Incidental pulmonary nodules are not infrequently identified on computed tomography imaging in the pediatric population and can be a challenge in suggesting appropriate follow-up recommendations. An evidence-based and practical imaging approach for diagnosis and appropriate directed management is essential for optimal patient care. This article provides an up-to-date review of the pediatric pulmonary nodule literature and suggests a practical algorithm to manage pulmonary nodules in the pediatric population.
Key points
- •
The status of an underlying malignancy is key in determining the appropriate algorithm to use in the pediatric population with an incidental pulmonary nodule.
- •
In the absence of an underlying malignancy, children with unexpected solid pulmonary nodules smaller than 5 mm should not require dedicated follow-up imaging, unless there is an underlying clinical concern.
- •
In children with extrathoracic malignancy, there remains conflicting literature and a variety of primary malignancies with different propensities for intrathoracic metastasis.
- •
Pulmonary nodules remain a diagnostic challenge in children with known extrathoracic malignancy, given the variable appearances and lack of standardized characteristics to determine benignity versus malignancy.
- •
Computed tomography is currently the best imaging modality to identify, evaluate, and perform follow-up of pediatric pulmonary nodules that cannot be visualized on chest radiography.
Introduction
Pulmonary nodules, when they are small, may be difficult to be identified on chest radiography. However, pulmonary nodules are not infrequently incidentally detected on computed tomography (CT) imaging in the pediatric population. The increased availability and improved CT techniques in recent years, such as the use of multidetector CT, resulting in thinner slice sections and decreased motion artifacts, have enabled the detection of smaller lung lesions, , , resulting in more pulmonary nodules being identified incidentally. Although the Fleischner guidelines for directing follow-up imaging based on nodule size and a patient’s risk for malignancy exists, this set of criteria is only applicable to the adult population older than 35 years old. , It currently recommends that the management of young patients should be made on a case-by-case basis and discourages the use of serial CT scans for follow-up.
Unfortunately, no comparable universally accepted criteria for evaluating children with pulmonary nodules currently exists. A main reason for why no established criteria exists is the relative paucity of literature available and the conflicting data for the determination of a benign versus malignant appearance. , , Additionally, owing to concerns regarding radiation exposure, a large proportion of the studies have been performed in children with extrapulmonary malignancies. , Only more recently have there been studies assessing the characteristics of pulmonary nodules incidentally detected in healthy children. ,
Without established recommendations tailored for pediatric population, radiologists and clinicians may be inclined to inappropriately extrapolate data from the Fleischner guidelines when they encounter pediatric patients with pulmonary nodules on imaging studies. A previous survey of the Society of Thoracic Radiology members demonstrated that radiologists tend to be overly aggressive in follow-up imaging, most commonly recommending follow-up examinations in 3 to 6 months for small nodules (3–5 mm) in young patients with no history of malignancy. , Additionally, a 2014 survey of pediatric pulmonologists in the United States demonstrated a tendency for a more aggressive approach in managing children with incidentally detected pulmonary nodules. , For example, when presented with a small (<4 mm), noncalcified nodule, 21% recommended a 3- to 6-month follow-up and 27% recommended a 12-month follow-up. , Given that the probability of an incidental pulmonary nodule being malignant in an otherwise healthy child is extremely low, this finding suggests that many radiologists and clinicians may be inappropriately recommending follow-up CT studies, resulting in unnecessary radiation exposure and anesthesia. , As previously established, the lifetime radiation risks attributable to CT examinations are considerably higher in children , and, thus, it is imperative to avoid unnecessary CT follow-up scans for unproven clinical indications.
In 2015, the Society of Pediatric Radiology (SPR) Thoracic Imaging Committee had presented a recommended algorithm to aid clinical management of incidentally detected lung nodules in children. , As new literature has since become available, especially with the updated research including healthy children, , the purpose of this article is to provide an updated literature analysis and evidence-based imaging recommendations, as well as discuss a practical approach to the imaging of pediatric pulmonary nodules.
Evidence-based imaging algorithm
Pulmonary Nodules in Healthy Children
Within the scarcely available literature on pediatric pulmonary nodules, incidental pulmonary nodules are not infrequently encountered. Alves and colleagues recently reviewed preoperative chest CT scans for pectus carinatum or pectus excavatum treatment and identified that 75% of the patients had at least 1 pulmonary nodule. Renne and colleagues retrospectively reviewed 259 trauma chest CT scans in pediatric patients and demonstrated 86 patients (33%) with incidental pulmonary nodules. Similarly, Samim and colleagues reviewed chest CT scans in children with high-energy trauma and demonstrated a total of 27 (37.5%) patients with incidental pulmonary nodules in the 72 included patients. Interestingly, Breen and colleagues retrospectively reviewed abdominal CT scans performed at a single institution over a span of 7 years, and only detected pulmonary nodules in 1.2% of the 5234 patients.
Assefa and Atlas previously reviewed 36 patients with incidentally identified pulmonary nodules on a variety of chest and abdominal CT scans and chest radiographs to review the natural history of incidentally identified pulmonary nodules in children with nonmalignant disease. A total of 46 nodules were identified, and 37 nodules (27 patients) were larger than 4 mm. Within this subset of patients, 22 of the patients had follow-up chest imaging 3 to 12 months later, and 14 (54%) of the nodules remained unchanged, 5 (19%) had decreased in size, and 7 (27%) had resolved on a follow-up CT scan, with no obvious growth or enlargement over the study period identified to suggest malignancy. Thus, the authors concluded the low risk of malignancy in these subcentimetric nodules and raised the concern for potential harm of repeated follow-up CT scans outweighing any potential clinical benefit. Breen and colleagues confirmed this finding, with no malignant pulmonary nodules identified in their pediatric patients with incidental pulmonary nodules without a history of known malignancy.
Recommendations in Healthy Children with Pulmonary Nodules
Recently, Barber and colleagues have questioned the usefulness of a diagnostic workup for an incidental solitary pulmonary nodule in patients without oncologic diagnosis or symptoms, because no definitive diagnosis was identified in 94% of cases. In light of this finding, in addition to the combination of a frequent occurrence and low associated malignancy risk in otherwise healthy children, it is imperative that follow-up imaging for incidental pulmonary nodules in this population be recommended cautiously to minimize the potential harm from repeated imaging, such as radiation exposure with risk of malignancy and caregiver anxiety.
The 2015 SPR Thoracic Imaging guidelines suggested that, if an unexpected asymptomatic solid pulmonary nodule was identified with a negative clinical history, and with the presence of classic features of benignity such as a popcorn calcification, fat, or features to suggest an intrapulmonary lymph node (peripheral, triangular, elongated, smoothly marginated, or a pleural tag), uniformly calcified or stability over time, no further imaging was required. Since then, more recent literature examining dedicated populations of children without malignancies have suggested the usefulness of size to suggest benignity. , Breen and colleagues identified that the malignant pulmonary nodules in their study were significantly larger than the benign pulmonary nodules, and a size of 7 mm or larger was recommended as an optimal cut-off to suggest high risk. Similarly, Alves and colleagues noted that 95% of the pulmonary nodules within their study were less than 6 mm and concluded that under these limits, incidental nodules are very unlikely to be pathologic. Finally, Renne and colleagues only identified incidental pulmonary nodules smaller than 5 mm, and thus concluded that 5 mm pulmonary nodules can be detected frequently in children without malignant disease. Therefore, upon review of the recent literature, a conservative recommendation would be that children with unexpected solid pulmonary nodules smaller than 5 mm in the absence of amalignancy, should not require dedicated follow-up CT scans unless there is an underlying clinical concern requiring further follow-up.
Pulmonary Nodules in Children with a Primary Extrathoracic Malignancy
In addition to the common presence of incidental pulmonary nodules in an otherwise healthy child, pulmonary nodules are also not uncommonly identified in the pediatric population with extrathoracic malignancies, including but not limited to Wilms tumors, osteosarcomas, soft tissue sarcomas, and embryonal tumors. Previously, Grampp and colleagues identified pulmonary nodules in 34% of patients with extrathoracic malignant tumors. Similarly, Silva and colleagues reviewed 488 patients with a new diagnosis of a non–central nervous system solid tumor or lymphoma and identified 111 patients (23%) with pulmonary nodules on the presentation CT scan. Rissing and colleagues prospectively reviewed 331 patients with sarcoma treated over a 5-year range, and identified 34 children, of whom 6 (18%) were identified to have indeterminate nodules on the initial CT scan. Recently, Vaarwerk and colleagues reviewed the clinical significance of pediatric patients with rhabdomyosarcoma with indeterminate nodules over an 8-year clinical span, and of the 316 patients included with chest CT imaging, 67 patients (21.2%) were identified with least a single pulmonary nodule. Thus, like the incidental pulmonary nodules identified in healthy children, the conundrum of what to do with the pulmonary nodule in patients with a primary extrathoracic malignancy is not infrequently encountered and becomes a challenging situation.
In contrast with the healthy child, this situation is complicated by a whole spectrum of primary malignancies with a variety of intrathoracic metastasis risk and a diverse range of pulmonary metastasis appearance. Although a greater proportion of the literature has been evaluated in children with malignancies, the ultimate challenge remains in the ability to determine a benign incidental pulmonary nodule versus metastatic disease, which can evidently alter the patient’s management, but unfortunately, which radiologists have done poorly. Although increased size on serial imaging has been widely accepted to suggest metastatic disease, given the concern for radiation risks, serial follow-up CT imaging should be avoided if possible.
The challenge remains in trying to direct management at initial diagnosis. Silva and colleagues reviewed 111 infants and children with extrapulmonary malignancies who had lung nodules, and found that the distribution, attenuation, shape, margin, and presence or absence of calcifications were not useful to reliably differentiate benignity from malignancy. This finding is like the initial studies performed by Robertson and colleagues in 1988, who concluded that pulmonary nodules smaller than 5 mm in diameter were no more likely to be benign than larger nodules. Similarly, McCarville and colleagues reviewed 41 young patients with 81 pulmonary nodules and demonstrated that size was not associated with malignancy. Grampp and colleagues have also cautioned the usefulness of size to determine malignancy; they demonstrated that 70% of solitary pulmonary nodules smaller than 5 mm in children at initial presentation with solid extrathoracic tumors may be benign.
In contrast, Rissing and colleagues reviewed 331 patients with sarcoma, including a subset of 34 pediatric patients, and concluded that pulmonary nodules greater or equal to 5 mm were at increased risk for metastatic disease in comparison with the patients without pulmonary nodules or with pulmonary nodules smaller than 5 mm. Brader and colleagues reviewed 30 pediatric patients with osteosarcoma who underwent a chest CT scan and had a total of 117 pulmonary nodules resected over a 5-year span. They demonstrated that, although most characteristics were not useful, nodule size of 5 mm or larger and the presence of calcifications were associated with a statistically significant increased probability of malignancy.
Recommendations in Children with Primary Extrathoracic Malignancy and Pulmonary Nodules
Although the imaging characteristics are important for ultimate diagnosis and management, it does raise the ultimate question, namely, “Does having the diagnosis really change outcome?” This clinically important concept was very recently questioned by Vaarwerk and colleagues of the Pediatric Soft Tissue Sarcoma Study Group who reviewed 316 patients with rhabdomyosarcoma, of whom 67 had indeterminate pulmonary nodules. Indeterminate pulmonary nodules were defined by the European Pediatric Soft Tissue Sarcoma Study Group rhabdomyosarcoma 2005 protocol as no more than 4 pulmonary nodules smaller than 5 mm size or 1 pulmonary nodule between 5 and 10 mm. The authors concluded that the indeterminate pulmonary nodules at diagnosis did not affect outcomes in patients with localized rhabdomyosarcoma, and thus recommended against the need to biopsy or upstage these patients. However, there has been conflicting data; in addition to demonstrating the increased risk of metastatic disease in patients with sarcoma with pulmonary nodules 5 mm or larger, Rissing and colleagues demonstrated that this group was also associated with a worse 3-year disease-free survival, suggesting that the ultimate diagnosis of these pulmonary nodules do matter. However, this dataset did include a large proportion of adult patients; therefore, this data should be applied cautiously. Similarly, Zhou and colleagues recently evaluated 88 patients with indeterminate pulmonary nodules in patients with high-grade localized osteosarcoma and demonstrated in their 34 pediatric patients a poorer event-free survival.
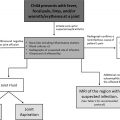
The 2015 SPR Thoracic Imaging guidelines previously suggested a conceptual framework to aid in management of pulmonary nodule on a CT scan in a child. Ultimately, this algorithm emphasized the importance of individualized care for pediatric patients with a history of malignancy. Even upon review of updated literature, given the conflicting results, a similar recommendation of a case-by-case individualized care is suggested. In challenging situations, a multidisciplinary discussion regarding the clinical team’s concern, tolerance of caregivers, interpreting radiologist’s comfort, surgical feasibility, and the possibility of altering the clinical outcome is recommended.
Practical imaging approach to pediatric pulmonary nodules
Because the recommendations of how to approach a child with an incidental pulmonary nodule has been previously discussed, this section discusses the benefits and challenges of the available imaging modalities and reviews the recommended definitions and descriptors and spectrum of imaging manifestations to allow the radiologist and clinician to become more familiar to practically approach the imaging of pediatric pulmonary nodules.
Imaging modalities
Three main imaging modalities currently used to evaluate pulmonary nodules in pediatric patients include chest radiography, CT scan, and MR imaging, which are discussed in this section.
Chest Radiography
Chest radiographs remain the first-line and most performed imaging examination in pediatric patients with suspected thoracic disorders, because it remains readily available, easy to acquire, relatively lower in radiation compared with a CT scan, and low in cost. Although the recent pediatric pulmonary nodule literature typically discuss the findings and usefulness of a CT scan, it is important to recognize that radiographs are still continuously being used for assessment of other suspected thoracic abnormalities, such as pneumonia or long-term cancer surveillance, and can also incidentally detect pulmonary nodules. In a recent study published in 2021 by Barber and colleagues, it was noted that, of the 88 children with pulmonary nodules, 38% of them were identified initially with chest radiographs. Therefore, it is imperative that radiologists continue to scrutinize chest radiographs carefully to ensure that pulmonary nodules are not missed.
Computed Tomography Scans
A CT scan remains the most used imaging modality for the assessment of pulmonary nodules in both pediatric and adult populations. With the advent of high-resolution multidetector CT allowing faster acquisition times and thinner collimation and resulting in a greater spatial resolution, and additional postprocessed visualization techniques such as multiplanar reconstructions, maximum intensity projections (MIPs), and computer-aided detection, this has markedly improved the radiologist’s ability to detect pulmonary nodules. , , , Encouragingly, CT technology continues to advance; recently Cho and colleagues successfully differentiated metastatic from benign nodules in patients with osteosarcoma using 3-dimensional computerized texture analysis.
However, even with technological advances, there remain the inherent challenges of pulmonary nodule detection, especially in children, such as a smaller thoracic diameter, as well as respiratory and motion artifacts from faster respiratory and heart rates. This challenge is compounded by the desire to aggressively minimize radiation dose, resulting in increased background noise and worsened image quality, , although recent promising literature has demonstrated similar nodular detection accuracy with a simulated 60% dose reduction. An additional challenge is that younger children, typically those less than 3 years old, may not be able to follow the breath-hold instructions, and thus require anesthetic to improve compliancy. In recognition of the 2016 Food and Drug Administration warning about the risk of general anesthesia in children younger than 3 years, whenever possible, anesthesia should be avoided; instead, in conjunction with the rapid acquisition times, gentle respiration should be encouraged.
Although MIPs have demonstrably improved pulmonary nodule detection, there remains conflicting literature regarding which plane and thickness is optimal. In 2013, Kilburn-Toppin and colleagues demonstrated the use of 10-mm axial MIPs in addition to axial source images to improve sensitivity and decrease reading time. However, Ozkan and colleagues subsequently demonstrated that, although 5-mm axial MIP images had the fastest reading times, the highest advantage was with 5-mm coronal 5 MIP images. Verhagen and colleagues recently confirmed that, although the detection of pulmonary nodules was improved with MIPs, inter-reader agreement remained only fair, and pulmonary nodule characterization remained best with 1-mm slice thickness CT images.
At our local institution, we typically perform chest CT studies via volumetric data acquisition protocol on a multidetector CT scanner (Siemens Somatom Definition Flash) at end-inspiration, with a reference peak kilovoltage range of 80 to 100 kVp, automatic tube current exposure control, collimation 128 × 0.6 mm, pitch of 3 (FLASH), and rotation time of 0.28 s. Multiplanar images are constructed with soft tissue and lung algorithms at 1 mm and 3 mm thickness, as well as axial and coronal 5-mm MIPs. If studies are performed with IV contrast, low-osmolar nonionic contrast material (iopamidol, Isoevue 370, Bracco) is administered on a weight-based dose (1.5–2.0 mL/kg) using a power injection method.
MR Imaging
Although CT scanning remains the gold standard for pulmonary nodule imaging, advances in chest MR imaging techniques have improved lung visualization, and remain an attractive, ionizing radiation-free alternative. The absence of ionizing radiation remains a desirable goal for pediatric patients with malignancies, because surveillance for metastases or recurrence is required routinely, and the cumulative dose may become damaging over time. Additionally, these pediatric patients may possess an underlying increased potential for developing new malignancies related to risk or treatment-related factors.
The current literature suggests that MR imaging can reliably detect pulmonary nodules 5 mm or larger with optimal conditions , ; however, pulmonary nodules smaller than 3 mm remain a challenge with poor sensitivity. , An added challengewith MR imaging remains the calcified pulmonary nodules, such as with osteosarcoma metastasis with a resultant susceptibly-related loss of signal intensity and thus appearing occult on MR imaging. Although the majority of the strategies have been adopted for 1.5 T scanners, with the increased availability of 3 T MR imaging, there has been discussion regarding the benefits of a higher magnetic field strength. However, although using a higher magnetic field strength would theoretically yield improved lesion detection and spatial resolution, this technique is thought to degrade image quality and decrease overall effectiveness owing to the increased susceptibility artifacts on MR imaging. Other MR imaging techniques have also been investigated; for example, diffusion-weighted imaging and dynamic contrast-enhanced MR imaging have demonstrated some usefulness in the detection and characterization of pulmonary nodules in the adult population.
Spectrum of pediatric pulmonary nodules
Definition of a Pulmonary Nodule
Before the characterization of a pulmonary nodule on CT imaging, a radiologist must be familiar with the widely accepted definitions, as previously detailed by the Flesichner Society. A pulmonary nodule is defined as a rounded opacity, well or poorly defined, measuring up to 3 cm in diameter, and can be ground glass, solid, or contain both solid and ground glass components. Ground glass components are defined as hazy with an increased opacity and preservation of the bronchial and vascular margins, whereas a solid component is defined as soft tissue attenuation.
Pulmonary Nodules in Healthy Children
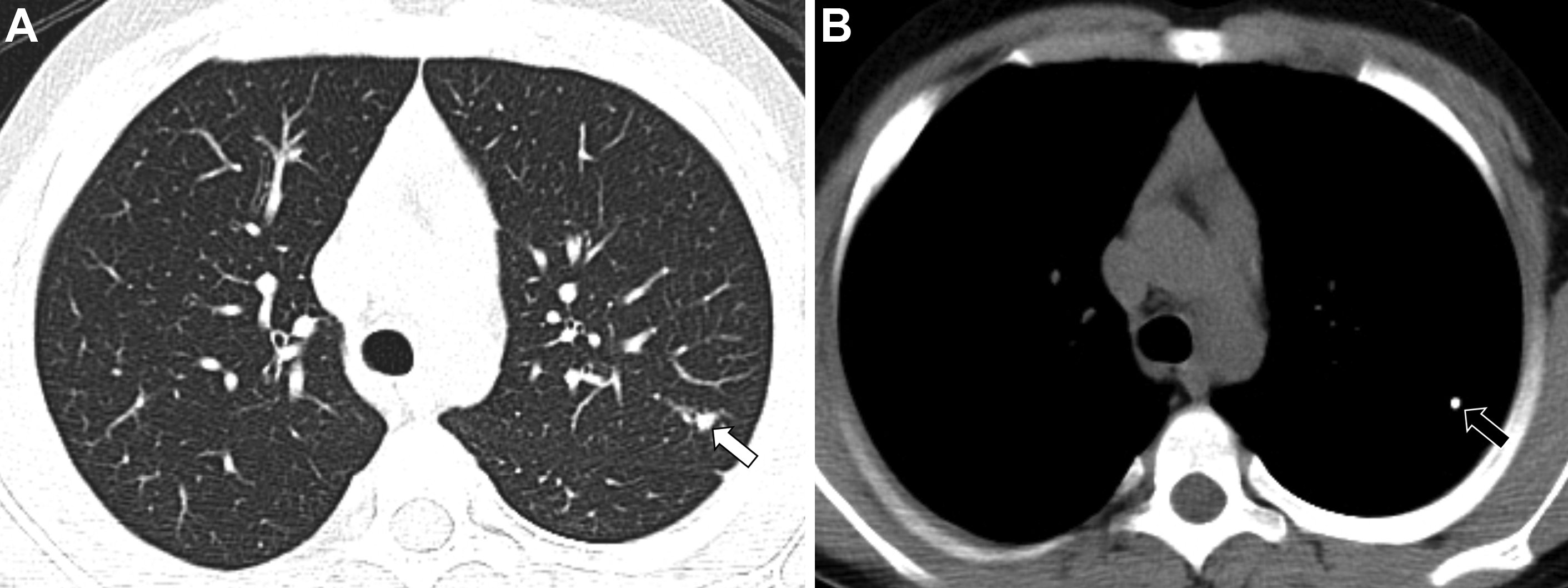
As discussed elsewhere in this article, incidental pulmonary nodules in healthy children are not infrequently seen and can be a diagnostic challenge. Pulmonary nodules in healthy children have been reported to have a large variety of characteristics, distributions, and sizes. Commonly reported shapes included round, triangular, and ovoid, and commonly reported margins include smooth, poorly defined, and irregular. The number of pulmonary nodules has also been reported to vary from a solitary nodule up to 4 nodules per patient. Although there is no consensus on the most common location, the peripheral location and lower lobes have been reported as the most frequent locations. , Incidental pulmonary nodules are commonly identified in the perifissural location in addition to within the lung parenchyma. The presence of calcification has also demonstrated to be variable ranging from 2% to 19% of the pulmonary nodules identified. ,
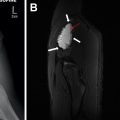
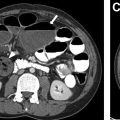
In addition to the smaller size described elsewhere in this article to suggest a benign appearance, additional classic features of a benign appearance include uniform calcification, suggesting a granuloma ( Fig. 1 ), the presence of fat and popcorn calcification, suggesting a hamartoma ( Fig. 2 ), and long-term stability. The presence of a nodule in a peripheral or perifissural location, with an oval or polygonal shape, and demonstrating a connection to pleura via linear extension (pleural tag) would be highly suggestive of an intrapulmonary lymph node ( Fig. 3 ).