CHAPTER 4 Percutaneous biopsy
Percutaneous biopsy is a widely used interventional technique for obtaining tissue samples.1,2 The crucial element of the procedure is image guidance, which facilitates accurate and safe needle passage. Percutaneous biopsy with image guidance is less invasive and less expensive than most surgical methods. The procedure has become safer and more effective with advances in imaging, specialized cytologic analyses, and small-gauge, thin-walled needles. Cross-sectional imaging with computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) permits safe diagnosis of small, remote lesions that once were completely inaccessible in this way. The accuracy of percutaneous biopsy for the diagnosis of malignancy in the chest and abdomen is about 85% to 95%.3 Definitive diagnosis of benign lesions is less accurate, however. Further advances in biopsy and cytopathologic methods should improve these results.
The most common indication for percutaneous biopsy is the diagnosis of malignancy, including primary neoplasms, metastatic disease, tumor staging, and recurrent disease after treatment. The procedure also is used for diagnosis of inflammatory or infectious processes, abnormal fluid collections, and diffuse organ disease. Relative contraindications include uncorrectable coagulation abnormalities, lack of a safe percutaneous pathway to the lesion, and an uncooperative patient in whom motion may increase the risk of bleeding.4 Biopsy usually is not indicated in a patient who will undergo surgery regardless of the biopsy results or if the results will not change the management of the patient (e.g., the patient is already in hospice care).
Technique
Patient preparation
The patient’s history, clinical status, and imaging studies should be reviewed and discussed with the referring physicians. Routinely performed coagulation studies include hematocrit, platelet count, prothrombin time, partial thromboplastin time, and international normalized ratio5 (INR) (see Chapter 2). Coagulopathies should be corrected with appropriate transfusions of platelets, fresh-frozen plasma, vitamin K, or packed red blood cells. Although institutional thresholds vary, transfusions should be considered when the INR is greater than 1.5 or when platelet count is less than 50,000/mm3. However, numerous studies have shown the relatively low predictive value of abnormal screening parameters in predicting bleeding.5,6 Surgical series also have indicated that preoperative coagulation studies correlate poorly with postoperative bleeding.6 Recent Society of Interventional Radiology practice guidelines offer useful coagulation and transfusion parameters for percutaneous biopsy depending on whether the lesions are superficial (minimal risk); deeper intraabdominal, thoracic, or retroperitoneal (moderate risk); or renal (significant risk).7 Interventionalists must be careful to screen their patients for use of potent antiplatelet agents such as clopidogrel (Plavix). These agents should be discontinued at least 5 days before moderate or significant risk biopsies. Care should be taken before discontinuing Plavix if it is prescribed to maintain patency of a coronary artery stent. A cardiology consult may be necessary to avoid stent complications based on type of stent used (drug-eluting vs. bare metal) and timing of stent placement.8,9
Materials
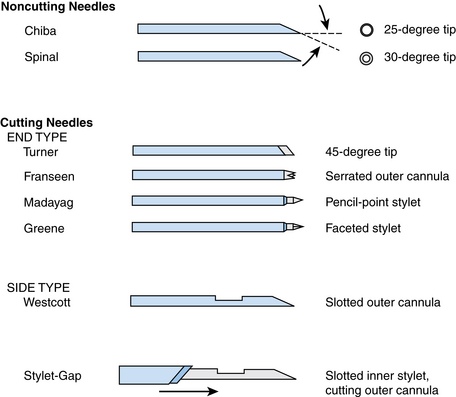
A wide array of needles are available for percutaneous biopsy procedures.10 Needles can be classified by caliber or gauge, tip configuration, and mechanism of sample acquisition. Needle sizes are divided broadly into smaller gauge (20 to 25 gauge) and larger gauge (14 to 19 gauge).3 Thinner-gauge needles often provide adequate cytologic material and often histologic material as well. Smaller-caliber needles also minimize bleeding, providing a margin of safety when multiple passes are required. Traversing bowel may at times be necessary, and complications can be minimized by using thin-gauge needles.4 However, they can be somewhat difficult to direct into lesions because they tend to deflect significantly, particularly in deep-seated lesions. Larger needles are easier to direct and provide better samples for cytology and histology, often with fewer needle passes. The risk for hemorrhage may increase as needle size increases.5 Needles also are classified by tip configuration10 (Fig. 4-1). Needles vary in the angle and bevel of the tip. The Chiba and spinal needles have bevel angles of 25 and 30 degrees, respectively. Greene, Madayag, and Franseen (or Crown) needles have 90-degree tips. Needle tips are classified also into noncutting (aspiration needles such as the Chiba or spinal) and cutting (end-cut or side-cut) types. Needles can be manual, spring-loaded, or automated.
Imaging guidance
The important factors in choosing an imaging modality for biopsy are lesion characteristics (e.g., size, location, depth), ability to visualize the abnormality, and operator experience and preference. Generally, the shortest path to the lesion is considered when planning the imaging approach, with the possible exception of peripherally located hypervascular hepatic lesions and when preprocedure imaging demonstrates better tissue yield from a different axis (e.g., elongated but thin lesion).
Fluoroscopy
Fluoroscopy is a common technique for biopsy of lung and pleural masses (Fig. 4-2). In the abdomen, fluoroscopy can be used for fine-needle biopsy of obstructing lesions of the biliary and urinary systems outlined by contrast material from percutaneously placed catheters. Disadvantages of this method include added radiation exposure to the operator and inability to visualize adjacent structures as the needle is advanced. Cross-sectional imaging studies (e.g., CT, ultrasound, MRI) can be helpful in planning an approach when fluoroscopy is used.
Sonography
Ultrasound is very useful for biopsy of intraabdominal lesions, including masses in the liver, pancreas, or kidney; bulky adenopathy in the retroperitoneum or root of the mesentery; and large adrenal masses.11 In the chest, ultrasound is used for aspiration of pleural-based masses and fluid collections and occasionally can be used in the biopsy of peripheral pulmonary parenchymal lesions.
A complete diagnostic ultrasound study is obtained first to determine the conspicuity of the lesion, its depth, and a safe angle of approach. Ultrasound-guided biopsy can be performed with a free-hand technique or with the use of ultrasound guides. Sterilized probes or sterile probe covers allow real-time imaging while the biopsy is performed. Advantages of ultrasound include the lack of ionizing radiation, real-time imaging of needle position, and the multiplanar imaging capability that facilitates complex angled approaches often required to biopsy upper quadrant abdominal masses. Disadvantages include impaired visualization of deep lesions and obscuration by overlying bowel gas or bone. Additionally, operator variability in experience and technique can play a part in sonographically guided biopsies.
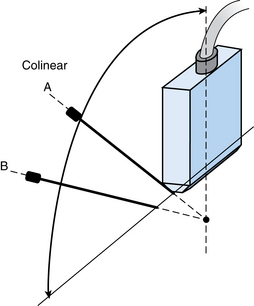
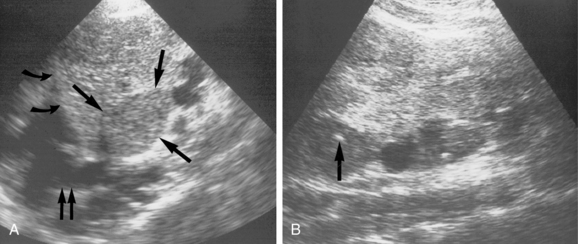
The needle can be inserted perpendicular to the transducer, which may facilitate visualization by improved reflections from the needle shaft11 (Fig. 4-3). Alternatively, the needle can be inserted in close to the ultrasound transducer. Ideally, the entire needle should be visualized during passage through the tissues into the mass (Fig. 4-4). If the needle is not visible, misalignment between the needle path and ultrasound beam is occurring. A slight rocking motion (or panning) back and forth of the ultrasound transducer may help visualize the needle. A gentle in-out motion of the needle or stylet may increase conspicuity of the needle tip as well. In every case, the needle tip should be documented as a discrete echogenic focus within the lesion in at least two views before biopsy (Fig. 4-5).
The advent of three-dimensional sonography has aided percutaneous biopsy by precise delineation of mass lesions and adjacent structures (e.g., blood vessels and bile ducts) in the anticipated needle pathway.12 It also has been useful in guiding transjugular liver procedures and regional tumor ablation procedures because orthogonal images can be obtained in planes that are anatomically useful to the interventionalist performing the procedure. Three-dimensional sonography, however, has not gained significant popularity in most practices.
Computed tomography
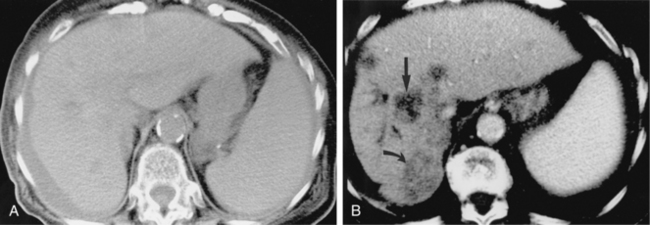
CT is used for biopsy of smaller intraabdominal and thoracic lesions not well visualized by ultrasound or fluoroscopy. The patient is first scanned in the anticipated biopsy position (e.g., prone, supine, oblique) with images obtained through the target area (Fig. 4-6). Intravenous contrast may be helpful in delineating vascular structures, in determining the vascularity of the target lesion, and for biopsy of lesions not well seen on unenhanced images (Fig. 4-7). A variety of devices is available to mark the skin during scanning and to determine the needle insertion site and angle.
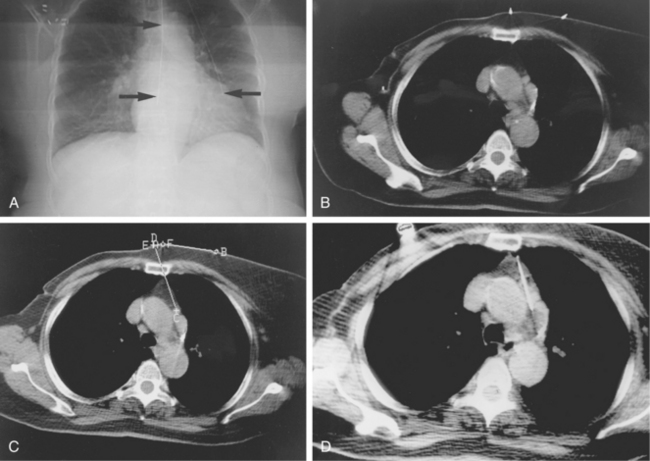
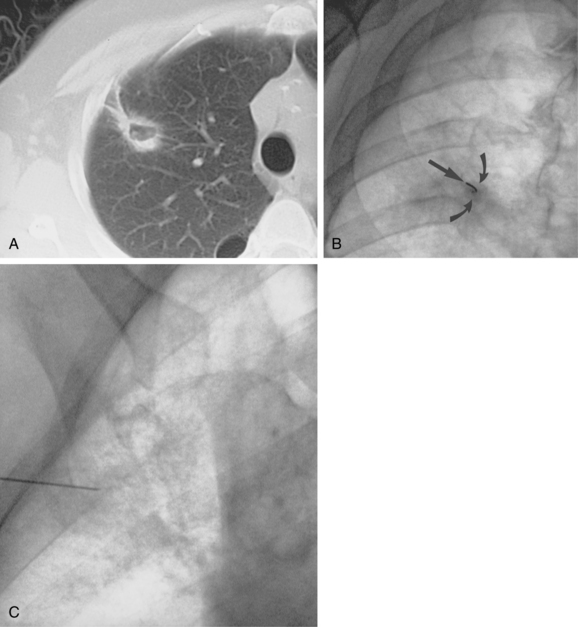
Figure 4-6 Technique for computed tomography (CT)-guided percutaneous biopsy. The patient had undergone resection for colon carcinoma. Rising carcinoembryonic antigen titers were found later along with mediastinal lymphadenopathy on a CT scan. A, Using the initial diagnostic CT scan as a guide, the patient is positioned in the scanner with a grid marker taped to the skin (arrows) near the anticipated needle entry site. B, Scans are obtained through the lesion, and the table position that best shows the lesion is identified. Note the grid bars on the skin overlying the mass. C, Cursors measure the distance between grid bars (F-B), which allows determination of the cephalocaudal location of the lesion. The planned skin entry site is labeled A, and the depth of lesion is the distance from A to C.D, The needle is advanced in stages as serial scans follow its progress into the lesion. The needle tip is seen as a beam-hardening artifact. In this case, no malignant tissue was recovered.
A vertical needle pathway is preferable because it avoids the need for triangulation or accurate angle measurements. The needle should be visualized in the axial plane during the biopsy. Occasionally, gantry angulation may be helpful to visualize cranial-caudal angled approaches (e.g., adrenal biopsy, transthoracic biopsy with overlying ribs).
CT fluoroscopy allows real-time monitoring of needle positioning during percutaneous biopsy. Since its introduction in 1996, various studies have outlined advantages and disadvantages of this technique for biopsy of various targets.13 Advantages of CT fluoroscopy over conventional CT include the ability to time a patient’s breathing to access moving lesions or to avoid ribs and other overlying structures. CT fluoroscopy allows the needle and lesion to be visualized during sampling to be sure the needle tip is properly located within the lesion. Using CT fluoroscopy in near real-time imaging requires either that the operator’s hands be in the radiation beam or that special needle holders be used. The technique also is useful to perform peripheral lesional biopsy when central necrosis is present. CT fluoroscopy is not used universally, because some investigators are concerned about added radiation exposure and conflicting studies about reduced procedure times. CT fluoroscopy has been used most often for transthoracic biopsy, for which high rates of technical success have been reported with sensitivity ranging from 89% to 95% and specificity of 100%.14 Several recent studies have shown that using intermittent “quick check” CT fluoroscopy between needle advancements instead of continuous (near real-time) fluoroscopy can reduce radiation doses significantly.15,16
Magnetic resonance imaging
Several investigators have described initial experiences with MR-guided percutaneous biopsies. Potential advantages include the ability to image lesions not readily seen by other modalities, multiplanar imaging capability, near real-time imaging during needle insertion, lack of ionizing radiation, and potential use of MR-guided tumor ablation in conjunction with biopsy.17 Disadvantages include the need for specially designed needles compatible with MR scanners, higher imaging costs, and special magnet configurations (i.e., open designs) to facilitate needle insertion.
Magnetic field–based electronic guidance systems are a special class of equipment that uses a low magnetic field with position sensors on the patient fused with previously obtained cross-sectional imaging to provide near real-time targeting.18,19
Sampling technique (online case 45)
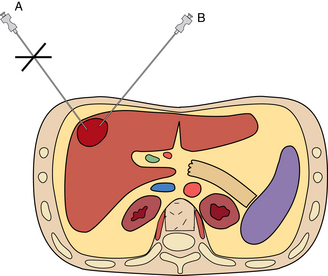
Most biopsies are performed with the patient in a supine position, although prone and oblique positions can be used when necessary. With a few exceptions, the shortest path to the lesion is preferred. Hypervascular hepatic lesions should be approached through a sizable parenchymal track to reduce hemorrhagic complications20 (Fig. 4-8). The likelihood of pneumothorax is reduced by minimizing the number of pleural surfaces crossed during chest and some abdominal biopsies. It is optimal to avoid traversing lung, pleura, pancreas, gallbladder, dilated or obstructed biliary ducts, and bowel during biopsy procedures. Small and large bowel can be crossed safely with small-gauge needles if no other pathway is available. However, sampling of intraabdominal fluid collections through bowel loops is not advisable.
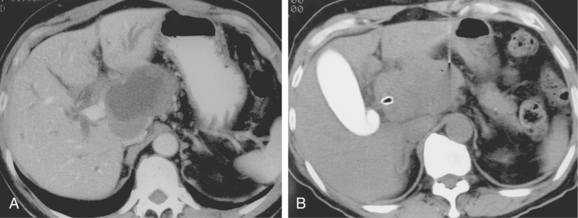
If the sample is predominantly composed of red blood cells, a better sample may be obtained with a smaller-gauge needle (e.g., 25-gauge). A nonaspiration technique also can be used in which the needle is advanced and retracted through the lesion without suction.21 Large lesions with necrotic centers may require biopsy of peripheral tissue to make a diagnosis (Fig. 4-9).
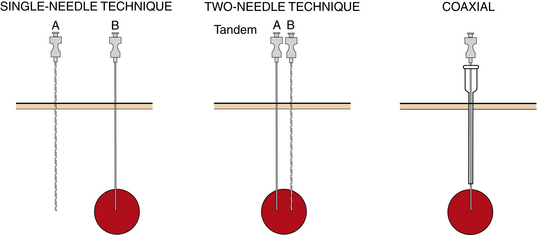
Single-needle technique
With the single-needle method, the needle is advanced into position, and its location is confirmed (Fig. 4-10). If the needle location is unsatisfactory, it is left in place to guide placement of a second needle. Each biopsy sample that is obtained requires a new needle be placed into the lesion, adding to the complexity of the case and increasing risks for complications such as bleeding. This technique allows sampling of different regions of a mass, which may improve biopsy yield. This technique is very useful for biopsy of superficial lesions such as thyroid nodules.
Two-needle technique
With the two-needle method, a needle is placed initially just superficial to the lesion to serve as a guide for subsequent needle passage and biopsy with a tandem or coaxial technique (see Fig. 4-10). Precise needle placement needs to be done only once. The coaxial method is particularly useful with smaller or deep lesions that are difficult to localize. It has the further advantage that a single puncture of the visceral organ capsule (or pleura) is made, regardless of the number of biopsy needle passes that are made. A disadvantage of this method is that the sampling path of the biopsy needle is limited by the direction of the outer guiding needle. Several different commercially available coaxial sets are now available and are particularly useful for biopsy of thoracic and deep abdominal lesions. Care should be maintained when using different needles from different vendors as slight size/length incompatibilities may arise, making biopsy difficult or impossible.
Additional special equipment is available, such as a modified coaxial system (e.g., 23-gauge needle with removable hub is used to guide a 19-gauge biopsy needle), for additional cost.
Specimen handling
An optimal cytologic specimen consists of a small amount of soft or semiliquid material with minimal bloody contamination.22 Smears should be composed of a thin layer of cells and fragments; blood dilutes and obscures the diagnostic material. Clotting of the specimen can be minimized by expeditious sample preparation and prerinsing the aspiration syringe and needle with a small amount of heparinized saline. Small-gauge needles (e.g., 25-gauge) also limit the blood content of the aspirate.
A small amount of aspirated material first is placed on a slide. The material is spread and then air-dried or fixed in alcohol. Air-dried samples are stained with Diff-Quik (Baxter Healthcare, McGraw Park, Ill.) for prompt diagnosis. Larger tissue fragments or material are placed in a tube or container of 10% neutral buffered formalin for processing as a cell block. This step is particularly important when immunohistochemical staining is required for diagnosis. Occasionally, the core sample can be used to perform a “touch preparation” in which the core sample is smeared across a slide or multiple slides and then the core is placed in formalin, the touch preparations are then processed similarly as other cytologic samples.23 The remaining core sample is processed for histologic examination. “Touch prep” or “core roll preparation” has been shown to improve the diagnostic yield during the biopsy when compared with fine-needle aspiration (FNA) alone.23–25 In combination with FNA, this allows for fewer passes to obtain a diagnostic sample without significantly altering the histologic sample.
Specific applications
Chest
Patient selection
Percutaneous chest biopsy is performed for evaluation of nodules or masses in the lung, hilum, mediastinum, pleura, and chest wall.26–28 In general, masses that involve lobar or segmental bronchi on chest radiography or CT suggest the presence of an endobronchial lesion, and these are best approached with bronchoscopy. On the other hand, peripherally located lesions are not that accessible with bronchoscopy and are easily reached with percutaneous biopsy. The most common indication for percutaneous chest biopsy is the diagnosis of a solitary pulmonary nodule (SPN), which is a rounded, well-defined mass of less than 3 cm that is largely surrounded by lung. Percutaneous biopsy, particularly core biopsy in conjunction with FNA, has been advocated for diagnosing pneumonia and diseases that mimic pneumonia (i.e., bronchiolitis obliterans organizing pneumonia, neoplasms [lymphoma, bronchoalveolar cell carcinoma], eosinophilic pneumonia, vasculitis [Wegener’s granulomatosis]).27 Percutaneous biopsy should be strictly avoided with suspected vascular lesions (e.g., arteriovenous malformation, pulmonary varix) and Echinococcus cysts. Relative contraindications to lung biopsy include severe obstructive pulmonary disease, moderate to severe pulmonary hypertension, contralateral pneumonectomy, ventilator dependence, and inability to cooperate with breathing instructions. These patients are at increased risk of or are less able to tolerate pneumothorax after biopsy.
The workup of pulmonary lesions varies significantly among institutions with regard to the use of percutaneous needle biopsy or surgical resection for an SPN. The probability that an SPN represents a primary lung tumor rather than a metastasis from a known malignancy depends largely on the primary tumor type (e.g., 1:1.2 for colon cancer, 3.3:1 for breast cancer, 3.3:1 for bladder cancer, and 8.3:1 for head and neck cancers).26 The false-negative rate for needle biopsy in patients with malignancy is relatively high (up to one third of procedures).28,29 The cases in which clinical suspicion of malignancy is high require repeat biopsy or close follow-up. In some centers, a potentially malignant SPN in a low-risk operative patient often is resected without prior needle biopsy.