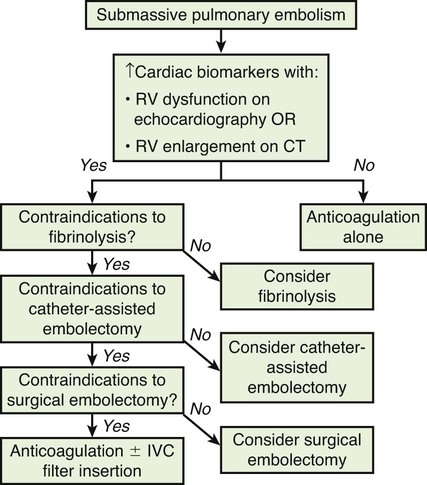
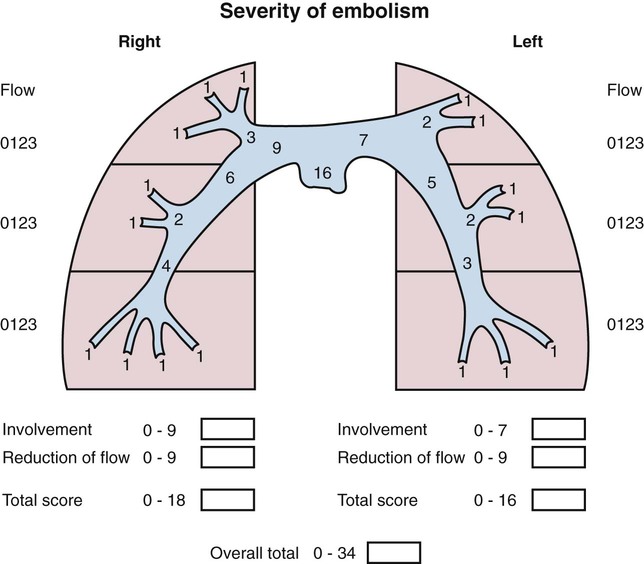
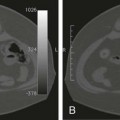
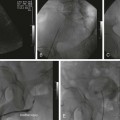
Chapter 86 Venous thromboembolism (VTE) is a global health problem that encompasses acute deep venous thrombosis (DVT) and acute pulmonary embolism (PE). Although the true incidence of PE is unknown, it is recognized as a significant cause of morbidity and mortality in hospitalized patients.1 In the United States alone, it is estimated there are between 500,000 and 600,000 cases per year,2 and approximately 300,000 people die every year from acute PE.3 Indeed, acute PE is believed to be the third most common cause of death among hospitalized patients.4 There are three basic categories of acute PE: (1) simple PE with no associated heart strain and no hypotension, (2) submassive PE with associated right heart strain, (3) massive PE with associated right heart strain and hemodynamic shock. Patients with simple acute PE require treatment with therapeutic anticoagulation alone. Patients with submassive PE may require treatment escalation beyond anticoagulation; and patients with massive PE certainly require treatment escalation beyond anticoagulation alone.5 The immediate mortality rate related to simple PE is less than 8% when the condition is recognized and treated with anticoagulation.1,6,7 However, patients with submassive PE have a higher cumulative mortality rate, reaching approximately 20% over a 90-day period.8 Patients with massive PE have the highest mortality rate, which can exceed 58%, including a high risk of sudden death.9 The pathophysiology of PE consists of direct physical obstruction of the pulmonary arteries, hypoxemic vasoconstriction, and release of potent pulmonary arterial vasoconstrictors that further increase pulmonary vascular resistance and right ventricular (RV) afterload. Acute RV pressure overload may result in RV hypokinesis and dilation, tricuspid regurgitation, and ultimately RV failure. RV pressure overload may also result in increased wall stress and ischemia by increasing myocardial oxygen demand while simultaneously limiting its supply. Ultimately, cardiac failure due to acute PE results from a combination of the increased wall stress and cardiac ischemia that compromise RV function and impair left ventricular (LV) output, resulting in life-threatening hemodynamic shock.9 Depending on underlying cardiopulmonary reserve, patients with acute massive PE may deteriorate over the course of several hours to days and develop systemic arterial hypotension, cardiogenic shock, and cardiac arrest. Owing to the risk of sudden death, these critically ill patients with massive PE should be quickly identified as candidates for rapid endovascular treatment as a lifesaving procedure.5 Because of the high mortality associated with acute massive PE, successful management requires prompt risk stratification and decisive early intervention (Table 86-1). Confirmation of hemodynamic shock attributed to central obstructing embolus should be present to justify treatment escalation beyond anticoagulation. The American College of Chest Physicians has recommended that percutaneous catheter-directed therapy (CDT) be considered in acute massive PE patients who are unable to receive systemic thrombolytic therapy because of bleeding risk.10 In addition, global meta-analytic data has demonstrated that percutaneous CDT can be considered as a first-line treatment option in lieu of intravenous (IV) tissue plasminogen activator (tPA) for patients with massive PE.11 TABLE 86-1 Risk Stratification and Indications for Aggressive Intervention to Treat Massive Pulmonary Embolism From Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol 2001;12:147–64. Pulmonary angiography was once considered the gold standard for diagnosing PE, but it has largely been replaced by the wide availability of cross-sectional imaging. Historically, many types of imaging studies have been used in diagnosing acute pulmonary embolism, including ventilation/perfusion (V/Q) scanning, magnetic resonance angiography (MRA), and computed tomographic angiography (CTA). CTA is the preferred modality and has proven to be advantageous thanks to its wide availability, superior speed, characterization of nonvascular structures, and detection of venous thrombosis. CTA has the greatest sensitivity and specificity for detecting emboli in the main, lobar, or segmental pulmonary arteries. Systematic reviews and randomized trials suggest that outpatients with suspected pulmonary embolism and negative CTA studies have excellent outcomes without therapy.12 If a patient has either acute or chronic renal insufficiency and contrast administration is undesirable, echocardiography may be used to evaluate for right heart dysfunction as an indication for underlying acute PE. The echocardiogram can be performed at bedside, and the study may reveal findings that strongly support hemodynamically significant pulmonary embolism,13 offering the potential to guide treatment escalation to thrombolytic and/or endovascular therapy. Large emboli moving from the heart to the lungs are occasionally confirmed with this technique. In addition, intravascular ultrasonography has also been used at the bedside to visualize central pulmonary emboli.14 Although the diagnosis of submassive PE follows a similar workup to evaluating massive PE, these patients do not present with systemic arterial hypotension, and particular attention must be paid to detecting the presence of right heart strain, which clinches the diagnosis of submassive PE. Identifying right heart strain allows risk stratification for possible treatment escalation beyond anticoagulation in normotensive PE patients.5 Echocardiography is the best imaging study to detect RV dysfunction in the setting of acute PE. Characteristic echocardiographic findings in patients with submassive PE include RV hypokinesis and dilatation, interventricular septal flattening and paradoxical motion toward the LV, abnormal transmitral Doppler flow profile, tricuspid regurgitation, pulmonary hypertension as identified by a peak tricuspid regurgitant jet velocity over 2.6 m/s, and loss of inspiratory collapse of the inferior vena cava (IVC).15 An RV-to-LV end-diastolic diameter ratio of 0.9 or greater, assessed in the left parasternal long-axis or subcostal view, is an independent predictor of hospital mortality.16 Detection of RV enlargement by chest CTA is especially convenient for diagnosis of submassive PE, because it uses data acquired during the initial diagnostic scan. Submassive PE can be diagnosed when RV enlargement on chest CT, defined by an RV-to-LV diameter ratio greater than 0.9, is observed17; RV enlargement on chest CTA also predicts increased 30-day mortality in patients with acute PE.17,18 Even if shock and death do not ensue, survivors of acute submassive PE remain at risk for developing chronic PE and thromboembolic pulmonary hypertension.19 Additionally, monitoring cardiac troponin T (cTnT) identifies the high-risk group of normotensive patients with submassive PE.20 A persistent increased cTnT level (>0.01 ng/mL) in PE patients with right heart strain predicts a significant risk of a complicated clinical course and fatal outcome, and these patients therefore require more aggressive treatment.20 Identifying submassive PE for treatment escalation is important because these normotensive PE patients demonstrate increased short-term mortality and high risk of adverse outcomes when the degree of heart strain results in elevations in levels of cardiac troponins and brain-type natriuretic peptide.21,22 The optimal protocol for treatment of acute submassive PE is still in evolution, but a proposed algorithm for managing submassive PE has been published23 describing treatment escalation beyond anticoagulation (Fig. 86-1). Based on the history, angiographic findings, and outcomes of patients undergoing CDT for acute PE, three groups of patients have been identified24: • Type I patients with fresh clots that have recently embolized should respond well to mechanical thrombectomy with increased peripheral flow and oxygenation. In general, 2 to 3 weeks is the upper age limit of thrombus when considering the option of CDT. • Type II patients with older and more organized clots respond less effectively to mechanical thrombectomy alone. Although more chronic clots are likely to remain, there is a chance for pulmonary flow improvement if pharmacologic thrombolysis of overlying acute thrombus can be achieved. • Type III patients with organized chronic PE do not respond well to the effects of CDT. Because of the risk of major hemorrhage, aggressive anticoagulation, systemic thrombolysis, and local catheter-directed thrombolysis may be contraindicated in patients with recent major general or intracranial surgery. In such cases, mechanical methods of percutaneous catheter-directed thrombectomy, fragmentation, and/or aspiration should be considered without pharmacologic agents. Since pulmonary hypertension is a relative contraindication to pulmonary angiography, the degree of pulmonary hypertension and underlying cardiopulmonary reserve are important considerations prior to performing pulmonary angiography. These factors must be used to determine a safe rate and volume of contrast injection into the pulmonary circulation. For instance, when systolic pulmonary artery pressure (PAP) exceeds 55 mmHg, or right ventricular end diastolic pressure (RVEDP) is greater than 20 mmHg, the mortality associated with pulmonary angiography using large-volume power injection has been reported to be as high as 3%.2,3 Therefore, such patients with massive PE should only receive a limited rate of power injection, controlled by hand. In the submassive PE patient, a selective contrast injection into the main left or right PA should not exceed a 20-mL volume at a rate of 10 mL/s. Lower injection parameters by hand may be considered depending on degree of heart failure, as determined by the operator’s judgment, to achieve adequate vessel opacification without endangering the patient. Pulmonary angiography can be used to calculate the degree of pulmonary obstruction before and after treatment, using the calculated Miller Index (Fig. 86-2). Finally, in PE patients with contraindications to anticoagulation and/or thrombolytic agents, placement of an IVC filter should be considered for prophylaxis of recurrent PE. Modern CDT for massive PE has been defined according to the following criteria: use of low-profile catheters and devices (≤10F), catheter-directed mechanical fragmentation and/or aspiration of emboli, and intraclot thrombolytic injection if a local drug is infused.11 Therefore, a variety of devices can be successfully used to treat PE, so long as they meet criteria for modern CDT (Table 86-2). TABLE 86-2 Catheter-Directed Therapy for Massive Pulmonary Embolism in 594 Patients
Percutaneous Interventions for Acute Pulmonary Embolism
Indications
At least one of the following criteria must be present:

Contraindications
Equipment
Author, Year (Reference)
Country
Patients, n
Sex-n
Age: Mean, Range
Technique-n
Local Intraclot Lytic During CDT-n
Local Intraclot Lytic, Extended Infusion-n
Minor Cxs
Major Cxs
Clinical Success (%)
Prospective Studies
Schmitz-Rode et al. 199848
Germany
10
M-6, F-4
54 (36-70)
PF-10
8
1
0
0
8/10 (80)
Schmitz-Rode et al. 200049
Germany
20
M-10, F-10
59 (48-60)
PF-20
0
0
1
0
16/20 (80)
Muller-Hulsbeck et al. 200150
Germany
9
M-4, F-5
55 (27-85)
ATD-9
0
5
0
0
9/9 (100)
Prokubovsky et al. 200351
Russia
20
na
51 (32-75)
PF-20
0
16
0
0
14/20 (70)
Tajima et al. 200452
Japan
25
M-8, F-17
61 (35-77)
PF & AT-25
25
21
0
0
25/25 (100)
Barbosa et al. 200853
Brazil
10
M-7, F-3
57 (39-75)
PF-10, (ATD-na)
0
0
0
0
9/10 (90)
Retrospective Studies
Brady et al. 199154
England
3
M-0, F-3
36 (18-71)
PF-1, MC-2
2
2
0
0
3/3 (100)
Rafique et al. 199255
South Africa
5
M-1, F-4
35 (21-47)
MC-5
5
5
1
0
5/5 (100)
Uflacker et al. 199624
U.S.A.
5
M-4, F-1
45 (25-64)
ATD-5
1
1
0
1
3/5 (60)
Fava et al. 199732
Chile
16
M-8, F-8
49 (20-68)
PF-16, (BA-na)
16
16
3
0
14/16 (88)
Stock et al. 199756
Switzerland
5
M-3, F-2
50 (21-80)
PF & BA-5
5
5
0
2
5/5 (100)
Basche et al. 199757
Germany
15
na
na (21-73)
PF & BA-2, BA-13
na
na
0
0
12/15 (80)
Hiramatsu et al. 199958
Japan
8
M-4, F-4
58 (42-87)
AT & WD-8
0
8
0
0
7/8 (88)
Wong et al. 199959
England
4
M-2, F-2
33 (18-46)
PF-1,PF & G-1, G-2
0
4
0
1
3/4 (75)
Murphy et al. 199960
Ireland
4
M-2, F-2
60 (46-66)
MC & WD-4
4
4
0
0
4/4 (100)
Voigtlander 199961
Germany
5
M-4, F-1
57 (25-72)
RT-5
0
0
4
0
3/5 (60)
Fava et al. 200062
Chile
11
M-3, F-8
61 (37-79)
Hy-11
0
4
0
0
10/11 (91)
Egge et al. 200263
Norway
3
M-2, F-1
49 (40-54)
PF-3
3
3
0
0
3/3 (100)
De Gregorio et al. 200233
Spain
59
M-25, F-34
56 (22-85)
PF-52, PF & BA-4, PF & DB -3
59
57
8
0
56/59 (95)
Zeni et al. 200336
U.S.A.
16
M-9, F-8
52 (30-86)
RT-16
0
10
2
1
14/16 (88)
Reekers et al. 200364
Netherlands
7
M-2, F-6
46 (28-76)
Hy-6, Oa-1
7
0
0
0
6/7 (86)
Tajima et al. 200465
Japan
15
M-4, F-11
60 (27-79)
AT-15
9
0
0
0
15/15 (100)
Fava et al. 200566
Chile
7
M-3, F-4
56 (30-79)
Hy-4, Oa-3
3
3
1
1
6/7/ (86)
Siablis et al. 200537
Greece
6
M-4, F-2
59 (42-76)
RT-6
4
0
2
0
5/6 (83)
Yoshida et al. 200667
Japan
8
M-4, F-4
61 (47-75)
PF & AT-8
na
na
0
1
7/8 (88)
Li J-J et al. 200668
China
15
M-11, F-4
56 (19-73)
PF & ATD-13, PF & Hy-1, PF & Oa-1
6
0
0
0
15/15 (100)
Pieri and Agresti. 200769
Italy
164
na
68 (35-78)
PF-164
164
164
0
0
138/164 (84)
Chauhan et al. 200770
U.S.A.
6
M-2 F-4
64 (49-78)
RT-6
2
0
5
2
4/6 (67)
Krajina 200771
Czech Rep
5
M-1, F-4
67 (52-80)
PF-3, PF & AT-2
3
0
0
0
2/5 (40)
Yang 200772
China
19
M-13, F-6
62 (22-87)
PF-10, PF & AT-5, PF+SR-4
19
na
0
0
18/19 (95)
Margheri 200873
Italy
20
M-12, F-8
66 (32-85)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access