Pediatric musculoskeletal infections often pose a diagnostic challenge due to their frequently vague and nonspecific clinical presentation. Imaging evaluation is a crucial component to diagnostic workup of these entities. Changed epidemiology of these infections over the past 2 decades has resulted in increases in both disease incidence and severity in the pediatric population. Prompt and accurate diagnosis is essential in order to reduce the risk of morbid sequelae, and to optimize patient management. In this article, the unique pathophysiology of musculoskeletal infections and characteristic imaging findings in children compared with adults are reviewed.
Key points
- •
Pediatric musculoskeletal infections and associated complications are increasingly encountered in daily clinical practice due to epidemiologic changes over the past 2 decades.
- •
Conventional radiographs continue to be the first-line imaging modality of choice for evaluating pediatric musculoskeletal infections, particularly early in the course of disease, despite their low yield toward detection of musculoskeletal infections.
- •
Ultrasound, which is fast, noninvasive, and uses no radiation, is ideal for evaluating superficial soft tissue infections and septic arthritis. In addition, ultrasound-guided joint aspiration can both facilitate diagnosis and provide symptomatic relief.
- •
MR imaging, due to its superior sensitivity and specificity, is the current gold standard for diagnosis of pediatric musculoskeletal infections and associated complications.
Introduction
Pediatric musculoskeletal infections can present a diagnostic dilemma due to their often-nonspecific presentation, particularly early in the course of the disease. Missed or delayed diagnosis is frequent and can lead to potentially severe, occasionally devastating consequences in the pediatric population. Imaging is integral to the diagnostic workup of these infections, complementing history, physical examination, and laboratory testing in the pediatric population. The overarching goal of imaging evaluation is to provide an early, accurate diagnosis and minimize unwanted sequelae.
In recent years, there has been an evolution in the epidemiology of pediatric musculoskeletal infections with both the incidence and severity of disease increasing, and this has further underscored the importance of imaging evaluation for timely and accurate diagnostic workup. Therefore, it is essential for both clinicians and practicing radiologists to be aware of how to best use diagnostic imaging toward workup of pediatric musculoskeletal infections.
In this article, the unique pathophysiology of musculoskeletal infections and characteristic imaging findings in children compared with adults are reviewed. Evidence-based imaging algorithms and up-to-date recommendations regarding optimal utilization of imaging modalities toward diagnostic workup of pediatric musculoskeletal infections and, when appropriate, imaging-guided intervention, are provided.
Evidence-based imaging algorithm
Diagnostic workup of pediatric musculoskeletal infections involves several imaging modalities. Although conventional radiography has a low sensitivity for detection of acute osteomyelitis, especially early in the course of disease, radiographs should always be the first study ordered, to exclude other similarly presenting differential diagnoses. MR imaging and technetium 99m ( 99m Tc)-MDP bone scintigraphy are both highly sensitive for the diagnosis of acute osteomyelitis. MR imaging has overtaken scintigraphy as the gold standard for diagnosis due to its lack of ionizing radiation, widespread availability, and superior delineation of extraosseous detail.
There are limited data on the exact sensitivity and specificity of imaging for diagnosis of septic joint, because imaging, by itself, cannot differentiate between sterile and infected joint fluid. Both ultrasound and conventional MR imaging are highly sensitive for detecting joint fluid. Ultrasound can guide joint aspiration, which is the gold standard for diagnosis. Dynamic contrast-enhanced MR imaging may aid distinction between a septic hip and transient synovitis.
Role of imaging in the evaluation of pediatric musculoskeletal infection: osteomyelitis
Epidemiology
Staphylococcus aureus has traditionally been the most common causative organism of pediatric musculoskeletal infections, especially since the advent of community immunization programs for previously common organisms such as Haemophilus influenzae type b (Hib). Over the past 2 decades, there has been an increase in the incidence and severity of osteomyelitis in children. During this time, there has been a concomitant increase in the prevalence of methicillin-resistant Staphylococcus aureus (MRSA). Most of the cases with severe complications are secondary to an MRSA infection and are likely related to the Panton-Valentine leucocidin or PVL gene. These complications include but are not limited to multifocal infection, deep venous thrombosis, subperiosteal and intraosseous abscesses, and adjacent pyomyositis. ,
Over the past 2 decades, there has been a substantial increase in the incidence of musculoskeletal infections from the facultative anaerobic gram-negative bacterium Kingella kingae, particularly in Europe and the Middle East, where it is now identified as the most common pathogen for osteomyelitis and septic arthritis in children between 6 months and 4 years of age. , This organism is highly difficult to culture. However, the increased availability of polymerase chain reaction–based assays has increased its detection. Other gram-negative organisms such as Escherichia coli and group B streptococci are primarily seen in neonates and young infants. Salmonella infection is common in patients with sickle cell disease. Pseudomonas aeruginosa infections are seen following puncture wounds in the feet.
Osteomyelitis is considered acute if symptoms have lasted 2 weeks or less. It is most often seen in young children, with half of all pediatric cases seen in children younger than 5 years. , Male children are affected at a higher rate than female children. The overall incidence of osteomyelitis in children has been increasing over the past 2 decades and now ranges between 1 and 13 per 100,000 and accounts for roughly 1% of all hospital admissions in children. , ,
Normal Anatomy and Pathophysiology
The pathogenesis of osteomyelitis in the pediatric population is unique compared with adults. Most cases of pediatric osteomyelitis occur secondary to hematogenous seeding of the metaphyseal bone marrow following an episode of transient bacteremia. Direct spread of bacteria to bone from overlying injury or infection, the predominant mechanism seen in adults, is much less common in children.
Metaphyses or metaphyseal equivalents represent the most common site of osteomyelitis in children due to distinct anatomic attributes of the metaphyseal vasculature, namely the open-ended structure of the terminal metaphyseal vessels and the large number of slow-moving metaphyseal sinusoidal veins. As the sluggish flow reaches the capillaries, bacteria can escape into the extravascular space via small gaps in terminal capillaries and become trapped at the junction of the metaphysis and physis. Osteomyelitis most often involves in the fast-growing long bones of the lower extremities including the femur, tibia, and fibula.
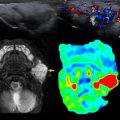
Over the first 12 to 18 months of life, the metaphyseal and epiphyseal vasculature communicate directly through the growth plate, permitting direct epiphyseal seeding from a metaphyseal focus of infection ( Figs. 1 and 2 ). Between 1 and 2 years of age, these transphyseal vessels begin to involute and the now avascular growth plate begins to serve as a barrier to spread of metaphyseal infection.


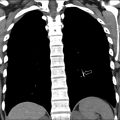
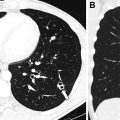
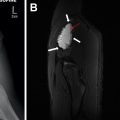
Subacute osteomyelitis is clinically defined as infection of the bone marrow that has lasted greater than 2 weeks but less than 6 weeks ( Fig. 3 ). On MR imaging, Brodie abscess, a focal region of subacute osteomyelitis is commonly characterized by the “penumbra” sign, which is a peripheral rim of vascular granulation tissue with high T1 signal surrounding the low T1 signal intensity abscess cavity ( Fig. 4 ). ,


Chronic osteomyelitis represents infection of greater than 6 weeks duration, often with intermittent, acute exacerbations. , Chronic infection can result in necrotic bone known as sequestrum with surrounding pus and reactive new bone called an involucrum. The sequestrum can limit antibiotic efficacy by preventing penetration into the infectious cavity. A cloaca is a sinus tract extending through the involucrum, which can allow debris to drain from the necrotic bone. ,
Imaging Techniques
The ultimate goal of imaging in a suspected case of osteomyelitis is to provide a prompt, accurate diagnosis in order to facilitate early and appropriate treatment and thereby decrease the likelihood of associated complications.
Radiography
Conventional radiographs are recommended as the first line of diagnostic evaluation in all cases of suspected osteomyelitis in the pediatric population. Radiographs should be obtained in conjunction with laboratory studies such as a hemogram, inflammatory markers (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]), and blood cultures. It is important to note that the diagnostic rate of the initial conventional radiographs is less than 20% for acute osteomyelitis, with radiographic findings seen only 1 to 2 weeks after symptom onset and following loss of approximately 33% of bone mineral. The most important role of the initial radiographs in cases of suspected acute osteomyelitis is to detect alternative causes such as trauma or malignancy, which may account for the pediatric patient’s symptoms ( Box 1 ).
- •
Ewing sarcoma
- •
Chronic nonbacterial osteomyelitis/chronic recurrent multifocal osteomyelitis
- •
Langerhans cell histiocytosis
The classic radiographic findings of acute osteomyelitis include ill-defined lytic changes with or without periosteal reaction and minimal surrounding sclerosis ( Box 2 ). In subacute osteomyelitis, admission radiographs are normal in 67% to 100% of patients. When positive, bone radiographs demonstrate bone destruction, lytic lesions, and periosteal reaction/ elevation.
- •
Conventional radiograph : ill-defined lytic changes with or without periosteal reaction.
- •
MR imaging : decreased marrow signal on T1; increased marrow signal on fluid-sensitive sequences (T2 and STIR). Enhancement of infected marrow on post–gadolinium-enhanced sequences. Hypoenhancement/nonenhancement of ischemic marrow.
- •
Nuclear medicine scintigraphy : focal increased 99mTc-MDP uptake on flow, blood-pool, and delayed images.
MR imaging
In all cases with a high clinical suspicion for acute osteomyelitis, obtaining prompt MR imaging of the region of concern, regardless of the findings seen on the initial radiograph, is recommended; this is especially important in children with elevated inflammatory markers. MR imaging is the imaging modality of choice for diagnosis of acute osteomyelitis because of equivalent sensitivity to bone scintigraphy for diagnosis (97%), along with superior spatial resolution and specificity. , Performing the MR imaging as soon as reasonably possible can expedite diagnosis and reduce the likelihood of complications.
MR imaging evaluation should be focused on the presumed site of infection. Non-contrast enhanced sequences include axial, coronal and sagittal T1,axial fat-suppressed T2, coronal and sagittal short-tau inversion recovery (STIR), and axial T1 with fat suppression. Gadolinium-enhanced fat suppressed T1 -weighted images should subsequently be performed in axial, coronal and sagittal planes ( Table 1 ). Gadolinium-enhanced fat-suppressed T1- weighted sequences can identify epiphyseal chondritis or ischemic bone. Infants and young pediatric patients (typically <5 years old) require sedation before imaging, and a multidisciplinary approach is paramount to prevent unnecessary delays in care.
| Sequence | Plane of Imaging |
|---|---|
| T1 | Axial, coronal, sagittal |
| Short-tau inversion recovery (STIR) | Coronal, sagittal |
| T2 + a FS | Axial |
| T1 + a FS | Axial |
| T1 + a FS + b GBCA | Axial, coronal, sagittal |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree