MS in as many as 18% of children, and in as few as 2% to 6%. These discrepant findings may be a result of inconsistent application of criteria for altered mental status or encephalopathy across studies. To mitigate this inconsistency and permit comparison of multiple cohorts, the IPMSSG proposed MS diagnostic criteria for children whose first attack met criteria for ADEM, requiring 2 non-ADEM events (which must exclude encephalopathy) more than 3 months after first attack.
An MS relapse is defined as neurologic symptoms that persist for at least 24 hours and are separated from a previous attack by a minimum of 28 days. Most children with MS experience complete recovery after the first demyelinating episode, remaining clinically stable between subsequent MS relapses. More than 95% of children with MS follow a relapsing-remitting disease course ; primary progressive MS is extremely rare in children. Annualized relapse rate, estimated between 0.38 and 0.87, is higher in children compared with adults. On average, the secondary progressive stage of MS occurs 10 years later in children compared with adults, but pediatric-onset patients are 10 years younger than adult-onset patients when they reach this phase of the disease.
Treatment
Corticosteroids are typically used to manage acute symptoms or relapses in children with MS, and intravenous immunoglobulin or plasma exchange may be administered to treat severe or life-threatening symptoms. Most disease-modifying therapies act on the immune-mediated inflammatory component of the disease. First-line immunomodulatory therapies used to treat pediatric MS include glatiramer acetate (GA), intramuscular and subcutaneous interferon (IFN) β1a, and subcutaneous IFN-β1b. At present, most treatment decisions for use of these first-line therapies in children are based on studies performed in adults. Recently, a European consensus for the use of IFN-β and GA in children with MS was published. Initiation of MS-targeted therapy should commence promptly after confirmation of MS diagnosis, because efficacy is higher early on in the disease, both IFN-β and GA are safe and tolerable in children and seem effective at reducing relapse rate in the early stages of the disease; relapses interfere with school attendance and negatively affect quality of life. Serious adverse effects to first-line therapies are rare in children, and flulike symptoms, increased liver enzyme levels, and reduced white blood cell counts are most common. Data on adequate treatment response are lacking; however, a reduction in relapse rate during the first 2 years of disease from 1.0 to less than 0.6 would be comparable to the 30% to 40% relapse rate reductions observed in adults.
Two second-line therapies, natalizumab and fingolimod, have recently been approved for use in adult patients. Natalizumab has shown relapse rate reductions of 68% in adult MS trials and more than 90% reductions in contrast-enhancing lesions compared with placebo ; however, it is associated with an increased risk for infection with JC virus, leading to progressive multifocal leukoencephalopathy (PML). Stringent monitoring and PML management guidelines are now in place. Fingolimod is the first orally administered disease-modifying therapy and has been shown to reduce MR imaging activity and clinical relapses in adults with MS. Fingolimod acts by binding to sphingosine-1-phosphase receptors on immune cells, impeding their ability to emigrate from the thymus. Whether this sequestration of immune cells in the thymus (a particularly active tissue in children that is responsible for educating the T-cell repertoire) results in premature immune senescence must be understood before it can be considered for treatment of pediatric MS.
Physical and Cognitive Outcome
Long-term outcome in children with MS is largely determined by rate of physical and cognitive disability accrual. The Expanded Disability Status Scale (EDSS) is a widely used measure of physical disability, ranging from 0 to 10, with increasing scores referring to increased disability. Children reach a disability score of 4 (deficits, but able to ambulate 500 m without aid or rest) a median of 20 years from onset, a score of 6 (able to walk with unilateral aid no more than 100 m without rest) after 28.9 years, and a score of 7 (able to walk no more than 10 m without rest) after 37 years. Compared with adults with MS, pediatric-onset patients take 10 years longer to reach irreversible disability scores, but do so at an age of 10 years younger.
Longitudinal studies have shown that more than half of children with MS show cognitive impairment at 10 years of follow-up. Compared with healthy controls, cross-sectional studies have shown that more than 30% of patients have cognitive impairment in the form of deficits in attention, memory, processing speed, expressive language, and visuomotor integration. Over 2 years of follow-up, 70% of children with MS enrolled in a multicenter longitudinal study showed worsening of cognitive impairment. Given that cognitive impairment affects quality of life and negatively affects school performance, special attention to school-related accommodations and emotional well-being is imperative to the multidisciplinary care of children with MS.
MR imaging appearance of relapsing-remitting MS
Typical MR Imaging Features of MS
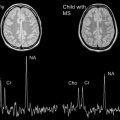
Conventional MR imaging has high sensitivity for macroscopic tissue abnormalities in MS, but low pathologic specificity within the lesions, because edema, inflammation, demyelination, remyelination, gliosis, and neuroaxonal loss all appear as hyperintense on dual-echo proton-density/T2-weighted, and fluid-attenuated inversion recovery (FLAIR) imaging. Dual-echo, FLAIR, and precontrast and postcontrast T1-weighted sequences provide important information for diagnosing MS, understanding the natural history of lesion accrual, and assessing whether treatment reduces accrual of new lesions.
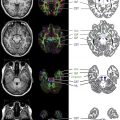
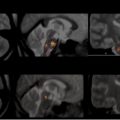
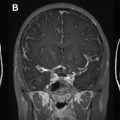
MS lesions typically appear as focal ovoid areas of hyperintensity on dual-echo and FLAIR images, ranging from a few millimeters to more than 1 cm in diameter. White matter lesions, such as those involving the juxtacortical and periventricular regions, the corpus callosum, as well as the brainstem and cerebellum, can be visualized on conventional T2-weighted or FLAIR images ( Fig. 1 ). In an analysis of supratentorial T2 lesion distribution in children with established MS, lesions have the highest probability of being located in the occipital periventricular white matter, followed by the frontal periventricular white matter, whereas cortical and deep gray matter T2 lesions are less commonly detected ( Fig. 2 ). T2 hypointensity has been detected in deep gray matter structures of pediatric patients with MS, specifically in the head of the caudate nucleus, and may suggest abnormal iron deposition related to diffuse underlying MS. Tumefactive lesions, defined as large lesions (>2 cm) with marked perilesional edema, may be difficult to distinguish from malignancy at onset, and therefore biopsy may be required for diagnosis. The presence of tumefactive lesions has been reported in children with acute demyelination who have subsequently been diagnosed with MS ( Fig. 3 ).
Gadolinium-enhanced T1-weighted imaging permits differentiation of active or newly formed lesions from inactive ones, because contrast enhancement occurs as a result of increased blood-brain barrier permeability and corresponds to active immune cell transmigration into the CNS. Enhancing lesions are typically focal or ringlike in contour ( Fig. 4 ). In a prospective cohort study of children with ADS, contrast-enhancing lesions were present in 22% of patients (10% of patients who had a monophasic illness and 70% of children subsequently diagnosed with MS). Lesions typically enhance for approximately 3 weeks, but the duration may be shorter in the context of treatment with methylprednisolone. Both number and volume of new T2 and contrast-enhancing lesions are often used as an outcome measure of in vivo inflammatory activity in treatment trials, because they capture subclinical disease activity with a higher frequency than clinical relapses.
A subset of T2 hyperintense lesions appears hypointense on T1-weighted imaging, ranging in intensity from isointense to gray matter to isointense to cerebrospinal fluid. These T1 lesions, or black holes, which may enhance with gadolinium initially but persist as nonenhancing lesions on serial T1-weighted images, represent focal areas of severe tissue damage and irreversible axonal loss ( Fig. 5 ). Decreasing brain volume over time can be observed in children when comparing serial MR imaging scans (see Fig. 5 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree