Fig. 6.1
Patient is placed in a supine position with a towel placed under the scrotum
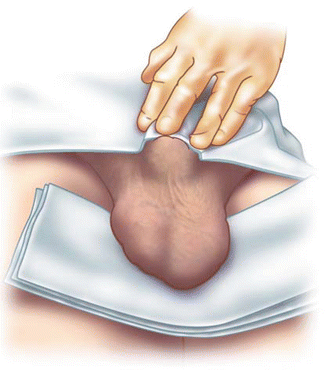
Fig. 6.2
Patient positioning: the phallus is positioned up on the pubis, held by the patient or a towel
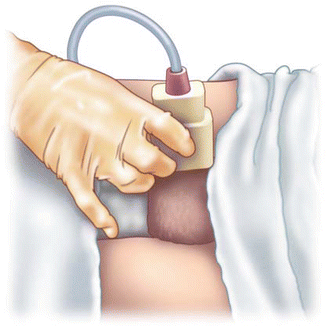
Fig. 6.3
Sonographer performing a longitudinal view of the testis. Note the use of the fifth finger on the patient’s thigh to help steady the transducer and minimize movement of the testis in the scrotum
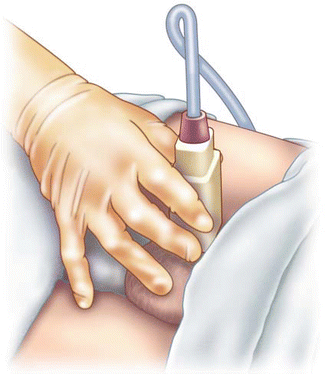
Fig. 6.4
Sonographer positioning the transducer for a transverse view of the testis. Note again, the use of the fifth finger on the patient’s thigh to help steady the transducer and minimize movement of the testis in the scrotum
Transducer Selection
The choice of the frequency used is determined by a balance between depth of penetration required and the detail of the image required. As the frequency increases the image resolution (axial resolution) improves and the depth of penetration decreases. Broad bandwidth transducers allow for multiple focal zones, eliminating the need for adjustment during the examination. Multiple frequency transducers allow the transducer to be set to several distinct frequencies. A high frequency (7–18 MHz) array transducer is most often used for scrotal scanning. A linear array probe with a “footprint” able to measure the longitudinal length of testis is ideal. A curved array probe can be used with a thickened scrotal wall or in the presence of scrotal edema or for a large testis. The curved array transducer is also useful to compare echogenicity of the testes; however, the frequency is usually lower with a curved array probe, resulting in decreased axial resolution. Color and spectral Doppler are essential elements of scrotal ultrasound because they provide documentation of testicular blood flow and paratesticular findings. The highest possible Doppler frequency, typically in the 5–10 MHz range providing the best axial resolution and blood flow detection, should be used [1].
Overview of the Exam
The American Institute of Ultrasound in Medicine (AIUM) guidelines recommend that scrotal ultrasound should be performed in at least two planes: longitudinal and transverse. In the longitudinal view, the standard orientation of the image should be with the superior pole of the testis to the left and the inferior pole to the right on the monitor screen (Fig. 6.5). In the transverse plane, the standard orientation is for the patient’s right testis to be the right side of the screen. Therefore, for the right testis, the lateral aspect is located on the left side of the screen and the medial aspect to the right. Conversely, for the left testis, the lateral aspect should be to the right and the medial aspect to the left (Fig. 6.6).
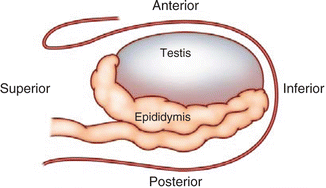
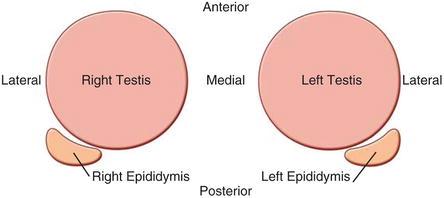

Fig. 6.5
Schematic view of longitudinal scrotal ultrasound

Fig. 6.6
Schematic view of transverse scrotal ultrasound as seen on the ultrasound screen with the right testis on the left and left testis on the right. The relative positions of each epididymis is also demonstrated
The evaluation of the scrotal contents should begin with a longitudinal survey scan, progressing medial to lateral to get an overall impression of the testis and paratesticular structures. If the testis is larger than the footprint of the transducer, it is important to document views of the superior and inferior portions of the testis including the epididymis in these regions. The transverse view is obtained by rotating the transducer 90°. A survey scan is performed using the mid-testis as a starting point and proceeding first toward the superior pole then back to the mid-testis before scanning to the inferior pole.
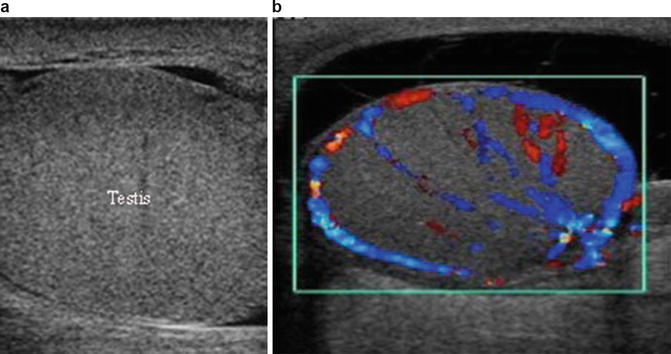
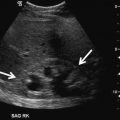
At least one image should visualize both testes to document the presence of two testes and their relative echogenicity (Fig. 6.7) [2]. Measurements of the testicular width and height are taken and documented at the mid-testis. A measurement should also be made of the long axis at the mid-testis and a testicular volume is calculated (Fig. 6.8). If the equipment being used has split-screen capabilities, comparative views of echogenicity can easily be made and documented.
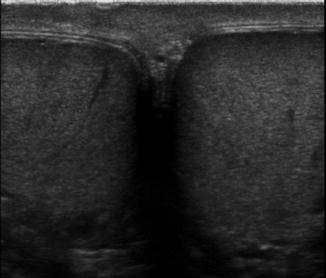
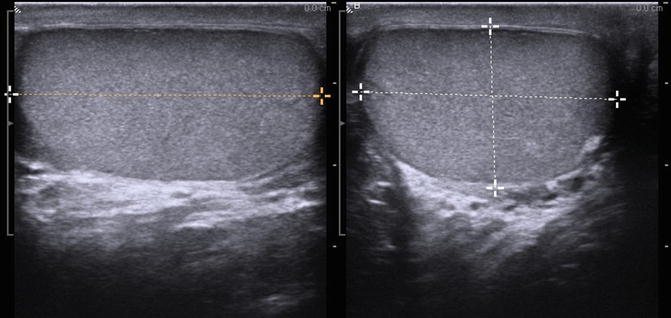

Fig. 6.7
Gray scale side-by-side view of both testes in a single image. This image is important to confirm the presence of two testes

Fig. 6.8
Gray scale ultrasound in transverse and longitudinal planes used to measure the testicular volume
All relevant extratesticular structures should be evaluated, including but not limited to the epididymis, spermatic cord, and scrotal skin. Techniques that improve visualization, such as Valsalva maneuver or upright positioning, may be used as needed.
Color and Spectral Doppler
Color and spectral Doppler should be considered an integral part of the scrotal US examination. Many inflammatory, neoplastic, and benign conditions have characteristic flow patterns that can assist in diagnosis. At least one side-by-side image containing both testes with identical Doppler settings should be included to evaluate symmetry of flow. If blood flow cannot be visualized on color Doppler (Fig. 6.9a), power Doppler may increase the sensitivity to detect blood flow (Fig. 6.9b) [1].
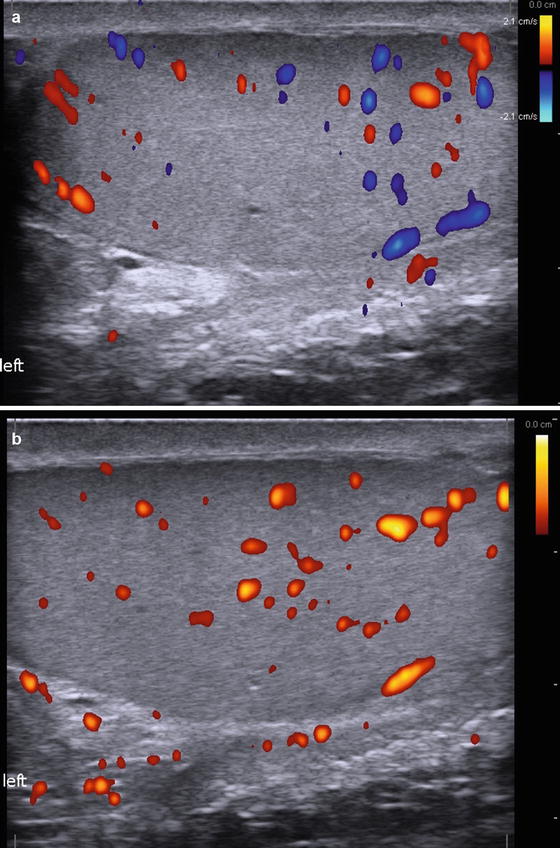

Fig. 6.9
(a) Color Doppler Ultrasound demonstrating testicular blood flow. (b) Power Doppler Ultrasound demonstrating testicular blood flow. Note lack of directionality with Power Doppler
Documentation
The written report and archived images are a reflection of the quality of the examination. The old adage “If it’s not documented, it wasn’t done” should guide the sonographer in developing a quality report. The static images obtained during the evolving ultrasound exam should represent the sonographer’s impression of the findings. If electronic storage space is available and the equipment allows, video clips, which demonstrate important findings and survey scans, can and should be obtained. A quality report can aid in diagnosis and is therefore in the best interest of our patients.
All the measurements and anatomical findings of the exam should be documented. Images should be attached to the report. It is essential to include patient identification information, the exam date, and the indications for performing the exam. The transducer used and its frequency should also be documented. The area of interest should be clearly identified. The orientation and measurements should be labeled along with the pertinent anatomy and any abnormalities. There is no minimum number of images that are required for proper documentation. It is a best practice to provide images that depict the measurements being taken and the pathology being described. The physician who performed the exam should sign the report.
Indications
There are many specific indications for scrotal ultrasound (Table 6.1 ). Scrotal ultrasound is often performed when the physical examination is inconclusive or difficult to complete (or both) because of patient discomfort or inability of the examiner to precisely identify the scrotal structures on palpation. In these instances the scrotal ultrasound examination is therefore an integral part of the physical examination of the male genitalia. In other situations, ultrasound evaluation is essential to diagnosis and treatment and is well supported in the literature. However, the decision on whether or not to obtain an ultrasound study is discretionary and without a clearly defined evidence-based approach [3, 4].
Table 6.1
Indications for scrotal ultrasound
1. Assessment of scrotal mass |
(a) Painful enlargement |
• Epididymitis/orchitis |
• Testicular abscess |
• Torsion |
(b) Nonpainful enlargement |
• Testicular tumor |
• Hydrocele |
• Varicocele |
• Spermatocele/epididymal cyst |
• Scrotal hernia |
• Cyst |
2. Evaluation of scrotal trauma |
(a) Testicular rupture |
(b) Hematocele |
3. Evaluation and management |
(a) Detection of occult primary tumors in patients with metastatic germ cell tumors |
(b) Follow-up of patients with prior primary testicular neoplasms, leukemia, or lymphoma |
(c) Evaluation of abnormalities noted on other imaging modalities (CT., MRI, PET, etc.) |
(d) Evaluation of intersex conditions |
4. Investigation of empty/abnormal scrotal sac |
(a) Undescended testis |
(b) Thickened scrotal skin |
5. Evaluation of male infertility and related issues |
(a) Varicocele |
(b) Intratesticular microcirculation |
(c) Atrophic testis |
(d) Microlithiasis |
(e) Impaired semen quality |
(f) Azoospermia |
(g) Antisperm antibody |
6. Postoperative follow up |
(a) Varicocele |
(b) Testis biopsy |
(c) Hydrocelectomy |
(d) Patients with indeterminate scrotal masses |
Normal Anatomy of the Testis and Paratesticular Structures
Scrotum
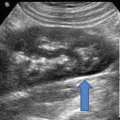
The normal scrotal wall thickness varies between 2 and 8 mm. The scrotal wall contains the following structures: rugated skin, superficial fascia, dartos muscle, external spermatic fascia, cremasteric fascia, and internal spermatic fascia. The scrotum is separated into right and left hemiscrotal compartments by a septum termed the median raphe. As the testis descends in utero from the abdomen, it acquires each layer of the scrotal compartment. The external spermatic fascia is derived from the external oblique fascia and is attached to the external inguinal ring. The cremasteric fascia and muscle derive from the internal oblique muscle. Encasing each testis is the tunica vaginalis, derived from the peritoneum, which consists of parietal and visceral layers. These layers are normally separated by 2–3 mL of straw colored fluid often referred to as a physiologic hydrocele. Ultrasound of this fluid is seen as a thin echo free rim around the head of the epididymis [5] (Fig. 6.10). The parietal and visceral layers join at the posterolateral aspect of the testes where the tunica attaches to the scrotal wall [6].
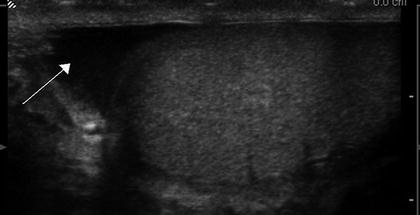

Fig. 6.10
Gray scale ultrasound showing physiologic hydrocele (arrow)
Testis
The size and shape of the testis changes with the age, influenced by gonadotropic hormones testicular volume gradually rises from birth to up to 5 months of age due to peak in gonadotropic hormones levels [7, 8]. After 5 months of age, the testicular volume steadily declines and reaches its minimum volume at approximately 9 months of age and remains approximately the same size until puberty [9]. In newborns, the testis is round and gradually becomes ovoid with growth. The echogenicity of the testis increases in puberty due to the development of germ cell elements [10]. The adult testis is a smooth ovoid gland, approximately 4–5 cm long, 3 cm wide, 2–3 cm in the anterior–posterior (AP) dimension, and typically between 20 and 30 mL in volume .
The testis exhibits medium homogenous echogenicity. A dense fibrous capsule the tunica albuginea envelops the testis, which is apparent as a thin echogenic line on ultrasound. Each testis has approximately 200–300 cone-shaped lobules each containing at least one seminiferous tubule [11] (Fig. 6.11). The lobules are separated by the fibrous septa of tunica albuginea that extend from the mediastinum of the testis in to the parenchyma of the testis [12]. Testicular lobules are occasionally identified on ultrasound as lines radiating from the mediastinum testis (Fig. 6.12). The seminiferous tubules contained within the lobules open into dilated spaces called rete testis within the mediastinum. The seminiferous tubules are long V-shaped tubules, both ends of which usually terminate in the rete testis. The rete testis is connected to the head (caput or globus major) of the epididymis with about 8–12 efferent ductules. The normal rete testis is sonographically evident in 18 % of patients as a hypoechoic area with a striated configuration adjacent to the mediastinum testes [13]. The mediastinum testes appear as a linear avascular echogenic band on ultrasonography [14] (Fig. 6.13).
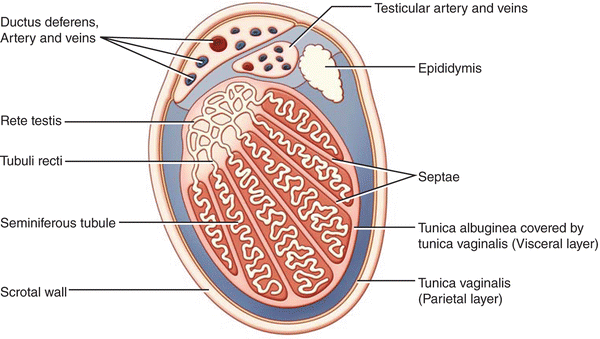
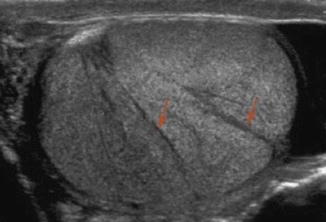
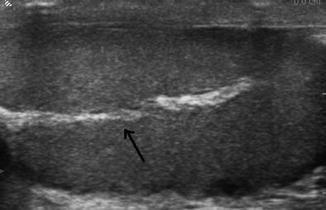

Fig. 6.11
Schematic cross section of the testis

Fig. 6.12
Gray scale ultrasound of a normal testis demonstrating testicular lobules separated by fibrous septa (arrows)

Fig. 6.13
The mediastinum testis appears as an avascular echogenic line (arrow)
Epididymis
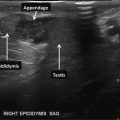
The adult epididymis is 6–7 cm long and has three parts, the head (caput) measuring 10–12 mm in diameter, the body (corpus) measuring 2–4 mm in diameter, and the tail (cauda) about 2–5 mm in diameter. In the normal epididymis, the head is routinely identified at posterolateral to the upper pole of the testis. The caput epididymis is triangular in shape, often has the same echogenicity as the testis (Fig. 6.14). However, it can be heterogenous with areas that are hyper or hypoechogenic. The smaller corpus epididymis can be seen as a hypoechoic structure containing multiple echogenic linear structures representing the coiled epididymal tubule and lies posteriorly along the long axis of the testis (Fig. 6.15).
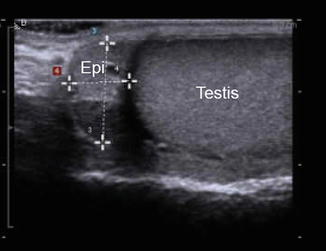
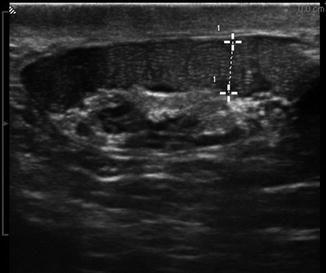

Fig. 6.14
The caput epididymis is triangular in shape (E) and is usually isoechoic or slightly hypoechoic compared to the testis

Fig. 6.15
Gray scale image of a dilated corpus epididymis in a vasectomized man
Vascular Anatomy
The spermatic cord is normally seen superior to the posteromedial aspect of the testis and contains the vas deferens, the testicular and cremasteric and deferential arteries, the pampiniform plexus of veins, genital branch of the genital femoral nerve, testicular plexus of the sympathetic trunk, and lymphatic vessels [12]. The blood supply to the scrotal structures is from three primary arteries: the testicular, deferential, and cremasteric arteries (Fig. 6.16). The testicular artery testis or gonadal artery, which arises from the aorta and courses through the scrotum with the spermatic cord, is the major supply to the testis. The deferential artery, which arises from the superior vesical artery and supplies the vas deferens and epididymis. The cremasteric artery, a branch of the inferior epigastric artery, which supplies the scrotal skin and coverings of the spermatic cord. As the testicular artery approaches the posterolateral aspect of the testis it divides. The branches pierce through the tunica albuginea to run in a layer called the tunica vasculosa. Capsular arteries run peripherally in the tunica vasculosa, supplying centripetal arteries that course toward the mediastinum and divide further to recurrent rami that flow away from the mediastinum [14]. The veins draining the testis and epididymis converge to form the pampiniform plexus at the mediastinum on the superior pole of the testis. The pampiniform plexus is primarily drained by the testicular and external pudendal veins [15]. The testicular vein on the left drains into the renal vein and the testicular vein on the right directly into the inferior vena cava [16].
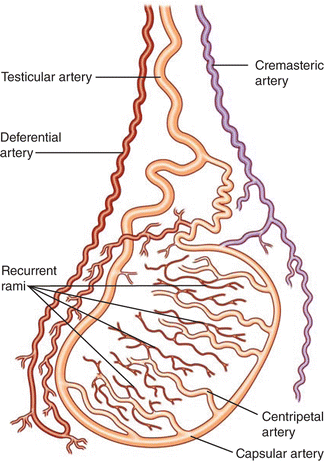

Fig. 6.16
Blood supply of testis . The testis is supplied by three sources of blood supply. (1) The testicular artery, which arises from the aorta. (2) The deferential artery, which arises from the superior vesical artery. (3) The cremasteric artery, a branch of the inferior epigastric artery
Ultrasonography with Color-Flow imaging provides visualization of the intratesticular, epididymal, and paratesticular blood flow. Under normal conditions, Color Flow images show equivalent vascularity of the bilateral testis. When vascularity is not well visualized, Power Doppler increases the sensitivity of detection. Spectral Doppler is used to calculate the Resistive Index (RI) of intratesticular arteries. The RI of intratesticular arteries has been found to correlate with testicular function .
Embryology Relevant to Ultrasound Imaging of the Scrotum
A basic understanding of the embryologic development male gonad and adnexal structures guide the interpretation of many abnormalities encountered in the scrotal ultrasound. Presented in this section is the embryology relevant to the ultrasound evaluation of the scrotum. There are many excellent textbooks available which present the known elements of male genitourinary development should the reader desire a more comprehensive treatise on the subject [17–20].
Early Gonadal Developmental Anatomy
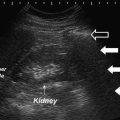
In the 3-week-old embryo (Fig. 6.17), primordial germ cells, originating in the wall of the yolk sac near the attachment of the allantois, migrate along the wall of the hindgut and through the dorsal mesentery into the urogenital ridge. The urogenital ridge is a protrusion of intermediate mesoderm that indents the coelomic cavity (precursor to the peritoneal cavity) on each side of the mesentery. The kidney precursors, gonads, and the proximal portions of the reproductive tracts develop from the urogenital ridge. The unilateral absence of the male reproductive ducts or gonad and reproductive ducts is associated with ipsilateral absence of the kidney in 20–85 % of cases [21]. It is therefore reasonable to evaluate the retroperitoneum for the presence of a kidney in a patient where the unilateral reproductive ducts are absent on ultrasound evaluation.
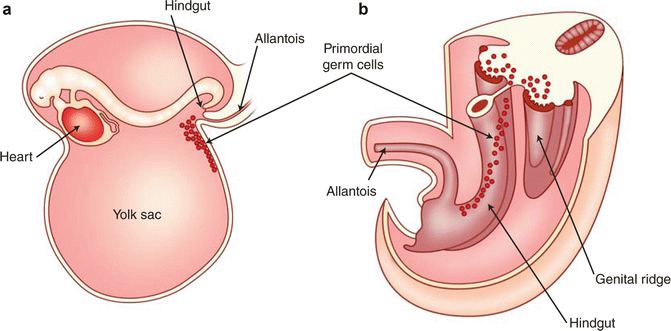

Fig. 6.17
Primordial germ cells at their location in yolk sac as they begin their migration (a). The primordial germ cells migrating through the mesentery to the genital portion of the urogenital ridge bulging into the coelomic cavity (b)
In the 5-week-old embryo (Fig. 6.18), the two early excretory organ systems (the pronephros and mesonephros ) begin to regress. The mesonephros constitutes the lateral portion of the urogenital ridge and consists of mesonephric tubules that interact with glomeruli-like vessels extending from the aorta at one end and drain into a mesonephric duct at the other end. The regression begins at the cranial aspects and continues toward the caudal end where the duct joins the cloaca (and at what will eventually diverge from the cloaca into the urogenital sinus). The ureteric bud (also referred to as the metanephric diverticulum) develops as a dorsal bud of the mesonephric duct near its insertion into the cloaca. The metanephros (precursor to the adult kidney) forms from the interaction of the metanephric diverticulum and a region of cells in the intermediate mesoderm known as the metanephric cell mass.
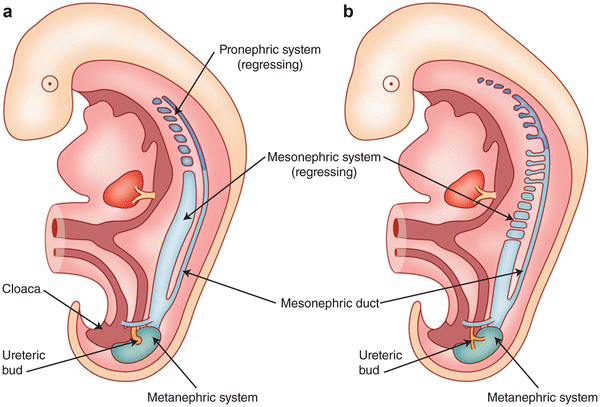

Fig. 6.18
Development of the early excretory system. Regression of the pronephros (a) and mesonephros (b) with the development of the metanephric system
At 7 weeks (Fig. 6.19), the reproductive ducts of embryo remain devoid of phenotypic manifestations of sexual differentiation. There are two, roughly parallel pairs of genital ducts: in addition to the mesonephric (Wolffian) ducts there are the paramesonephric (Müllerian) ducts, which are located more laterally. Along the medial portion of the urogenital ridge, cords of coelomic epithelium termed sex cords have invaginated in to the ridge and house the primordial germ cells. This portion of the urogenital ridge becomes the gonadal ridge and the most superficial aspects of the sex cords regress to completely separate the sex cords from the rest of the coelomic epithelium.
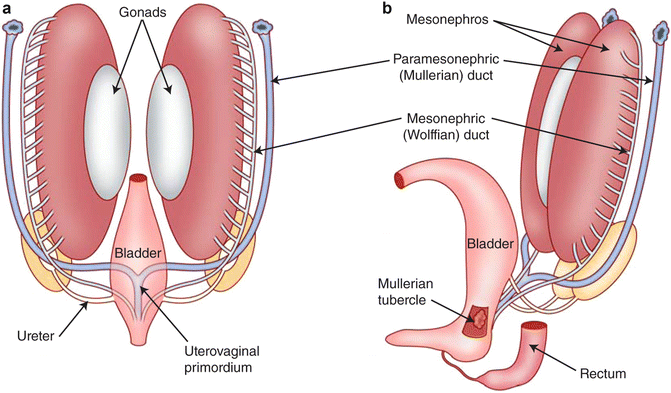

Fig. 6.19
The reproductive ducts and their relationship to the developing gonads after the incorporation of the distal mesonephric ducts into the urogenital sinus
It is the presence of a Y chromosome gene, called the sex-determining region Y gene (SRY ), that results in the development of testes and the initiation of phenotypic sexual differentiation. The testis forms in the gonadal ridge and Sertoli cells differentiate from cells within the epithelial sex cords. By week 8, the developing fetal testis produces at least two hormones. The first has been referred to as Müllerian-inhibiting substance or factor (MIS, MIF) and, in other reports, as anti-Müllerian hormone (AMH). It is produced by the fetal Sertoli cells and suppresses the development of the paramesonephric duct (which in the absence of this suppression would develop into the upper female reproductive tract organs). The other, testosterone, stimulates development of the mesonephric ducts into components of the male genital tract. Vestigial remnants of portions of these ducts (Fig. 6.20) often can be visualized sonographically.
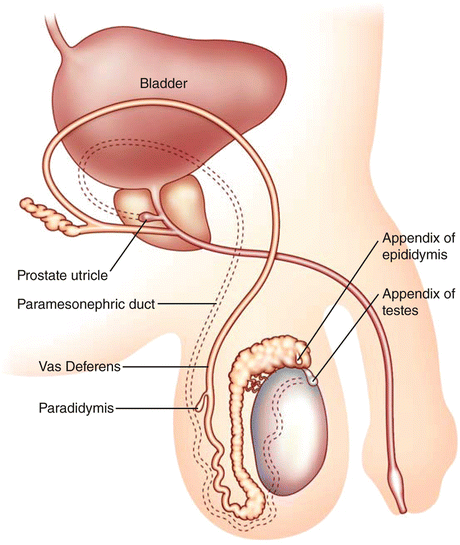

Fig. 6.20
Schematic showing the most frequent location of testicular and epididymal appendages and other vestigial remnants of the mesonephric and paramesonephric ducts in the male genitalia
Remnants of the most cranial aspects of the regressed paramesonephric ducts can persist beyond the embryologic period as the appendix of the testis in and be demonstrated on ultrasound (Fig. 6.21). The most caudal vestiges of the paramesonephric ducts form midline structures and are often found in the prostate, which is derived from the embryonic urogenital sinus. The prostatic utricle is such a structure and when enlarged, be demonstrated on ultrasound. In addition, midline cysts derived from vestiges of the paramesonephric ducts may also be demonstrated. These midline paramesonephric remnants have also been found to obstruct the ejaculatory ducts and result in dilation of the seminal vesicles (anterior–posterior distance greater than 15 mm).
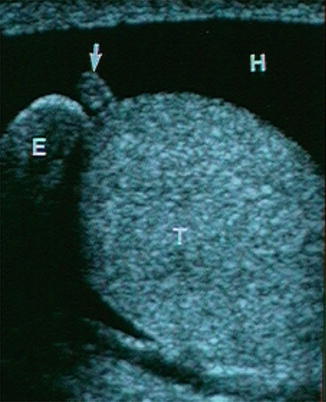

Fig. 6.21
Ultrasonic appearance of the appendix testis
The deepest aspects of the sex cords converge on what will become the mediastinum testis. The mesenchyme between each of the cords gives rise to the interstitial cells of Leydig and the septa of the testis that radiate out from the mediastinum testis. The sex cords develop into tubules composted of the germ cells and Sertoli cells and the inner ends of these tubes are referred to as the tubuli recti. The gonadal ridge, throughout its development, slowly separates from the mesonephros. The mesonephric structures develop under the paracrine influence of the testosterone produced in the neighboring testis. The mesonephric tubules adjacent to the testis develop into the ductuli efferentia and meet the tubuli recti at the rete testis. The mesonephric ducts develop into the epididymides, vasa deferentia, and ejaculatory ducts. The seminal vesicles develop as a diverticulum of this tube. Residual mesonephric structures can persist as a vestigial remnant of the mesonephric ducts. Those tubules located cranial to the tubules that become the ductuli efferentia of the testis may protrude off the epididymis as a polypoid vestige called the appendix epididymis (Fig. 6.22). In addition, remnant mesonephric tubules can persist proximal to those draining the testicle as well. This remnant is known as the paradidymis (aka organ of Giraldés), a small collection of convoluted tubules lined by ciliated epithelium, that can be found anywhere along the epididymis or vas deferens.
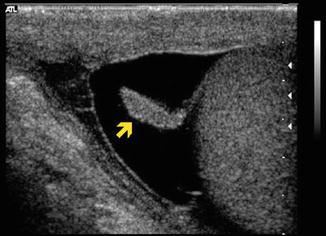

Fig. 6.22
Ultrasonic image of the caput epididymis with adjacent appendix epididymis
Torsion of the appendix testis, appendix epididymis, and paradidymis are all in the differential diagnosis of the acute scrotum . The appendix testis, appendix epididymis, and ectasia of the rete tubules can be visualized during the scrotal ultrasound and their characteristic appearance should be kept in mind to avoid confusion of these structures with testicular and extratesticular masses.
Testicular Descent
The process of testicular descent begins prior to 7 or 8 weeks in development. At this time, the gonadal position in the dorsal abdominal wall is similar in both sexes. In males, the fetal testis begins to produce MIS from the Sertoli cells. In addition, androgens and an insulin-like hormone 3 (INSL3) are produced from the Leydig cells [22]. These hormones work in concert to control descent of the testis, which is held by a suspensory ligament at the upper pole, and, at the lower pole by the genito-inguinal ligament, or “gubernaculum.”
During the initial phase of descent, the cranial suspensory ligament progresses and the gubernaculum thickens allowing the testis to be held near the inguinal region as the abdomen enlarges. The inguinal canal forms as the abdominal wall muscles develop around the thickened gubernaculum.
The subsequent phase of descent occurs several weeks later. At about 20–25 weeks an out-pouching of the peritoneal membrane, which is known as the processus vaginalis, travels with the testis toward its final position in the scrotum. The processus vaginalis maintains a connection with both the epididymal tail and the lower pole of the testis. At about 25 weeks, testis and attached processus vaginalis begin to pass through the inguinal canal along with fascial coverings from the abdominal wall (Fig. 6.23). The processus vaginalis is continuous with the peritoneum and tunica vaginalis of the testis. Between 25 and 30 weeks the testis descends rapidly through the inguinal canal and then more slowly across the pubis into the scrotum. The fascial coverings from the abdominal wall that travel with the testis during its descent become the layers covering the spermatic cord and testis. This process is usually completed by 35 weeks of gestation and it is followed by the obliteration of the proximal portion of the processus vaginalis to close the connection between the scrotum and peritoneum. Closure may occur during prenatal development or in early infancy. The gubernaculum does not become anchored to the scrotum until descent is completed [23–25].
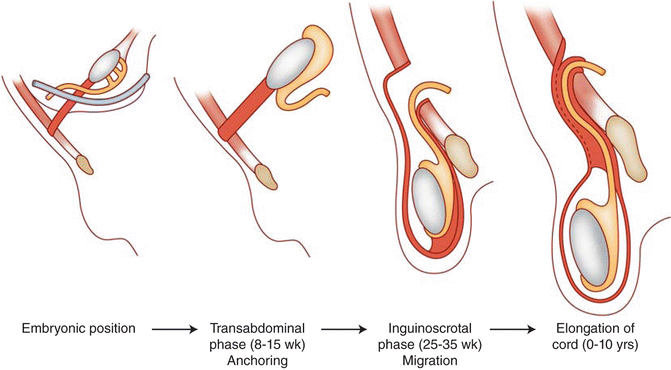

Fig. 6.23
The phases of testicular descent . At about 25 weeks, testis and attached processus vaginalis begin to pass through the inguinal canal along with fascial coverings from the abdominal wall
The embryology of testicular descent becomes important for the sonographer when evaluating the undescended or nonpalpable testis. A majority of undescended testes are found in the inguinal canal which is accessible to diagnostic ultrasound [26–29]. Ultrasound examination of the inguinal region is essential when a testis is not found on routine scrotal ultrasound .
Development of the Scrotum
The embryonic precursor to the scrotum is the labioscrotal folds. These structures originate as the embryonic cloaca develops and differentiates. The cloaca is a chamber shared by the allantois (which extends anteriorly from the cloaca into the umbilical cord) and the hindgut (Fig. 6.18a). The cloacal membrane makes up the ventral wall of the chamber and is located at the caudal end of the developing hindgut as a bilaminar apposition of ectoderm and endoderm located on the ventral midline. A septum develops as an ingrowth of folds from the lateral walls and a caudal extension of the intervening mesenchyme from the branch point of the allantois and hindgut, which ultimately divides the cloaca into the anterior/ventral urogenital sinus and the posterior/dorsal developing rectum. While the septum develops, mesodermal mesenchyme also encroaches between the two layers of the cloacal membrane. The septum also divides the membrane into a urogenital membrane and anal membrane. Both of these membranes ultimately rupture to create continuity between the ectoderm and both the urogenital sinus and rectum that will persist as the urethral meatus and anus. The mesenchymal tissues that have encroached around these orifices develop into the muscles and bones of the lower anterior abdomen and pubis.
The urethra prostate and bladder all develop from the urogenital sinus . The remnants of the caudal ends of the mesonephric ducts become incorporated into the urogenital sinus (Mullerian Tubercle, Fig. 6.19) and become aspects of the trigone and posterior urethra. The incorporated portions of the mesonephric ducts include the branch points of the metanephric ducts, which become the ureteral orifices. The unincorporated portions of the mesonephric ducts end up entering the urethra at the prostatic urethra as the ejaculatory ducts which channel the path of the testicular adnexal structures into the prostatic urethra.
The external male genitalia are indistinguishable from the female external genitalia until the eighth or ninth week of gestational. They both include the genital tubercles at the craniolateral edges of the cloacal membrane. The tubercles develop from mesoderm as it infiltrates the cloacal membrane. As the cloacal membrane divides to separate its anterior portion into the urogenital membrane, the tubercles fuse in the midline. The urogenital membranes and ultimately the urogenital sinus are flanked by collections of infiltrating mesoderm termed the urogenital folds with labioscrotal swellings located laterally on either side (Fig. 6.24). Masculinization of the indifferent external genitalia occurs under the endocrine influence of testosterone produced by the interstial Leydig cells of the fetal testis [30–32]. The tubercle becomes the future phallus and glans. The scrotum is formed by extension of the labioscrotal swellings between the pelvic portion and the anus. With testicular decent and migration of the gubernaculum into these labioscrotal swellings during the second phase of testicular descent, the scrotal sac takes shape around the testes and associated structures. The scrotal skin will ultimately become the point of contact with the ultrasonographer’s transducer and the window into which clinical questions about the scrotal contents can be explored.
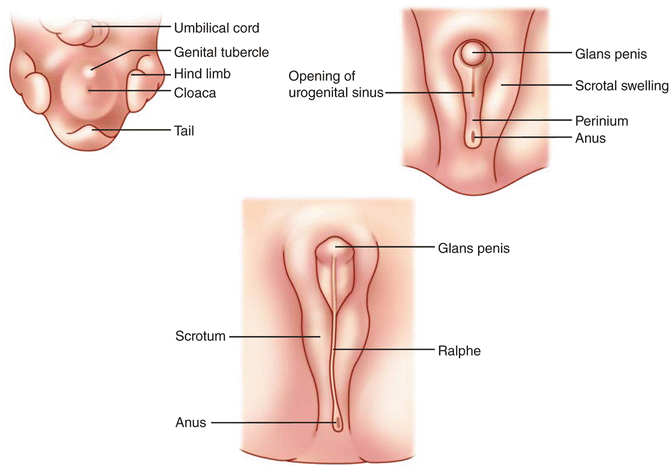

Fig. 6.24
The male external genital development progressing clockwise from the left. The genital tubercle develops into the glans penis. The upper right panel shows the urogenital sinus opening within the urogenital folds, which are between the scrotal swellings
Pathologic Conditions and the Scrotal Ultrasound
Extratesticular Findings
Hydrocele
Hydrocele is the most common cause of painless scrotal swelling. A hydrocele is a serous fluid collection between the parietal and visceral layers of the tunica vaginalis. The tunica vaginalis is a mesothelium-lined sac that results from closure of the superior portion of the processus vaginalis. This fascial structure normally covers the entire testis except the posterior border. It has a visceral layer and an outer parietal layer that lines the internal spermatic fascia of the scrotal wall. Hydroceles can be congenital or acquired. The congenital hydrocele or communicating hydrocele occurs when a patent processus vaginalis allows fluid to pass from the peritoneal space into the scrotum [33]. The acquired hydrocele may be idiopathic with no identifiable cause. The incidence of hydroceles is about 1 % of adult males. Hydroceles are usually anechoic on ultrasonography (Fig. 6.25). They may contain echogenic cholesterol crystals. The presence of septations is often associated with infection, trauma, or metastatic disease. Hydrocele may develop secondary to venous or lymphatic obstruction caused by infection, trauma, torsion, or tumor. About 10 % of testicular tumors are accompanied by a hydrocele; clinical suspicion increases with new onset of hydrocele in men in their 30s or 40s [34]. Scrotal ultrasound is essential to rule out testicular pathology in these patients. The testis is often posteriorly displaced by the hydrocele. A massive hydrocele exerts a pressure effect that may compromise blood flow within the testis. Vascular resistance in intratesticular arteries is increased, and color Doppler ultrasound may demonstrate an increase in the caliber of capsular arteries. Fluid aspiration and surgical excision of hydrocele sac has been shown to restore normal blood flow to the testis [35].
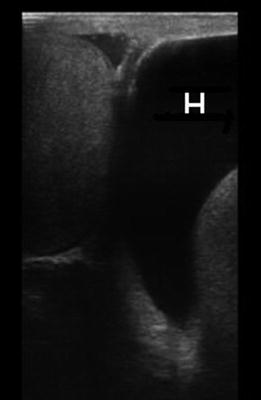

Fig. 6.25
Gray scale ultrasound showing a left hydrocele (H)
Pyocele
Pyocele is an accumulation of purulent material within the tunica vaginalis and is most often occurring because of untreated epididymo-orchitis. Pyoceles present with acute scrotal pain and symptoms of sepsis. A pyocele also appears heterogeneous on the ultrasonogram, and gas may be identified, causing hyperechoic reflections and shadowing [36] (Fig. 6.26).
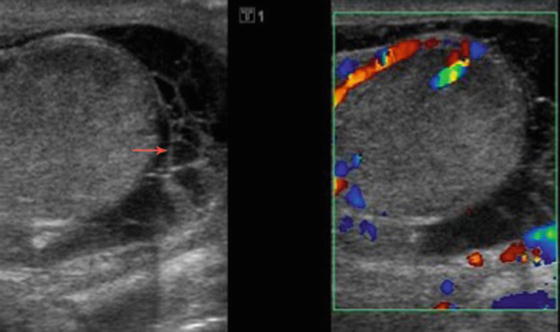

Fig. 6.26
A pyocele is seen as a complex heterogeneous fluid collection within the tunica vaginalis on gray scale ultrasound and without blood flow on Doppler study
Scrotal Hernia
Congenital inguinal hernia is due to failure of the processus vaginalis to obliterate and result in passage of intestinal loops or omentum or peritoneal fluid in the scrotal sac [12, 37, 38]. Right inguinal hernias are more common as the right processus vaginalis closes later. Scrotal ultrasound can be helpful for inconclusive physical examination. Clinically occult contralateral hernia can also be assessed with the ultrasound [39]. Patients with a scrotal hernia usually present with mesenteric fat and/or bowel loops seen superior to the testis. Real-time imaging can identify peristaltic activity or intestinal gas bubbles with their characteristic echogenic interfaces. Ultrasound of an omental hernia will demonstrate highly echogenic fat [40] (Figs. 6.27a, b and 6.28).
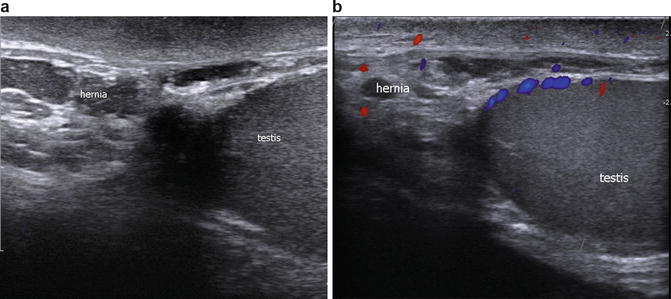
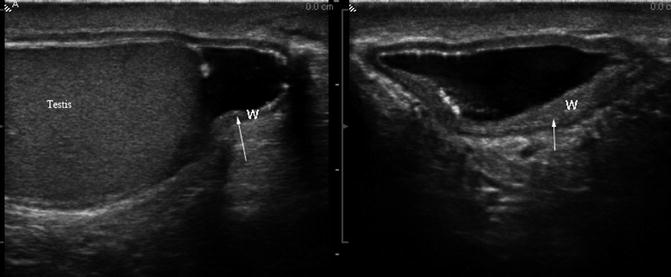

Fig. 6.27
(a) Gray scale ultrasound showing the highly echogenic omental fat of an omental hernia. (b) Color Doppler study showing no increased blood flow to the inguinal hernia

Fig. 6.28
Gray scale ultrasound showing thickened hernia sac (W) in chronic inguinal hernia (arrows)
Sperm Granuloma
Spermatazoa are highly antigenic, and an intense inflammatory reaction occurs when they exit the vas difference [40]. Sperm granulomas occur in at least 40 % of men following a vasectomy [41] (Fig. 6.29). Sperm granulomas are rarely symptomatic. However, 2–3 % of vasectomy patients will have pain attributed to sperm granulomas, usually occurring 2–3 weeks postoperatively [42].
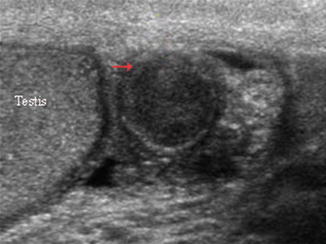

Fig. 6.29
Gray scale ultrasound showing a sperm granuloma (red arrow)
Tumors of the Spermatic Cord
Lipomas of the spermatic cord are very common benign lesions of the spermatic card. They can be unilateral or bilateral, and often present as asymptomatic fullness of the spermatic cord. Ultrasound of a lipoma demonstrates homogeneous echogenicity similar to subcutaneous fat without internal color flow. The echogenicity of lipomas may be variable, and MRI may be helpful to confirm diagnosis, showing nonenhancing, fat saturated areas [43]. It is also important to differentiate a lipoma from an inguinal hernia by noting the intact external inguinal ring on physical examination and assessing for the presence of a hernia on ultrasound.
Rhabdomyosarcomas of the spermatic cord is a malignant lesion in children, and liposarcoma is the most common malignant tumor arising in the spermatic cord in adults, although both are rare. Leiomyosarcomas in the paratesticular space also have been reported. The ultrasound appearance of these lesions is an ill-defined solid mass with heterogeneous echotexture and increased vascular flow on Doppler color study (Fig. 6.30).
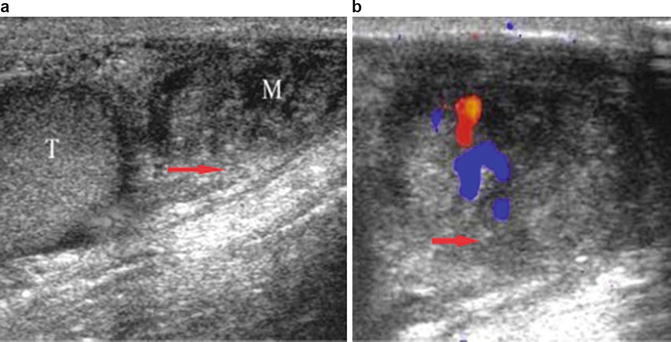

Fig. 6.30
Leiomyosarcoma of the scrotum: (a) Gray scale ultrasound appearance as an ill-defined solid mass with heterogeneous echotexture in the paratesticular space (M mass, T Testis). (b) Doppler color flow shows increased vascularity with in the mass
Epididymal Findings
Epididymo-Orchitis
Epididymitis is the most common cause of subacute unilateral scrotal pain in preadolescent and adolescent boys and adult men. On physical exam the epididymis can often be identified as an enlarged and tender structure posterolateral to the testis. The pain is often relieved with elevation of the testis over the symphysis pubis, known as Prehn’s sign. Among sexually active men younger than 35 years old, epididymitis often results from sexually transmitted infections, particularly Chlamydia trachomatis and Neisseria gonorrhoeae. In older men, bacterial epididymitis can result from retrograde transit of bacteria from the vasa, and therefore the most common organisms are urinary pathogens: Escherichia coli and Proteus mirabilis. Rare infectious causes include brucellosis, tuberculosis, cryptococcus, syphilis, and mumps. Epididymitis in prepubertal boys normally has a benign course, and these boys commonly are found to have positive titers for enteroviruses and adenoviruses and M. pneumonia. Rare noninfectious causes include sarcoidosis and amiodarone. Blunt trauma as well as congestion following vasectomy is potential cause of epididymal inflammation [44, 45].
In patients with acute epididymitis, the epididymis is enlarged with increased vascularity. Epididymitis may lead to focally or global enlargement and thickening of the epididymis. Gray scale ultrasound demonstrates a hypoechoic or heterogeneous enlarged epididymis (Fig. 6.31). The color flow Doppler shows increased vascularity with high-flow, low-resistance pattern (Fig. 6.32). A reactive hydrocele is often present. Complications of epididymitis include infectious spread to the testis resulting in epididymo-orchitis, testicular abscess formation, and testicular infarction due to obstruction of venous flow which may result in testicular atrophy. Patients with chronic epididymitis often present with persistent pain. In these men, ultrasound examination reveals an enlarged epididymis with increased echogenicity and possible areas of calcifications (Fig. 6.33).
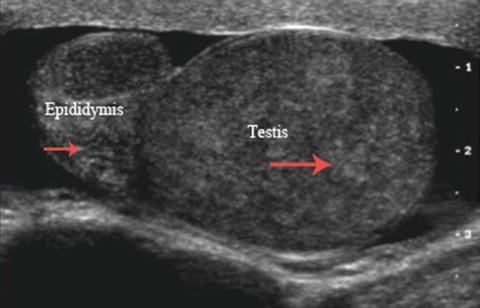
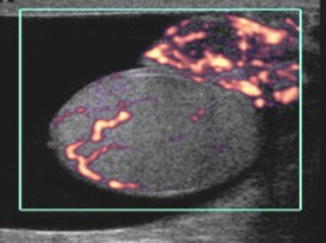
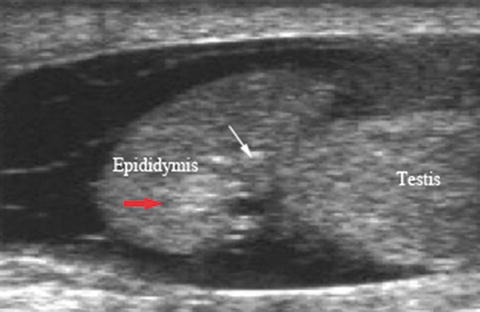

Fig. 6.31
Epididymo-orchitis : gray scale image demonstrates enlarged and heterogeneous epididymis and testis

Fig. 6.32
Epididymo-orchitis : Power Doppler ultrasound showing increased vascularity of the epididymis and the testis

Fig. 6.33
Chronic epididymitis : Gray scale ultrasound showing increase of echogenicity and microcalcifications seen in the caput epididymis (arrows)
Torsion of the Appendix Epididymis and Testis
Torsion of the appendix testis is important to differentiate from torsion of the spermatic cord (testicular torsion), as this condition is self-limiting and does not threaten testicular viability. Clinically, the cremasteric reflex is preserved and a palpable nodule with bluish discoloration (blue dot) is often detected [46]. Ultrasound shows a hyperechoic mass with central hypoechoic area adjacent to the testis or epididymis. Other associated findings include scrotal wall edema and epididymal enlargement. Blood flow in the peritesticular structures may be increased. Doppler ultrasound is helpful as blood flow within the testis is normal in torsion of the appendix testis.
Benign Epididymal Lesions
An epidydimal cyst is a nonpainful cystic structure that, when large, displaces the testis inferiorly. Cysts of the epididymis occur in up to 40 % of the men and contain lymphatic fluid. They are typically thin walled and well defined, usually with strong posterior acoustic enhancement and no internal echoes. These men will often have multiple cysts occurring present throughout the length of the epididymis.
Spermatoceles are benign cystic lesions, which contain spermatozoa, lymphocytes, and debris. Spermatoceles form as a result of efferent duct obstruction and usually located in the head of the epididymis. Ultrasonography cannot differentiate between epididymal cysts and spermatocele, but the spermatocele often has septations (Fig. 6.34).
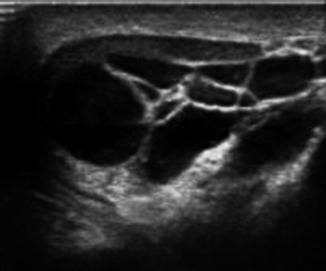

Fig. 6.34
Gray scale ultrasound showing multiple anechoic epididymal cysts
Adenomatoid tumors are the most common tumors of the paratesticular tissues, accounting 30 % of these lesions and up to 77 % of the benign tumors arising from the epididymis. They are most commonly identified in men in their 20s to 40s. It has been suggested that they derive from vascular endothelium, the mesonephros, or müllerian epithelium, although most recent reports consider them to be mesothelial in origin [47]. They are round, firm, smooth, discrete masses measuring 0.5–5 cm in diameter that are usually asymptomatic and slow growing. Ultrasonography can confirm the extratesticular nature of these masses. Ultrasound of adenomatoid tumors reveals an isoechoic mass with increased vascularity (Fig. 6.35).
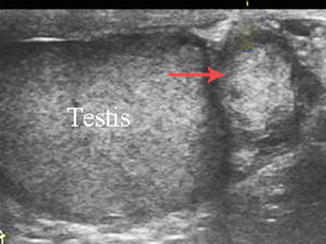

Fig. 6.35
Adenomatoid tumor seen on Gray scale imaging as an as isoechoic paratesticular mass
Papillary cystadenoma is a rare benign tumor of epithelial origin believed to arise from the efferent ductules of the head of the epididymis [48]. Papillary cystadenoma presents clinically as a firm, nontender palpable mass in the epididymis. Two-thirds of papillary cystadenomas occur in patients with von Hippel-Lindau (VHL) syndrome and are frequently bilateral [49]. Unilateral presentation is seen very rarely in sporadic cases. Sonographically, small papillary cystadenoma are usually solid and echogenic, but when large may appear vascular and cystic [49].
Leiomyomas are benign epididymal solid tumors. These lesions are most commonly seen in men over the age of 50. The ultrasound appearance is a well-defined solid mass with heterogeneous echotexture located in paratesticular space separate from the epididymis [50].
Malignant Epididymal Lesions
Malignant tumors arising from the epididymis are very rare, with the exact incidence of malignant tumors of the epididymis uncertain because of the small number of reported cases. Sarcoma of the epididymis comprises of more than half of the reported malignant neoplasms of the epididymis [51]. Fibrosarcoma of the epididymis has been reported in isolated case reports. Dowling et al. reported fibrosarcoma in a 60-year-old male confined to the epididymis on final pathology [52]. Leiomyosarcoma of the epididymis on ultrasound appears as a large hypoechoic mass (Fig. 6.36). Clear Cell carcinoma of the Epididymis is very rare and has been reported in individual case reports [53]. Ultrasound findings may include large cysts, necrosis, and invasive margins.
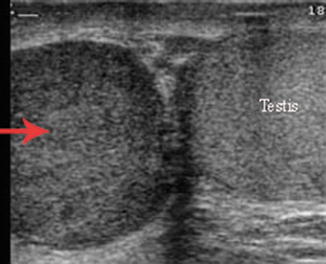

Fig. 6.36
Leiomyosarcoma of the epididymis. Gray scale ultrasound showing large hypoechoic mass in the epididymis with normal testis
Scrotal Wall Lesions
Scrotal Infectious Findings
Patients who are diabetic or immunocompromised are more susceptible to infection and scrotal wall cellulitis or abscess. Ultrasonography demonstrates thickening of the subcutaneous tissue and heterogeneity with increased blood flow on color Doppler study. The scrotal wall abscess appears on ultrasound as a well-defined hypoechoic lesion within the scrotal wall and no Doppler flow within the lesion [5].
Fournier’s gangrene is a polymicrobial rapidly progressing necrotizing fasciitis commonly involves perineum and genital regions. Fournier’s gangrene is a urologic emergency with mortality up to 50 % [54, 55]. Computer tomography remains the imaging modality of choice [56]. However, ultrasonography can provide valuable clues at the time of initial presentation. Ultrasonography shows marked thickening of the scrotal skin with multiple hyperechogenic foci associated with shadowing, which are consistent with the presence of subcutaneous gas, pathognomonic of Fournier’s gangrene [57].
Benign Scrotal Lesions
Epidermoid Cysts of the Scrotal Wall
Epidermoid cysts or epidermal inclusion cysts are the most common cutaneous cysts of the scrotal wall. Epidermoid cysts result from the proliferation of epidermal cells within a circumscribed space of the dermis at the infundibulum of the hair follicle [58]. Epidermoid cysts may become infected and form scrotal wall abscess.
Henoch–Schonlein purpura (HSP ) is a systemic vasculitis of unknown origin. It is characterized by a palpable skin rash, abdominal pain, and polyarthralgia. HSP has been reported to have scrotal wall swelling and ecchymosis in up to 38 % of cases [59].
Scrotal fibrous pseudotumors are uncommon and are thought to be reactive, benign lesions. The sonographic appearance of the fibrous pseudotumor of the scrotum is variable depending on the contributing fibrous tissue components, presence or absence of calcification, and the scrotal structure involved [60]. Pseudotumor of the scrotum is a benign condition and local excision of the mass is the treatment of choice; however, preoperative diagnosis is seldom made due to the nonspecific clinical and sonographic findings [61].
Acute idiopathic scrotal edema (AISE) is a self-limited disease of unknown origin. It presents with unilateral or bilateral scrotal swelling without pain and is associated with unilateral or bilateral inguinal lymphadenopathy. It is thought to be a variant of angioneurotic edema, often associated with eosinophilia. Physical examination findings include scrotal skin swelling and erythema that extends to the inguinal and perianal area. AISE is a diagnosis of exclusion. The characteristic ultrasound findings for AISE, include edema of the scrotal wall with hypervascularity and compressibility with enlargement of the inguinal lymph nodes, and normal testis and epididymis (Fig. 6.37) [62, 63].
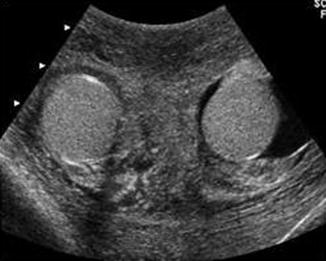

