Fig. 9.1
Curved array transducer position
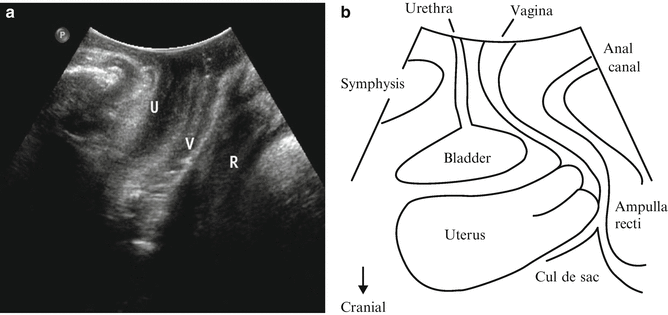
Fig. 9.2
(a) Two-dimensional orientation of translabial ultrasound image in midsagittal line. U urethra, V vagina, R rectum (b) Illustration demonstrating pertinent anatomy in midsagittal plane
Normal Ultrasound Anatomy
Urethra
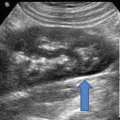
The urethral complex appears immediately caudal to the pubic symphysis and includes the urethral mucosa, smooth muscle, and surrounding vasculature (Fig. 9.3). The normally oriented urethra is parallel to the incident ultrasound beam and appears hypoechoic. With increasing degrees of urethral hypermobility (as with Valsalva maneuver), the proximal urethra will begin to rotate in the posteroinferior direction, more perpendicular to the ultrasound beam, and the urethral complex progressively appears less hypoechoic.
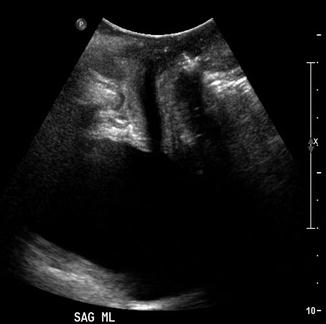

Fig. 9.3
Hypoechoic urethra
The fibers of the rhabdosphincter are oriented transversely to the incident beam in the normally oriented urethra, and the rhabdosphincter thus appears hyperechoic. With progressive urethral hypermobility, rhabdosphincter orientation shifts in relation to the ultrasound energy and may become less hyperechoic [2]. Periurethral connective tissue appears hyperechoic relative to the urethral complex, though clearly less echogenic than the adjacent inferoposterior margin of the pubis. Frequently observed punctate echogenicities within the urethra that are likely calcified periurethral glands appear to be of no clinical significance [3].
Bladder Neck
Multiple techniques are described, but the position of the bladder neck may be the most reliably described relative to the inferoposterior margin of the symphysis pubis [4]. Though bladder fullness influences examination findings, volumes have not been standardized. A full bladder may reduce sensitivity in demonstrating the degree of bladder neck mobility, and an empty bladder makes it more difficult to identify the location of the bladder neck [5].
Bladder neck descent (BND ) is conventionally determined by measuring the displacement of the bladder neck from rest to maximum Valsalva relative to the inferoposterior symphysis pubis margin (Fig. 9.4). The normal amount of BND has yet to be defined with numerous proposed cutoffs ranging between 15 and 30 mm. It does appear that the greater the BND, the more likely the patient is to have stress urinary incontinence. Many variables confound the measurement of BND, including Valsalva maneuver effort, bladder fullness, parity, and recruitment of levator ani contraction at the time of Valsalva [6]. Alternatively, bladder neck mobility may be measured by retrovesical angle (RVA), defined as the angle formed by the proximal urethra and the bladder trigone. This angle measures urethral rotation around the urethral axis as demonstrated (Fig. 9.5). Normal RVA values, as commonly observed in continent patients, range from 90° to 120° [7]. RVA can be used to radiographically classify cystoceles using the criteria proposed by Green et al., which has been shown to have moderate to good correlation with clinical examination [8].
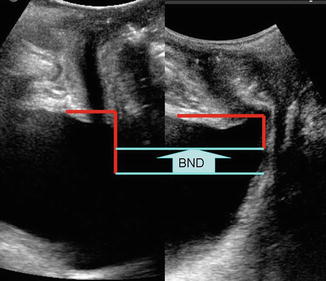
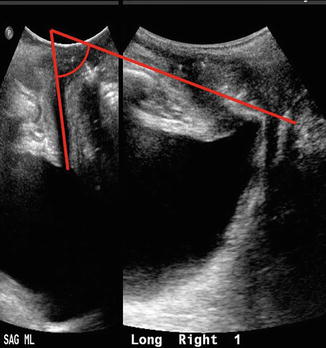

Fig. 9.4
Bladder neck descent . Left side image without Valsalva maneuver, right side image with Valsalva

Fig. 9.5
Retrovesical angle measurement describing urethral rotation
Abnormal funneling of the bladder neck and proximal urethra may also be observed both at rest and with Valsalva maneuver and with or without significant BND or enlarged RVA. Furthermore, bladder neck funneling is related to urethral hypermobility, greater rotation of the bladder neck, low Valsalva leak point pressures, and low urethral closing pressures [9].
Bladder
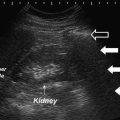
The bladder is examined for position, wall thickness, intravesical volume, intravesical lesions, and adjacent ureterectasis of the distal ureters. In patients without prolapse, the bladder is positioned above the inferior margin of the symphysis pubis. Cystocele may be understaged if the bladder is examined only when fully distended, particularly in patients with stenosis of the vaginal introitus, as this will prevent the bladder from descending during Valsalva maneuvers. If the bladder is examined while partially or completely distended, the luminal surface of the posterior wall in a partially filled bladder appears hyperechoic due to through transmission of the incident beam. The bladder lumen should be examined for echogenic material such as debris secondary to infection, or urolithiasis. While bladder ultrasound is inadequate for complete oncologic screening, the urothelial surface should be examined for neoplasm. The inter-ureteric ridge is also commonly apparent. The distal ureter may be visualized posterior to the ridge with lateral movement of the transducer. Inspection of the ureter at this level may reveal ureterectasis, possibly indicative of vesicoureteral reflux, ureteric obstruction, or normal physiology. Doppler flow ultrasonography may reveal the presence of a ureteral urine jet. The absence of a ureteral jet does not establish ureteral obstruction; thus the clinical utility of a ureteral jet is debated [10].
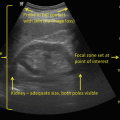
Much has been made of detrusor wall thickness (DWT ). Association between DWT and the presence of urodynamically proven detrusor overactivity, obstruction, stress incontinence, and p det/Q max has been described [11]. Likewise, DWT has been investigated as a predictor of de novo detrusor overactivity following anti-incontinence surgery [12]. In a compliant bladder, DWT lessens with increased bladder filling. No consensus exists regarding bladder volume at which DWT is calculated. DWT is calculated most commonly by measuring the wall thickness at three discreet locations: posterior wall, dome, and anterior wall (Fig. 9.6). Some authors consider normal DWT to be 5 mm or less [13], but this is controversial [14].
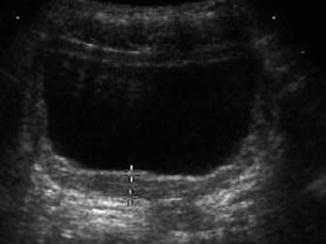

Fig. 9.6
Bladder wall thickness
3D/4D Assessment of Levator Ani Complex
With the adoption of 3D/4D ultrasound, practitioners now have an alternative to magnetic resonance imaging in the evaluation of the levator ani complex in the axial plane. Advantages of 3D ultrasound in comparison to MRI include low cost, reproducibility, and real-time imaging where imaging planes can be modified for optimal visibility of anatomic structures. One recent multi-institutional study has found a sensitivity of 0.078 and specificity of 0.86 in detecting levator ani defects detected on MRI; however, because of interobserver disagreement the authors concluded widespread implementation may be limited [15]. The technique is similar to 2D imaging, with orientation set in the midsagittal plane. To obtain volumes, an acquisition angle between 70° and 85° is needed to capture pertinent anatomy (levator hiatus, vagina, paravaginal tissue, urethra, puborectalis muscle, anorectal angle). Higher acquisition angles may be needed in women with significant prolapse, as displacement of lateral structures may occur. Display modes for 3D ultrasound are best depicted in three cross-sectional planes including the axial, coronal, and midsagittal plane (Fig. 9.7) [16]. A thorough assessment of the pelvis can be conducted in real time, assessing for musculofascial defects, measuring downward displacement of pelvic organs, and the assessment of the levator hiatus with Valsalva maneuver. Special attention should be placed to the inferomedial aspect of the puborectalis muscle which can account for delivery-related trauma predisposing women to pelvic organ prolapse . The levator hiatus lies between the two muscle bellies of the puborectalis muscle’s attachment to the pubic bone. Within this space the urethra can be found anteriorly, the rectum posteriorly, and the vaginal canal medially. Measurements are taken at the midsagittal plane, measuring the anteroposterior distance, then an axial plane is used to obtain the anteroposterior and transverse diameter. Levator hiatus area measuring >25 cm2 has been defined as abnormal on Valsalva maneuver [17]. Defects in the levator ani complex can be quantified using tomographic ultrasound imaging (TUI), which displays an axial multislice image. This modality is performed while the patient is performing a pelvic contraction and begins at 5 mm below to 12 mm above the plane of minimal hiatal dimensions [18]. The slices are obtained in 2.5 mm intervals and eight images are produced. A score of zero is used if there are no defects graded with 16 being consistent with bilateral avulsion. Imaging in the 4D mode allows a real-time dynamic investigation of the pelvic floor. Valsalva maneuver can be done in real time to assess defects in the rectovaginal septum and detachment of the puborectalis from the pubis can be appreciated during voluntary levator contraction (as with Kegel exercise). Pelvic floor muscle contractility can be assessed in the midsagittal view and measured by the displacement of the bladder neck and reduction of levator hiatus anteroposterior diameter. Although pelvic floor muscle tone is important for continence, one recent study did not show a strong association with contractility measured on ultrasound or physical exam and urinary continence [19]. However, defects in the pubovisceral musculature delineated by 3D/4D ultrasound are associated with larger levator hiatus dimensions, and severity of pelvic organ prolapse [20]. Despite this clinical relation , one recent study found no association between pubovisceral muscle avulsion in cadavers undergoing translabial ultrasound and pubovisceral muscle avulsion at dissection [21]. Thus, further investigation is necessary to correlate sonographic findings with functional and anatomical pelvic floor disorders .
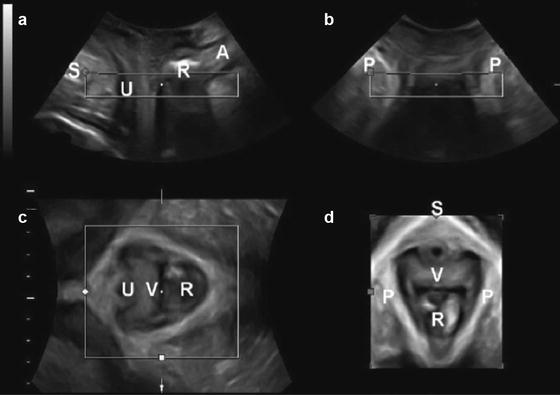

Fig. 9.7
3D pelvic floor ultrasound . (a) midsagittal plane; (b) coronal plane; (c) axial planes; (d) rendered axial plane. *A anal canal, P puborectalis muscle, R Rectal ampulla, S symphysis pubis, U urethra, V vagina. Source: American Journal of Obstetrics and Gynecology, Pelvic floor ultrasound: a review, Dietz HP, April 2010, Vol 202, Issue 4, Fig 2, with permission from Elsevier
Common Abnormal Findings
Urethra
As mentioned above urethral hypermobility may be suspected by static ultrasound imaging and confirmed with dynamic ultrasonography with Valsalva maneuvering. Periurethral tissues should be homogenous and without hypoechoic, cystic-appearing lesions. Periurethral lesions may include urethral diverticulum as well as nearby Gartner’s duct cysts. Large urethral diverticula are readily imaged with ultrasound. Typically, urethral diverticula arise from the dorsal aspect of the urethra. Less frequently, they arise ventrally from the urethra. Ultrasound is not as sensitive as MRI for small diverticula, but ultrasound can confirm an otherwise palpable suburethral fluctuance suggestive of diverticulum [22, 23].
Gartner’s duct cysts and Bartholin’s gland lesions may be readily distinguished from urethral diverticula. The former lesions are stationary on Valsalva maneuver while the diverticula are affixed to the urethra and thus commonly mobile on Valsalva. Diverticula of the urethra are commonly septated and contain heterogeneously echogenic material. Bartholin’s and Gartner’s cysts are typically homogenous in appearance [24]. Urethral ultrasonography may also reveal the presence and extent of periurethral fibrosis. This may be important in patients with urethral stricture desiring reconstruction, or in planning extent of urethrolysis, or anti-incontinence surgery.
Bladder
Bladder ultrasonography may reveal bladder diverticula as well. These may appear as periureteral cystic lesions near the trigone and inter-ureteric ridge or along the posterolateral walls and dome. Ureteroceles may also be visualized, particularly with ureterectasis. Bladder calculi may also be identified as hyperechoic intravesical filling defects with posterior shadowing. Papillary bladder lesions may be identified as intravesical echogenic foci attached to the bladder wall. These lesions are best imaged with a full bladder and may easily be missed without bladder distension. Bladder ultrasound may reveal significant detrusor fibrosis and thinning of the wall, perhaps suggestive of patients at risk for bladder rupture during planned hydrodistension. Ultrasonography may also be valuable in office-based imaging of vesicovaginal fistulae. Particularly in radiation-induced fistula where physical exam is often precluded by patient discomfort and distal vaginal stenosis, office-based ultrasound may prove useful in identifying extent of fibrosis and ischemia. With increasing use of mesh in vaginal reconstruction, hyperechoic mesh is increasingly encountered. With 3D reconstruction, the lattice structure of the mesh is visible. This is more thoroughly explored later in the chapter.
Apical and Posterior Compartments
Basics of Apical and Posterior Prolapse Assessment
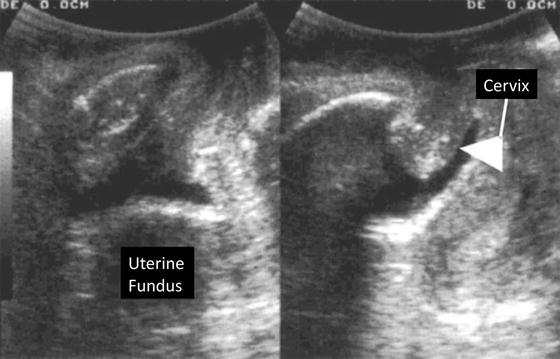
Translabial ultrasound can be used for the assessment of apical and posterior wall prolapse [25, 26]. A full sonographic assessment of the apical portion of the vagina includes the uterus. The uterus can be difficult to visualize due to a few factors: it is iso- to hypoechoic making it similar and difficult to discern from vaginal tissues, a retroverted position can be obscured by a rectocele or hidden by rectal contents, and overall it is small in size in postmenopausal women. There are some clues that can be used to localize the uterus. The cervix is typically seen as a specular echo which represents its leading edge. A specular reflector reflects sound waves with minimal scatter usually producing a bright linear echo. The uterus can also be identified by locating nabothian follicles which are oftentimes seen within the cervix. Translabial ultrasound can be readily used to locate the cervix, especially in patients with prolapse (Fig. 9.8). The same can be said for identifying a prolapsed vault posthysterectomy.