Percutaneous Placement and Management of Hemodialysis Catheters
Intravenous catheters, both nontunneled (temporary) and tunneled, provide immediate venous access for hemodialysis patients. The use of such catheters continues to increase along with this growing patient population. Familiarity with the advantages and disadvantages of each type maximizes the potential benefit to patients. This chapter discusses hemodialysis catheters, the indications for and the contraindications to their use, appropriate sites of access, catheter placement, the immediate results and long-term outcome of catheterization, catheter maintenance, and management of catheter-related complications, with specific reference to the recommendations of the National Kidney Foundation Dialysis Outcomes Quality Initiative (NKF-DOQI).1
Optimum permanent hemodialysis access is through a surgically created upper-extremity arteriovenous (AV) fistula, or, if a fistula cannot be created or fails, through a surgically created AV graft. Each form of access can be expected to provide longer patency and fewer complications than that afforded by catheters; however, surgically created accesses require maturation prior to use. A new primary fistula should be allowed to mature for at least one month, and ideally for three to 4 months, before cannulation, and dialysis grafts should be placed at least 3 to 6 weeks before an anticipated need for hemodialysis in patients who are not candidates for primary AV fistulae. When a patient requires hemodialysis before creation or maturation of such access, a dialysis catheter is indicated. Similarly, hemodialysis may be performed through a catheter in a patient awaiting renal transplantation or initiation of continuous ambulatory peritoneal dialysis (CAPD). In a minority of patients in whom other access is not possible, a tunneled catheter may function as permanent access.
Dialysis catheters have many attributes that make them attractive for hemodialysis access: They are universally available; they can be inserted into many different sites; maturation time is not required (i.e., they allow immediate hemodialysis); unlike fistulas or grafts, their presence results in no hemodynamic consequence; and they can be inserted relatively easily by experienced physicians. These advantages do not come without cost, however; their use is associated with high morbidity relative to that of other accesses from both thrombosis and infection; there is a risk of permanent central venous stenosis or occlusion from catheterization; the presence of the catheter may cause patient discomfort; their expected duration of use is shorter than that of other accesses; and current catheters provide lower blood flow rates than other accesses.2–5
The initial success, ease of use, and painless access to the patient’s blood provided by a dialysis catheter may engender reluctance in the patient to consider other access options, despite the greater risk of infection and potentially inadequate dialysis (if lesser blood flows are not compensated by increased dialysis time) associated with chronic catheter access use. Further, the incidence of complications associated with cuffed tunneled dialysis catheters increases with their duration of use.3,6 Therefore, it is the opinion of the DOQI Work Group that fewer than 10% of chronic maintenance hemodialysis patients should be maintained on catheters as their permanent chronic dialysis access.1
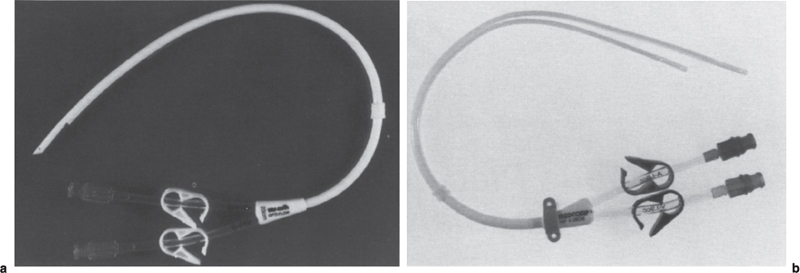
FIGURE 22–1. (a) 14.5F Opti-Flow double-lumen dialysis catheter (Bard Access Systems, Salt Lake City, UT) and (b) 14F Ash Split double-lumen catheter (Medcomp, Harleysville, PA). Blood is aspirated through the shorter red lumen and returned through the longer blue lumen on each catheter.
Although hemodialysis can be performed through a single-lumen catheter, in the United States, hemodialysis is performed through a double-lumen catheter (Fig. 22–1) or two single-lumen catheters (Fig. 22–2). Hemodialysis catheters, both nontunneled and tunneled, usually are made of either silicone or polyurethane, both of which are biocompatible and durable. Because high blood-flow rates are required for hemodialysis, these catheters must have large lumens (Poiseuille’s law shows that flow increases as the fourth power of the radius of the lumen of a conduit). Current hemodialysis catheter outer diameters range from about 10F to 14.5F.
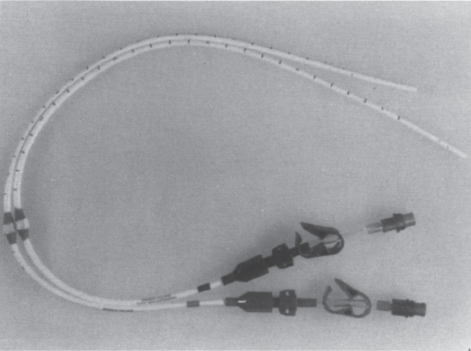
FIGURE 22–2. Tesio dialysis catheters (Medcomp, Harleysville, PA). Blood is aspirated through the shorter 10F catheter (red hub) and returned through the longer 10F catheter (blue hub).
Polyurethane has a higher tensile strength than silicone, which allows catheters to be constructed with a higher inner lumen to outer diameter size ratio and, consequently, higher flow rates in polyurethane catheters versus silicone catheters with the same outer diameter. Perhaps because of their thinner walls, polyurethane catheters have been more susceptible to kinking than those made of silicone; however, polyurethane catheters now on the market purport to have overcome this problem, and our initial experience suggests that flows greater than 400 mL per minute are possible through relatively kink-resistant double-lumen polyurethane catheters.7 Similarly, the Tesio twin-catheter system, consisting of two 10F silicone catheters, has been reported to consistently allow flow rates over 375 mL per minute.8
Nontunneled and tunneled hemodialysis catheters differ in several respects. The most obvious is whether a subcutaneous tunnel is used for their placement. Catheters intended for longer use are tunneled through a subcutaneous tract on the patient’s chest wall before their entrance into the jugular or subclavian vein, whereas nontunneled catheters exit the skin immediately above their insertion site. Most tunneled catheters have Dacron cuffs (Fig. 22–3) at a fixed distance from their tips. Tissue ingrowth into the fibers of the cuff anchors the catheter into position. Additionally, cuffs decrease the incidence of infection,9 theoretically because they prevent the migration of bacteria along the catheter into the bloodstream. Some catheters have a second cuff (see Fig. 22–3) that is impregnated with silver, which has been shown to inhibit bacterial growth10; but in our experience, use of this cuff is associated with an increased incidence of inadvertent catheter removal, perhaps as a result of local fibroblast cytotoxicity11 and resultant decreased tissue ingrowth. Similarly, coating of the catheter itself with silver did not prevent clinical infection or catheter colonization in a randomized study comparing uncoated and coated tunneled catheters.12

FIGURE 22–3. The smaller white cuff is made of Dacron. The larger, tan cuff is impregnated with silver.
The presence of the cuff(s) on a tunneled catheter mandates that the lengths of the catheter and the subcutaneous tunnel be matched carefully to the patient in whom the catheter is placed so that following placement the catheter tip is positioned at or below the caval-atrial junction and the cuff remains within the subcutaneous tract. Careful measurement of the intravascular portion of the catheter is necessary because, unlike infusion catheters, double-lumen hemodialysis catheters cannot be cut to size. The “Venous” lumen, through which blood is returned to the patient after hemodialysis, usually marked with a blue hub, is longer than the “arterial” lumen (red hub), through which blood is aspirated, to minimize recirculation (see Fig. 22–1). In two-catheter systems, the cuffs should be placed at the same level on the patient’s chest wall because such placement will ensure that the venous lumen tip extends beyond the arterial lumen tip.
Nontunneled hemodialysis catheters have no cuff and have relatively streamlined tips compared with those of tunneled catheters (Fig. 22–4). A nontunneled catheter’s tapered tip allows it to be placed directly over a wire rather than through the sheath, which is necessary for placement of a tunneled catheter. Because the nontunneled catheter has no cuff, measurement of the portion that will be intravascular is not necessary because the amount of catheter that can be left outside the patient is not limited. Nontunneled catheters, therefore, can be placed at the patient’s bedside easily, without fluoroscopy.
The choice between placement of a nontunneled catheter or of a tunneled catheter should be based on several factors:
1. The expected duration of need for catheter access: Nontunneled hemodialysis catheters can be inserted at the patient’s bedside; hence, they are less expensive to place than tunneled catheters. Numerous studies have demonstrated, however, that the rate of infection of catheters increases greatly with time. Suchoki et al3 reported a bacteremia rate lower than 5% for cuffed catheters used for less than 3 months compared with a 50% removal rate for infection for cuffed catheters at 12 months of use. Therefore, nontunneled jugular or subclavian catheters should be used for no longer than 3 weeks.13,14 Noncuffed femoral catheters, because of their high infection and dislodgment rates, should be left in place no longer than 5 days, and then only in bed-bound patients with good exit site care. We adhere to these DOQI guidelines1; however, it is important to note that others believe that nontunneled catheters can be safely used for significantly longer durations. For example, Montagnac et al15 showed a 41.5 ± 30-day mean duration of use of 64 silicone femoral catheters in 55 patients, and Takeda et al16 demonstrated a mean duration of use of 22.4 ± 13.1 days for 81 8F polyurethane single-lumen femoral catheters in 64 patients.
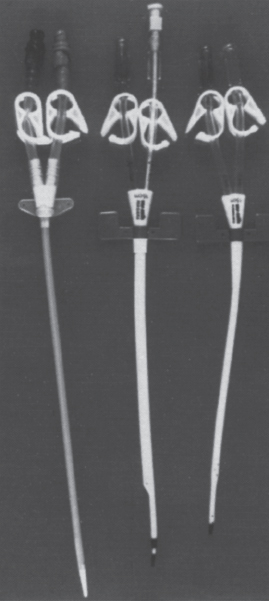
FIGURE 22–4. Nontunneled dialysis catheters.
2. Coexistent coagulopathy/thrombocytopenia: Although tunneled catheters can be expected to yield more lasting dialysis access than nontunneled catheters, their placement is more difficult and costly and should be avoided in some patients. In a coagulopathic or thrombocytopenic patient, the risk of bleeding from the subcutaneous tract mitigates against placement of a tunneled catheter. We attempt to correct the patient’s International Normalized Ratio (INR) to less than 1.4 times control and his or her platelet count to above 50,000 mm-3 before placement of a tunneled catheter. If hemodialysis is required before adequate correction, we place a nontunneled catheter. We have found that, using appropriate techniques [i.e., ultrasound- and fluoroscopic-guided cannulation of the jugular vein with a small-bore (21-gauge) needle], placement of nontunneled catheters is safe, even in markedly coagulopathic patients. We have placed nontunneled catheters in many such patients and have yet to encounter clinically significant bleeding.
3. Bacteremia: The risk of infection of a newly placed catheter in a bacteremic patient is high enough to mitigate against placement of a tunneled catheter. We mandate that a patient be afebrile on organism-specific antibiotics and that blood cultures have been negative for at least 48 hours before placement of a tunneled catheter, whereas we will place a nontunneled catheter in a septic patient if dialysis is needed. The catheter may become infected, but the access it allows may achieve sufficient temporization.
These considerations stress the differences in contraindications to tunneled or nontunneled catheter placement. In general, there are no contraindications to nontunneled catheter placement. If the patient requires dialysis, and catheter access is the only option, other considerations become secondary. Contrary to that simple equation addressing the immediate need to prevent death from renal insufficiency, the decision to place a tunneled catheter should take into account consideration of the aforementioned factors as well as future access planning.
Having chosen the desired catheter, the next step in placement is choosing the desired access site. As noted, nontunneled catheters can be inserted at the bedside into the femoral, internal jugular, or subclavian veins. There is ample evidence, however, demonstrating that subclavian catheterization is associated with a high incidence (20 to 50%) of resultant central venous stenosis or occlusion.17–19 Therefore, the subclavian vein should not be accessed in any patient who later may require surgically created access in the ipsilateral upper extremity. The group of patients in whom a subclavian catheter should not be placed includes not only those who already have chronic renal insufficiency but also those patients with borderline renal function, who eventually might develop renal insufficiency.
Either common femoral vein is an acceptable access site, but any catheter inserted therein should be at least 19 cm long to ensure that its tip reaches the inferior vena cava. Such placement will minimize recirculation.20 The longer expected duration of use (3 weeks versus 5 days)1 afforded by a nontunneled jugular catheter might lead one to choose jugular over femoral access. If ultrasound guidance is not used for catheter placement, however, the greater risks of jugular catheter placement versus those of femoral placement might reasonably lead to use of the latter vein. If the jugular vein is chosen for nontunneled catheter placement, the portion of the jugular vein optimally punctured might differ from that punctured for tunneled catheter placement: whereas the jugular vein should be punctured as close to the clavicle as possible when placing a tunneled catheter (to prevent kinking of the catheter at its forced bend at the point where it enters its subcutaneous tract), it can be punctured higher when placing a nontunneled catheter. A higher (i.e., more peripheral) puncture site has two additional potential advantages. First, the risk of pneumothorax may be decreased because the accessing needle is further removed from the cupula of the lung; second, the central portion of the jugular vein is preserved for future tunneled catheter placement, if indicated.
The preferred insertion site for a tunneled catheter is the right internal jugular (RIJ) vein21–23 because catheter insertion via this site is associated with the fewest complications.21,24 The left internal jugular (LIJ) vein is thought to be a less attractive choice because catheters placed through this vein have been shown to be associated with worse blood flow rates and higher rates of stenosis and thrombosis than RIJ catheters21,22,25; however, each of these studies included fewer than 15 LIJ catheters. Our preliminary examination (not yet published) of a larger group of LIJ catheters placed by interventional radiologists using ultrasound and fluoroscopic guidance found no difference in the flow rates achieved by RIJ or LIJ catheters when appropriate positioning of the catheter tip is ascertained on an upright chest radiograph obtained immediately following catheter placement. To date, whether LIJ catheters are more likely to incite central vein stenosis than RIJ catheters remains unanswered. The LIJ catheter potentially can place the left upper extremity’s vasculature in jeopardy by virtue of its course through the left innominate vein. Additionally, it is technically easier to place a RIJ catheter because of the shorter, straight course necessary for its placement compared with the longer, curved course mandated by a LIJ catheter. Thus, although no real difference has been found in flows obtainable through right or left jugular catheters, we continue to prefer to place right-sided catheters.
Many patients, especially those who have had multiple catheterizations in the past, have bilateral internal jugular vein occlusion. Ultrasound examination in these patients may demonstrate a patent external jugular vein. Either external jugular vein is acceptable for access and is preferable over either subclavian vein. If the external jugular veins are occluded as well, the choice of catheter placement is more difficult. It may be possible to recannulate the occluded vein or to cannulate a jugular collateral vein, thus achieving jugular placement. Such placement is more difficult than de novo vein puncture, however, and is not always possible. The remaining access sites are the subclavian and femoral veins and the inferior vena cava (IVC; translumbar placement).26,27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree