© American Academy of Pain Medicine 2015
Timothy R. Deer, Michael S. Leong, Asokumar Buvanendran, Philip S. Kim and Sunil J. Panchal (eds.)Treatment of Chronic Pain by Interventional Approaches10.1007/978-1-4939-1824-9_3030. Percutaneous Disc Decompression
(1)
Department of Anesthesia, Pain Management, Surgery, Cape Canaveral Hospital, Cocoa Beach, FL, USA
(2)
Space Coast Pain Institute, 4770 Honeyridge Lane, Merritt Island, FL 32952, USA
(3)
Division of Pain Medicine, Department of Anesthesiology, Case Western Reserve University, University Hospitals Case Medical Center, 11100 Euclid Ave., Clevland, OH 44106, USA
(4)
Department of Pain Management, Institute of Pain Diagnostics and Care, Ohio Valley General Hospital, 1104 Ventana Drive, McKees Rocks, PA 15136, USA
Key Points
At any time during the procedure, if a patient reports any lower extremity sensation (radicular pain or burning foot), the procedure should be stopped and the position of the trocar or probe assessed with anteroposterior and lateral fluoroscopic views and repositioned as necessary.
Like all intradiscal procedures, multiplanar fluoroscopy should be used for confirmation of needle and probe placement. Sedation should be optimized to maintain meaningful communication between the operator and patient.
If back pain occurs during PLDD, it may be due to the heating of adjacent vertebral end plates or increased pressure within the disc from trapped gas. In such cases, the position of the optical fiber should be checked to ensure it is away from the end plates, and the interval between the pulses should be increased. Aspiration could also be applied through the sidearm fitting to avoid the trapping of gas.
If no aspirated material is present in the Dekompressor probe after 3 min of activation, the procedure should be discontinued.
Introduction
Approximately two-thirds of individuals living in western countries suffer from an episode of low back pain during their lifetimes [1]. Low back pain is one of the leading reasons for multiple visits to physicians, and its rising prevalence is a significant factor in lost productivity, disability, and increased healthcare use [2–4]. Further, low back pain has had a substantial impact on the US economy, with healthcare expenditures swelling by 65 % from 1995 to 2005 [5].
“Nonspecific” low back pain with an unexplained etiology is currently the most prevalent low back pain group [6]. According to the best available evidence and proven diagnostic techniques, structural disorders of intervertebral discs, facet joints, and the sacroiliac joint are the three most important etiologies of the types of low back pain collectively known as “specific” low back pain [7]. Based on studies that employed controlled diagnostic injections, the relative prevalence of intervertebral discs, facet joints, and the sacroiliac joint as a source of low back pain has been estimated at 39 % [8], 15 % [9], and 19 % [10], respectively.
Lumbar disc prolapse accounts for less than 5 % of all low back problems, yet is the most common cause of radicular symptoms [11]. Given the incomplete understanding of the exact natural course of disc herniation and inconsistent findings in trials that compared surgery and conservative care, clinicians are often faced with the challenging choice of surgery versus nonsurgical care for the treatment of patients.
Historical Background
Historically, there have been paradigm shifts between operative and nonoperative treatment, and no single modality has been proven superior in long-term studies. In 1934, William Jason Mixter, a neurosurgeon, and Joseph Barr, an orthopedic surgeon, published a landmark article in the New England Journal of Medicine that established an association between the intervertebral disc and sciatica [12]. Their work led to a paradigm shift from conservative to surgical management for sciatica. This shift spurred innovations in diagnostic and surgical techniques designed to minimize the trauma of therapeutic interventions.
Conversely, the famous retrospective study of Saal and Saal supported conservative management and showed the resolution of pain in more than 90 % of the subjects treated nonoperatively [13]. This result is comparable to the 4-year outcomes in the nonoperative arm of a landmark study by Henrik Weber [14]. However, in a combined (randomized and observational cohort) as-treated 4-year analysis of large multicenter trial (Spine Patient Outcomes Research Trial: SPORT), patients who underwent surgery for lumbar disc herniation fared better than patients treated nonoperatively in all primary and secondary outcomes except work status [15]. Unfortunately, methodological weaknesses in both trials, including significant crossover between the operative and nonoperative arms and the use of as-treated analysis (vs. intention to treat analysis), undermine the validity of any conclusions drawn from this analysis.
Hence, in light of the generally favorable natural course of lumbar radiculopathy associated with lumbar disc herniation, conservative care and minimally invasive treatment modalities should be considered as the first-line treatment options, while an immediate referral to surgery should be made if the patient exhibits a progressive neurologic deficit or the signs and symptoms of cauda equina syndrome. The relative advantages for surgical decompression include rapid pain relief and functional improvement in those who have failed conservative management.
Evolution of Minimally Invasive Percutaneous Disc Decompression
Traditionally, conventional discectomy has been the gold standard treatment for sciatica refractory to conservative management. With the introduction of surgical microscopes in the 1970s, comparable results could be achieved with “microdiscectomy,” which has the advantages of a smaller surgical incision and enhanced operative field view [11].
In the 1960s, three decades after Mixter and Barr’s publication, there was once again a paradigm shift, as the field returned to a minimally invasive approach in the treatment of lumbar disc disease. Lyman Smith was the first to perform percutaneous injection of chymopapain (a proteolytic enzyme) for the treatment of unrelenting sciatica, a technique he called chemonucleolysis (CNL) [16]. In 1975, Japanese orthopedic surgeon Hijikata introduced “percutaneous manual nucleotomy,” a technique that decompressed a herniated disc by the fenestration of the annulus and the partial resection of the nuclear material [17].
Over time, CNL and percutaneous manual nucleotomy fell out of favor due to fatal enzymatic complications and technical limitations, respectively. However, the desire of clinicians for minimally invasive therapies in the field of spine surgery has continued to lead to breakthroughs in percutaneous intradiscal therapies.
Minimally Invasive Percutaneous Disc Procedures
As one would expect, minimally invasive procedures are associated with smaller surgical scars, rapid convalescence, less postoperative analgesic consumption, lower costs, and less spinal instability. In an updated Cochrane review, Gibson and Waddell concluded that, in general, surgical discectomy procedures are superior to chemonucleolysis and other forms of percutaneous discectomy [11]. However, in several trials, most nonrandomized and uncontrolled, the success rate of percutaneous disc decompression ranged from 50 to 90 % [18–20].
Percutaneous Disc Decompression
The postulated mechanism of indirect decompression techniques entails that the excision or degradation of a portion of the central nucleus results in the reduction of intradiscal pressure and prolapsed disc retraction, thus allowing for indirect nerve decompression and the potential resolution of radicular pain [21].
Understandably, the selection of the appropriate patients with specific disc pathoanatomy would be crucial in a study to obtain successful outcomes with the chosen percutaneous disc decompression technique. Carragee and others have demonstrated that clinical (symptom duration, litigation status), demographic (age), morphometric (disc size and shape evident on MRI/CT scans), and intraoperative (type of disc herniation) variables have prognostic significance in terms of treatment outcomes [22, 23]. Small (<6 mm) and contained disc protrusions (intact outer annulus and posterior longitudinal ligament) are less likely to resorb spontaneously and are associated with fair or worse surgical outcomes after discectomy [22–24]. Individuals with contained disc protrusions may potentially benefit from percutaneous disc decompression. Percutaneous decompression can be accomplished through several techniques, including chemical (chemonucleolysis and ozone), thermal (Radiofrequency Coblation®, Acutherm®, and light amplification by stimulated emission of radiation (laser)), and mechanical (automated percutaneous lumbar discectomy and Dekompressor®) means. However, each technique has its limitations, and the full efficacy of each is unknown due to a paucity of high-quality evidence.
Procedural Anatomy
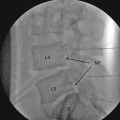
For any percutaneous disc procedure, access to the intervertebral disc is achieved with an extrapedicular posterolateral approach performed under fluoroscopic guidance (an oblique view) through a triangular working zone known as Kambin’s triangle [25]. The exiting nerve root, superior articular process of the facet joint, and superior end plate of the distal vertebra make the three dimensions: more specifically, the hypotenuse, perpendicular, and base, respectively, of Kambin’s triangle. Further fluoroscopic maneuvers are performed to access the disc as follows:
1.
The target disc is identified via fluoroscopy with an anteroposterior view in which the vertebral end plates are aligned perfectly (“squared off view”).
2.
An oblique view of the target disc is obtained in which the superior articular process (SAP) of the facet joint of the target segment lies against the midpoint of the intervertebral disc.
3.
A puncture point is identified over the target point at the mid-height of the target disc and ventral to the SAP projection. The introducer (trocar) entered in a coaxial fashion at this insertion point should avoid the spinal nerve as the nerve passes superolaterally.
Patient Selection
The radiologic identification of a disc herniation congruent with a patient’s history and physical examination is mandatory before the contemplation of a percutaneous disc procedure. Magnetic resonance imaging (MRI) is both sensitive and specific and has been reasonably reliable in the diagnosis of lumbar disc herniation [26]. Additionally, as mentioned above, MRI findings (the size of disc herniation and the containment status of the herniation) have been found to have significant impact on surgical outcomes. In a prospective study by Weiner et al., MRI was found to be 70 % accurate in identifying the containment status of a lumbar disc herniation [27].
The other important prerequisite is the demonstration that a patient’s symptoms emanate from the level of the disc proposed to be targeted for decompression. In the case where morphological changes are evident on MRI at multiple disc levels, the culprit level can be determined by the performing of selective nerve root blocks and the identification of the nerve root block that leads to the resolution of pain symptoms. In cases where there are equivocal findings on MRI or a lack of pain relief after selective nerve root block, provocative discography is warranted to find the symptomatic disc and assess its containment [28].
Indications [28]
1.
Predominately radicular pain lasting more than 6 months.
2.
The failure of conservative treatment.
3.
A small contained disc herniation evident on MRI or computed tomography (CT)/discography.
4.
The residual disc height of the involved disc is more than 50 % of the original disc height.
Contraindications [28]
In addition to the usual contraindications for any neuraxial intervention (such as systemic infection, local infection, coagulopathy, and patient refusal), contraindications particular to percutaneous disc decompression are as follows:
1.
Severe disc degeneration, as evidenced by residual disc height <50 % of the original disc height on imaging
2.
Large disc herniation that occupies more than one-third of the spinal canal
3.
Extruded or sequestered nucleus pulposus at the proposed level of intervention
4.
Previous lumbar back surgery (laminectomy, discectomy, or fusion) at the proposed level of intervention
5.
Progressive neurologic deficit
6.
Structural deformities such as spondylolisthesis, spinal canal stenosis, scoliosis, tumor, or fracture
Automated percutaneous lumbar discectomy, laser discectomy, Radiofrequency Coblation®, and Disc Dekompressor are the most common percutaneous disc decompression techniques used and are discussed below.
Automated Percutaneous Lumbar Discectomy (APLD)
After “percutaneous manual nucleotomy” fell out of favor and the use of CNL diminished due to adverse effects, refinements in surgical techniques led to the emergence of APLD in 1984. To overcome the inherent limitations of manual nucleotomy with a large cannula (5–8 mm diameter) and the cumbersome manual removal of nucleus pulposus, Onik et al. developed a smaller 2-mm probe with a single side port that potentially reduces the risk of nerve root injury and facilitates the easier removal of tissue with an all-in-one suction cutting device [29].