Fig. 6.37
Gray scale ultrasound showing diffuse scrotal wall thickening in a patient with scrotal wall edema
The other noninflammatory causes of scrotal wall edema including congestive heart failure, renal failure, anasarca, hepatic failure, cirrhosis, nephrotic syndrome, and poor nutritional status. The scrotal wall appears thickened in chronic venous or lymphatic obstruction secondary to filariasis, radiation, and trauma or surgery. Ultrasound demonstrates scrotal wall thickness with layers of alternating hypo and hyperechogenicity [64, 65].
Malignant Scrotal Lesions
Squamous cell carcinoma (SCC ) of the scrotum is an uncommon neoplasm. SCC is associated with occupational exposure to chemical or oil industries, radiation, chimney sweepers, human papilloma virus, chronic scar, and immune-related conditions such as psoriasis [66]. The literature concerning scrotal SCC is limited. Ultrasound evaluation of these lesions is not well defined.
Testicular Pathology
Nonmalignant Abnormalities of the Testis
Torsion of the Spermatic Cord or Testicular Torsion
Ultrasound is often used to assess boys and adolescents with acute scrotal pain when the urologist is concerned for testicular torsion. Testicular torsion can be classified as extravaginal or intravaginal. The extravaginal form of torsion is found exclusively in newborn infants. Intravaginal torsion is more common and is due to a bell-and-clapper deformity in which the tunica vaginalis has an abnormally high insertion on the spermatic cord and completely encircles the testis, leaving the testis free to rotate within the tunica vaginalis. The deformity is bilateral in most cases. Intravaginal testicular torsion occurs most frequently in adolescent boys, with two-thirds of cases occurring between 12 and 18 years of age. Intravaginal torsion may occur in testes that are retractile or are not fully descended. Blunt trauma, sudden forceful rotation of the body, or sudden exertion also predispose to testicular torsion.
Ultrasound is very effective in differentiating testicular torsion from other causes of acute scrotal pain. The severity of torsion of the testis can range from 180° to 720°, but complete occlusion of blood flow is thought to occur after 450° of torsion [12]. Transient or intermittent torsion with spontaneous resolution sometimes occurs. Venous congestion or occlusion progresses to arterial occlusion, testicular ischemia, and infarction. The collateral blood flow is typically not adequate to provide viability to the testicle if the testicular artery is occluded. There is a 90 % chance of salvaging the testicle when ischemia has been present for less than 6 h, which decreases to 50 % at 12 h and 10 % at 24 h [67]. While irreversible testicular damage is presumed after 4 h of torsion, only 50 % of men who were detorsed less than 4 h after their symptoms began were noted to have normal semen quality [68].
On gray scale ultrasound, the affected testis usually appears hypoechoic (Fig. 6.38a) and Doppler color flow study shows decreased or no flow in the affected testis (Fig. 6.38b). Testicular size can vary from increased to decreased when compared to its counterpart depends upon the duration of the torsion. The sonographer should always compare the affected testis with the contralateral side using longitudinal, transverse, and coronal views. When the sonographer attempts to align the transducer parallel to flow, apical views can be particularly informative. In patients with acute torsion, the epididymis may appear hypoechoic and enlarged, similar to epididymitis. With testicular torsion ultrasound may also demonstrate that the spermatic cord immediately cranial to the testis and epididymis is twisted, which gives it a characteristic ‘torsion knot’ or ‘whirlpool appearance ’ (Fig. 6.39a, b).
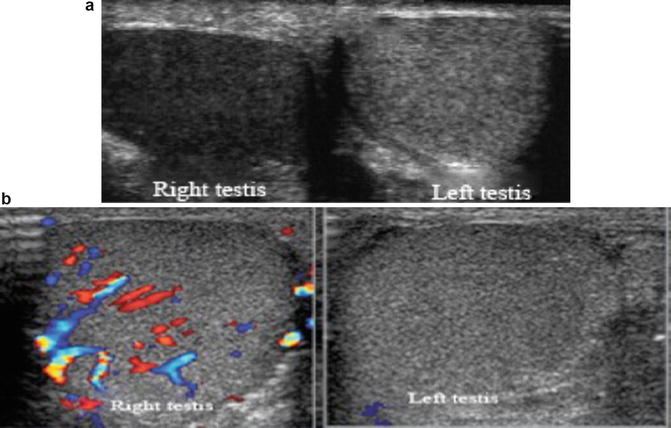
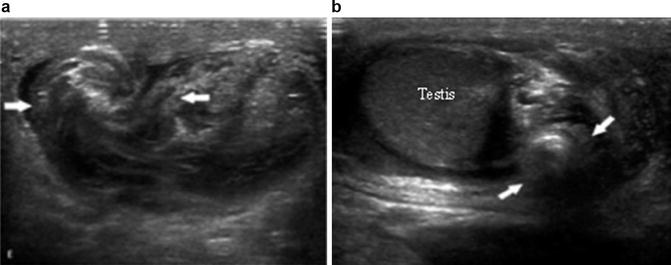

Fig. 6.38
(a) Right testicular torsion with normal left testis for comparison. The torsed testis has decreased echogenicity on gray scale ultrasonogram compared to the contralateral healthy testis. (b) Color Doppler ultrasound shows absent blood flow in the left testis with testicular torsion and normal flow in the healthy right testis

Fig. 6.39
(a, b) The spermatic cord immediately cranial to the testis and epididymis is twisted, which gives it a characteristic ‘torsion knot’ or ‘whirlpool appearance’ on gray scale ultrasound
Acute unilateral scrotal pain may be of a nonemergent etiology, due to epididymitis or torsion of a testicular or epididymal appendage. Waldert et al. retrospectively reviewed the charts of 298 boys who presented with an acute scrotum and underwent color Doppler ultrasonography followed by exploratory surgery, regardless of the sonographic findings. Twenty percent were diagnosed with testicular torsion, 56 % with torsion of an appendage, 8 % with epididymitis, and 11 % with no definite diagnosis. Color Doppler sonography sensitivity, specificity, positive predictive value, and negative predictive value for testicular torsion were 96.8 %, 97.9 %, 92.1 %, and 99.1 %, respectively. The two boys in this study misdiagnosed as epididymo-orchitis were both found to have 90° of torsion and no venous drainage but with residual arterial flow [69].
Despite the findings that color Doppler sonography has a high sensitivity and specificity, it is our feeling that torsion remains a clinical diagnosis proven only at surgery. Ultrasound should only be used to document findings. Many conditions including torsion–detorsion, intermittent torsion, persistent capsular flow, and color flow artifacts can suggest apparent flow in cases where none exists. Therefore, ultrasound does not diagnose or “rule out” torsion, only surgical exploration is indicated when the diagnosis of testicular torsion is suspected.
Primary Orchitis and Testicular Abscess
The ultrasound findings of patients with orchitis are often an enlarged testis with homogenous appearance. Orchitis may be diffuse or focal, with focal orchitis appearing as multiple hypoechoic lesions with increased testicular blood flow (Fig. 6.40a, b). Additionally, the RI of the epididymal and testicular artery has been shown to be significantly lower in patients with epididymo-orchitis than in control subjects [70]. If inflammation progresses, the pressure of intratesticular edema may compromise blood flow leading to infarction; the ultrasound will demonstrate absence of blood flow and surrounding reactive hyperemia [71].